Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Low-dimensional materials facilitate the conjugation between fluorogenic boronic acids and saccharides†
Shi
Guo‡
a,
Jie
Chen‡
a,
Bi-Ying
Cai
a,
Wen-Wen
Chen
a,
Yu-Fei
Li
a,
Xiaolong
Sun
b,
Guo-Rong
Chen
a,
Xiao-Peng
He
*a and
Tony D.
James
 *c
*c
aKey Laboratory for Advanced Materials & Institute of Fine Chemicals, School of Chemistry and Molecular Engineering, East China University of Science and Technology, 130 Meilong Rd., Shanghai 200237, P. R. China. E-mail: xphe@ecust.edu.cn
bDepartment of Chemistry and Biochemistry, The University of Texas at Austin, Austin, Texas 78712, USA
cDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: T.D.James@bath.ac.uk
First published on 12th October 2016
Abstract
Here we demonstrate that low-dimensional materials (LDMs) enhance the conjugation between fluorogenic boronic acids (BAs) and saccharides. Among the LDMs investigated, 1D carbon nanotubes significantly lower the limit of detection and enhance the binding of the BA with D-fructose.
The development of synthetic receptors capable of imitating the active sites of natural biological receptors are of particular interest in the advancement of sensor development.1–6 Fluorophore-tagged boronic acids (fluorogenic BAs) are artificial receptors that are synthesized extensively for sensing biologically important diols such as saccharides, glyco/glycated proteins and dopamine by fluorescence and electrochemical techniques.7–11 In addition, many other types of BAs have been developed for building polymeric materials, separation systems and imaging probes.9–11 However, due to the moderate affinity in forming cyclic boronate esters with diols, the sensitivity of BAs for complex saccharides remains unsatisfactory.
With the rapid advancement of materials science and technology, a number of new functional materials have been produced over the past few decades. Among them the low-dimensional materials (LDMs) including the one-dimensional (1D) carbon nanotube (CNT), the 2D graphene and 2D graphene analogues have been the most attractive because of their exceptional optical, electronic and mechanical properties. Recently there has been an emerging trend to employ these LDMs (especially graphene oxide [GO] with good water solubility and biocompatibility) for biomedical applications.12–19 While, graphene analogues such as 2D transition metal dichalcogenides and oxides have also been shown to be useful in the construction of biosensors, drug delivering platforms and theranostic materials.12–19 We have shown in a recent study the possibility of constructing a BA/GO composite material for sensing saccharides.20 Here we demonstrate that the presence of a proper amount of LDMs can enhance fluorogenic BA–saccharide conjugation.
Two fluorophore-tagged phenylboronic acids (BA120 and BA2) were synthesized (Fig. 1). We employed typical donor–π–acceptor (D–π–A)21 chromophores, 1,8-naphthalimide and dicyanomethylene-4H-pyran (DCM), to couple with BA for the construction of the fluorogenic probes (Scheme S1, ESI†).22,23 Herein, we demonstrate that the fluorescence emission produced as a result of the BA–saccharide conjugation is enhanced in the presence of a small amount of LDMs including: 1D CNT, 2D GO, 2D molybdenum disulphide (MoS2) and 2D manganese dioxide (MnO2).
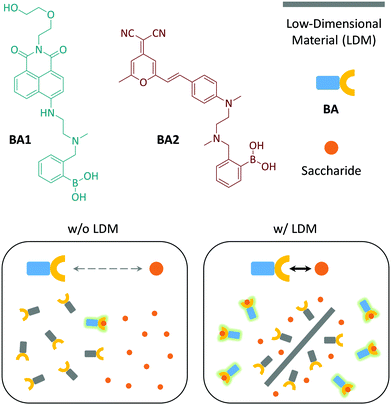 | ||
| Fig. 1 Structures of the fluorogenic boronic acids (BA1 and BA2) used in this study and schematic illustration of the low-dimensional material (LDM) enhanced conjugation between BA and saccharides. | ||
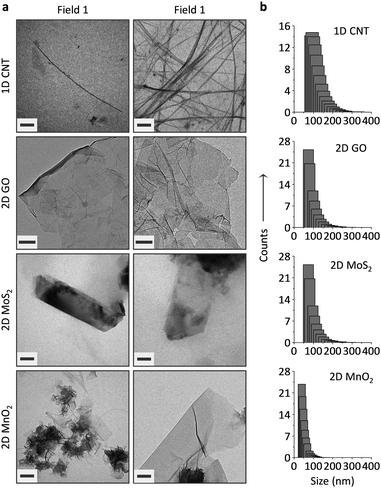
The carbon materials (single-walled CNT and GO) were purchased and the 2D MoS224 and 2D MnO225 prepared according to previous reports. The LDMs were characterized using transmission electron microscopy (TEM), dynamic light scattering (DLS), Raman spectroscopy and UV-vis spectroscopy. While tube-like objects were observed for CNT, GO appeared to be flakes of atomic-thickness (Fig. 2a). Meanwhile, thin-layer sheets were observed for 2D MoS2 and 2D MnO2 (Fig. 2a), and the morphology is in agreement with those observed in previous reports.24,25 DLS indicates that 2D GO and 2D MoS2 have a similar size distribution, whereas the particle size of 1D CNT was larger and that of 2D MnO2, and was slightly smaller than GO (Fig. 2b).
While typical absorbance peaks were observed for the LDMs (272 nm for 1D CNT,26 230 nm for GO,27 687/617 nm for 2D MoS2,24 and 370 nm for 2D MnO225) (Fig. S1, ESI†), Raman spectroscopy also suggests the formation of the LDMs (Fig. S2, ESI†). For example, in-plane stretching of sp2-carbon atoms (G band) were observed for the LDM carbon materials (1593 cm−1 for CNT and 1604 cm−1 for GO).28 The E12g and A1g vibration modes corresponded to the in-plane (382.6 cm−1) and out-of-plane (406.6 cm−1) vibrations of Mo and S atoms, proving the formation of thin-layer MoS2 sheets.29 In addition, a specific fingerprint of the Mn–O vibration in the MnO2 framework was observed at 572 cm−1.30
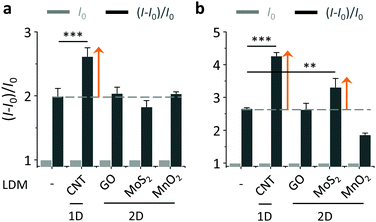
With the LDMs prepared, we then evaluated their ability to enhance the BA–saccharide conjugation (Fig. 3). As expected, we first determined that the fluorescence of both BA1 and BA2 increased in a concentration-dependent manner with D-fructose (fru) in a Tris-HCl buffer (Fig. S3, ESI†).20 A blue shift was observed for BA2 after conjugation with D-fructose. DCM has a typical D–π–A structure with a broad absorbance band because of an intramolecular charge transfer (ICT) process, therefore saccharide conjugation could compromise the planarity due to enlarged steric demand, resulting in a blue-shifted emission spectrum.21 Subsequently, with their initial fluorescence (I0) normalized, we tested the fluorescence enhancement [(I − I0)/I0, where I is the fluorescence intensity after conjugation with fru] of the BAs with or without LDMs. Interestingly, we determined that the presence of 1D CNT rather than other 2D LDMs led to an additional fluorescence enhancement of BA1 with fru (with respect to BA1 with fru alone) (Fig. 3a). In contrast, the additional fluorescence enhancement was produced by both 1D CNT and 2D MoS2 for the conjugation between BA2 and fru (Fig. 3b).
Subsequently, we investigated the effect of loading concentration with LDMs towards the BA–fructose conjugates. We calculated the limit of detection (LOD) for the fluorogenic BAs with D-fructose (3σ/k). The LODs of BA1 and BA2 in the absence and presence of the different LDMs are shown in Fig. 4. With low concentrations of CNT the LOD of BA1 for D-fructose was lowered (which means an improved sensitivity). Likewise the LOD of BA2 for D-fructose could also be lowered when using low concentrations of CNT and 2D MoS2. However, a further increase of the material loading reduces the sensitivity. According to our previous study,20a we also determined that the fluorescence of both BAs decreased in a concentration-dependent manner with the LDMs and that the quenched fluorescence of the BA/LDM composites could be recovered gradually with increasing fru (data not shown). This precludes the possibility that the fluorescence enhancement was a result of the interaction between BA probes and LDMs alone. We have observed a similar behaviour previously, where a low concentration of GO could improve the binding between a fluorophore-tagged ligand and a protein receptor.31,32 Low-concentration GO33 and 2D graphene analogues34 have also been shown to enhance the aggregation-induced-emission (AIE) of different AIEgens.
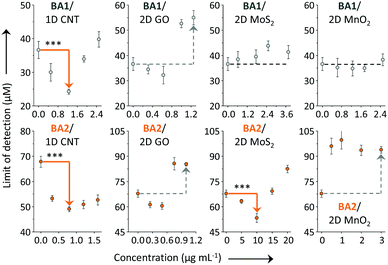 | ||
| Fig. 4 Limit of detection (LOD) of BA1 (20 μM) and BA2 (20 μM) for D-fructose in the absence and presence of a low-dimensional material (LDM) including carbon nanotube (CNT, 0–2.5 and 0–1.6 μg mL−1 for BA1 and BA2, respectively), graphene oxide (GO, 0–1.25 and 0–1 μg mL−1 for BA1 and BA2, respectively), molybdenum disulphide (MoS2, 0–3.75 and 0–20 μg mL−1 for BA1 and BA2, respectively) and manganese dioxide (MnO2, 0–2.5 and 0–3 μg mL−1 for BA1 and BA2, respectively) in Tris-HCl (0.01 M, pH 7.4) (***P < 0.001; two-tail P values were calculated by GraphPad software). For original plots for LOD calculation, see Fig. S4–S7 (ESI†). Excitation wavelength: 450 and 470 nm for BA1 and BA2, respectively. | ||
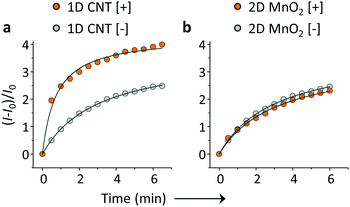
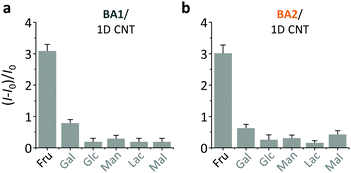
On the basis of the previous reports and data collected here we propose that the LDMs might serve as a platform to cluster both the fluorogenic BA probes and saccharides, and the approaching BA and saccharide molecules show an enhanced conjugation (Fig. 1). This has been further corroborated by a kinetic experiment that the presence of 1D CNT (Fig. 5a) rather than 2D MnO2 (Fig. 5b) evidently accelerated the binding between BA2 and D-fructose over a short time period. The ratio of the reaction rate constant of BA2 in the presence of CNT to that in the absence of CNT was determined to be 3.4. Additionally, both BA1 (Fig. 6a) and BA2 (Fig. 6b) showed good selectivity for D-fructose over a range of other saccharides in the presence of CNT, suggesting that the sensing performance of BA is not compromised in the presence of the LDM. However, a further increase of the materials might enhance the adsorption of the fluorophores to the material surface, thus leading to fluorescence quenching, since LDMs are typically quenching materials for fluorophores.16,17 Therefore, the smaller fluorescence enhancement of BA1 than BA2 with CNT (Fig. 3) might be a result of a stronger binding affinity of the former (0.79 L g−1) with the material than the latter (0.18 L g−1), leading to a stronger fluorescence quenching of the former. We are following up on these preliminary observations with more detailed investigations in order to probe the exact mechanism by which LDMs enhance the conjugation between the BA and saccharides and other diols.
To conclude, we have demonstrated, for the first time that BA–saccharide conjugation can be improved by LDMs when loaded at an appropriate concentration. This unique feature of LDMs enables the development of other types of BA/LDM composite materials with enhanced diol binding properties for a wide range of biomedical applications.
This research is supported by the 973 project (2013CB733700), the Science and Technology Commission of Shanghai Municipality (15540723800), the National Natural Science Foundation of China (21572058) and the Shanghai Rising-Star Program (16QA1401400). The Catalysis And Sensing for our Environment (CASE) network is thanked for research exchange opportunities. TDJ thanks ECUST for a guest professorship.
Notes and references
- E. Galbraith and T. D. James, Chem. Soc. Rev., 2010, 39, 3831–3842 RSC.
- T. J. Mooibroek, J. M. Casas-Solvas, R. L. Harniman, C. M. Renney, T. S. Carter, M. P. Crump and A. P. Davis, Nat. Chem., 2016, 8, 69–74 CrossRef CAS PubMed.
- H. Li, H. Valkenier, L. W. Judd, P. B. Brotherhood, S. Hussain, J. A. Cooper, O. Jurček, H. A. Sparkes, D. N. Sheppard and A. P. Davis, Nat. Chem., 2016, 8, 24–32 CrossRef CAS PubMed.
- C. Ke, H. Destecroix, M. P. Crump and A. P. Davis, Nat. Chem., 2012, 4, 718–723 CrossRef CAS PubMed.
- S.-K. Ko, S. K. Kim, A. Share, V. M. Lynch, J. Park, W. Namkung, W. V. Rosson, N. Busschaert, P. A. Gale, J. L. Sessler and I. Shin, Nat. Chem., 2014, 6, 885–892 CrossRef CAS PubMed.
- N. Busschaert, L. E. Karagiannidis, M. Wenzel, C. J. E. Haynes, N. J. Wells, P. G. Young, D. Makuc, J. Plavec, K. A. Jolliffe and P. A. Gale, Chem. Sci., 2014, 5, 1118–1127 RSC.
- S. D. Bull, M. G. Davidson, J. M. H. van den Elsen, J. S. Fossey, A. T. A. Jenkins, Y.-B. Jiang, Y. Kubo, F. Marken, K. Sakurai, J. Zhao and T. D. James, Acc. Chem. Res., 2013, 46, 312–326 CrossRef CAS PubMed.
- X. Wu, Z. Li, X.-X. Chen, J. S. Fossey, T. D. James and Y.-B. Jiang, Chem. Soc. Rev., 2013, 42, 8032–8048 RSC.
- M. Li, W. Zhu, F. Marken and T. D. James, Chem. Commun., 2015, 51, 14562–14573 RSC.
- X. Sun, W. Zhai, J. S. Fossey and T. D. James, Chem. Commun., 2016, 52, 3456–3469 RSC.
- X. Sun and T. D. James, Chem. Rev., 2015, 115, 8001–8037 CrossRef CAS PubMed.
- M. Pumera and A. H. Loo, Trends Anal. Chem., 2014, 61, 49–53 CrossRef CAS.
- Y. Chen, C. Tan, H. Zhang and L. Wang, Chem. Soc. Rev., 2015, 44, 2681–2701 RSC.
- D. Chimene, D. L. Alge and A. K. Gaharwar, Adv. Mater., 2015, 27, 7261–7284 CrossRef CAS PubMed.
- Y. Zhang, B. Zheng, C. Zhu, X. Zhang, C. Tan, H. Li, B. Chen, J. Yang, J. Chen, Y. Huang, L. Wang and H. Zhang, Adv. Mater., 2015, 27, 935–939 CrossRef CAS PubMed.
- X.-P. He, Y. Zang, T. D. James, J. Li and G.-R. Chen, Chem. Soc. Rev., 2015, 44, 4239–4248 RSC.
- X.-P. He and H. Tian, Small, 2016, 12, 144–160 CrossRef CAS PubMed.
- C. Zhu, Z. Zeng, H. Li, F. Li, C. Fan and H. Zhang, J. Am. Chem. Soc., 2013, 135, 5998–6001 CrossRef CAS PubMed.
- H. Fan, Z. Zhao, G. Yan, X. Zhang, C. Yang, H. Meng, Z. Chen, H. Liu and W. Tan, Angew. Chem., Int. Ed., 2015, 54, 4801–4805 CrossRef CAS PubMed.
- (a) X. Sun, B. Zhu, D.-K. Ji, Q. Chen, X.-P. He, G.-R. Chen and T. D. James, ACS Appl. Mater. Interfaces, 2014, 6, 10078–10082 CrossRef CAS PubMed; (b) X. Sun, Q. Xu, G. Kim, S. E. Flower, J. P. Lowe, J. Yoon, J. S. Fossey, X. Qian, S. D. Bull and T. D. James, Chem. Sci., 2014, 5, 3368–3373 RSC.
- H. Zhu, B. Liu, J. Liu, W. Zhang and W.-H. Zhu, J. Mater. Chem. C, 2015, 3, 6882–6890 RSC.
- S. Banerjee, J. A. Kitchen, S. A. Bright, J. E. O’Brien, D. C. Williams, J. M. Kelly and T. Gunnlaugsson, Chem. Commun., 2013, 49, 8522–8524 RSC.
- R. B. P. Elmes, M. Erby, S. A. Bright, D. C. Williams and T. Gunnlaugsson, Chem. Commun., 2012, 48, 2588–2590 RSC.
- U. Halim, C. R. Zheng, Y. Chen, Z. Lin, S. Jiang, R. Cheng, Y. Huang and X. Duan, Nat. Commun., 2013, 4, 2213 Search PubMed.
- D. He, X. Yang, X. He, K. Wang, X. Yang, X. He and Z. Zou, Chem. Commun., 2015, 51, 14764–14767 RSC.
- A. G. Ryabenko, T. V. Dorofeeva and G. I. Zvereva, Carbon, 2004, 42, 1523–1535 CrossRef CAS.
- D. Li, M. B. Müller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS PubMed.
- Z. Ni, Y. Wang, T. Yu and Z. Shen, Nano Res., 2008, 1, 273–291 CrossRef CAS.
- C. Lee, H. Yan, L. E. Brus, T. F. Heinz, J. Hone and S. Ryu, ACS Nano, 2010, 4, 2695–2700 CrossRef CAS PubMed.
- C. Julien, M. Massot, R. Baddour-Hadjean, S. Franger, S. Bach and J. P. Pereira-Ramos, Solid State Ionics, 2003, 159, 345–356 CrossRef CAS.
- D.-K. Ji, Y. Zhang, X.-P. He and G.-R. Chen, J. Mater. Chem. B, 2015, 3, 6656–6661 RSC.
- D.-K. Ji, Y. Zhang, Y. Zang, W. Liu, X. Zhang, J. Li, G.-R. Chen, T. D. James and X.-P. He, J. Mater. Chem. B, 2015, 3, 9182–9185 RSC.
- X. Qi, H. Li, J. W. Y. Lam, X. Yuan, J. Wei, B. Z. Tang and H. Zhang, Adv. Mater., 2012, 24, 4191–4195 CrossRef CAS PubMed.
- C. Tan, X. Qi, X. Huang, J. Yang, B. Zheng, Z. An, R. Chen, J. Wei, B. Z. Tang, W. Huang and H. Zhang, Adv. Mater., 2014, 24, 1735–1739 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional figures, experimental section and original spectral copies. See DOI: 10.1039/c6qm00158k |
| ‡ Equal contribution. |
| This journal is © the Partner Organisations 2017 |