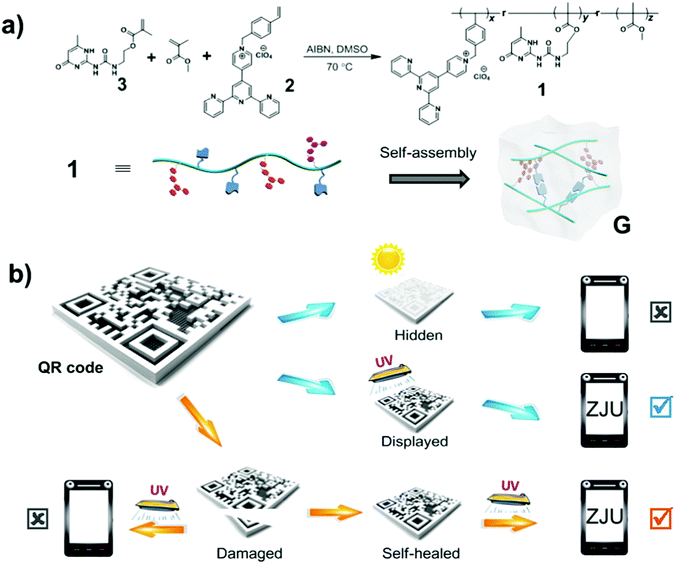
Preparation of a white-light-emitting fluorescent supramolecular polymer gel with a single chromophore and use of the gel to fabricate a protected quick response code†
Hu
Wang
a,
Xiaofan
Ji
a,
Zhengtao
Li
a,
Chao Nan
Zhu
b,
Xuxu
Yang
c,
Tiefeng
Li
c,
Zi Liang
Wu
*b and
Feihe
Huang
*a
aState Key Laboratory of Chemical Engineering, Center for Chemistry of High-Performance & Novel Materials, Department of Chemistry, Zhejiang University, Hangzhou 310027, P. R. China. E-mail: fhuang@zju.edu.cn; Fax: +86-571-8795-3189; Tel: +86-571-8795-3189
bKey Laboratory of Macromolecular Synthesis and Functionalization of Ministry of Education, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou, 310027, P. R. China. E-mail: wuziliang@zju.edu.cn
cSoft Matter Research Center, Key Laboratory of Soft Machines and Smart Devices of Zhejiang Province, Department of Engineering Mechanics, Zhejiang University, Hangzhou, Zhejiang 310027, P. R. China
First published on 6th October 2016
Abstract
We report here a novel yet facile approach to prepare white-light-emitting fluorescent polymeric materials resulting from the aggregation of a single fluorescent chromophore. This aggregation resulted from the energetically favorable self-assembly of the polymer through intermolecular quadruple hydrogen bonding. This advanced material was shown to be an ideal candidate for constructing intelligent information display/storage devices. A protected quick response code was fabricated by using this white-light-emitting fluorescent supramolecular gel, which was shown to hide the information under natural light yet to display it under UV light. Therefore, the code was protected and only readable under a specific condition. Furthermore, due to the dynamic nature of hydrogen bonds, the supramolecular polymer gel and the resulting quick response code showed self-healing abilities, which is crucial for their applications. The information of an intentionally damaged QR code was incomplete and could not be read out under UV light irradiation, but we were able to mend the code, based on the interfacial self-assembly of the gels through multiple hydrogen bonding, and then recover the protected information.
Introduction
The quick response (QR) code,1 mostly represented in binary form (black and white pixels), is a two-dimensional code in a square shape and attached to web pages or posters. The QR code was developed to overcome the inconveniences of using a mobile phone keypad, in particular for linking to web pages.1a,c With the rapid development of network information technology, information security has become increasingly more important and a focus for many scientists. For example, Aida and co-workers reported a novel photoluminescent ink for rewritable media that dichroically phosphoresces due to a structural bistability of the self-assembled luminophor.2a Long-lasting images were developed by using conventional thermal printers, and were readable only on exposure to ultraviolet light; and more importantly, the images were thermally erasable, allowing for rewriting. Similarly, securing QR code information has become an increasingly important issue in information science. In addition, the QR code cannot be read out once damaged. Therefore, it is desirable to develop protected codes with improved durability and security. First, the security would be improved if the code can be hidden and displayed on demand. In this regard, fluorescent materials,2 which only emit light when irradiated with light of a specific wavelength, are promising materials for constructing protected QR codes. Conventional QR codes are made of zebra stripes to maximize chromatic aberration,3 which favors reading the code. Therefore, to prepare protected QR codes, white-light-emitting fluorescent materials4 would be ideal candidates. Secondly, to improve the durability of QR codes, the material applied should have a self-healing ability, so that, under specific conditions, any damaged codes could be mended and their functions recovered (i.e., so that the information can still be read out after the recovery). A white-light-emitting fluorescent material can be endowed with such a self-healing ability by noncovalently crosslinking the polymeric constituents of the material. By applying these methods with a white-light-emitting fluorescent supramolecular material, protected codes can be developed.Despite the myriad of available fluorescent materials, white-light emission is not easily achieved. White fluorescence is usually attained by adjusting the molar proportion of fluorescent chromophores that emit different colors, such as red, green, and blue (RGB). For example, Nandi and co-workers successfully prepared a white-light-emitting gel by incorporating 6,7-dimethoxy-2,4[1H,3H]quinazolinedione (Q), riboflavin (R), and rhodamine B (RhB) in a requisite proportion of Q![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R
R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RhB = 100
RhB = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02 (molar ratio).5a Meijer and co-workers used blue-light-emitting oligofluorene (OF), green-light-emitting oligo(phenylene-vinylene) (OPV), and red-light-emitting perylene bisimide (PB) as the RGB emitting chromophores to prepare a white-light-emitting photoluminescent film, in which the molar ratio of OF to OPV to PB was 84
0.02 (molar ratio).5a Meijer and co-workers used blue-light-emitting oligofluorene (OF), green-light-emitting oligo(phenylene-vinylene) (OPV), and red-light-emitting perylene bisimide (PB) as the RGB emitting chromophores to prepare a white-light-emitting photoluminescent film, in which the molar ratio of OF to OPV to PB was 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.5b However, there are two disadvantages in this traditional approach to develop white-light-emitting fluorescent materials: (1) the synthesis of the various fluorescent chromophores involves multi-step reactions and is therefore complex and requires much work; and (2) it is difficult to accurately control the molar ratio of the different fluorescent chromophores.
6.5b However, there are two disadvantages in this traditional approach to develop white-light-emitting fluorescent materials: (1) the synthesis of the various fluorescent chromophores involves multi-step reactions and is therefore complex and requires much work; and (2) it is difficult to accurately control the molar ratio of the different fluorescent chromophores.
To address the above issues, we report a novel yet facile approach to obtain a supramolecular polymer gel6 showing white fluorescence based on the aggregation of a single fluorescent chromophore. The aggregation was assisted by self-assembly via multiple hydrogen bonding of ureidopyrimidinone (UPy), which endowed the fluorescent gel with self-healing properties. Moreover, due to the dynamic nature of hydrogen bonding, which can be destroyed by heating (or by adding a polar solvent) and then recovered by cooling (or removing the polar solvent), this fluorescent gel showed reversible gel–sol transitions accompanied by changes in the fluorescence.
Polymer 1 used intermolecular quadruple hydrogen bonding to self-assemble into a translucent supramolecular polymer gel in chloroform (see Scheme 1a). The pyridinium salt monomer 2, which is a donor–acceptor molecule, emits intense white light upon aggregating.4f Self-assembly of polymer 1 to form a supramolecular polymeric gel apparently resulted in the pyridinium salt units becoming close to each other and forming charge-transfer complexes, giving rise to an additional low-energy emission that combined with the non-quenched high-energy monomer emission to produce white fluorescence. Then, a protected QR code was fabricated by carrying out laser carving of this fluorescent supramolecular gel coated on a glass plate (Scheme 1b). Under natural light, the code information was found to be hidden, due to the absence of fluorescence and low contrast between the patterned gel and the background. However, the code information was displayed under UV light (wavelength of 365 nm) and could be read out quickly after being scanned by a mobile phone. Furthermore, the damaged QR code could be mended due to the dynamic nature of hydrogen bonding in the fluorescent supramolecular gel. As a consequence, the healed QR code displayed the original information and could be read out under UV light. Therefore, this protected QR code made from the fluorescent supramolecular gel can hide/display the information on demand and self-heal after damage.
Experimental
Polymer 1 was prepared by free radical polymerization of methyl methacrylate (MMA), chromophore monomer 2, and UPy monomer 3 in DMSO. The chromophore was a pyridinium salt derived from 2,2′:6′,2′′-terpyridine. Polymer compositions were calculated based on the integrations of the proton NMR peaks of H1g in the chromophore, H1d in the UPy unit, and H1q in the methoxy group of the MMA unit (Fig. S5, ESI†). These data combined with gel permeation chromatography (GPC) data were analyzed to determine the number of each repeat unit per polymer chain. For the details of the synthesis and characterization data, see ESI,† (Fig. S1–S4 and Table S1).Results and discussion
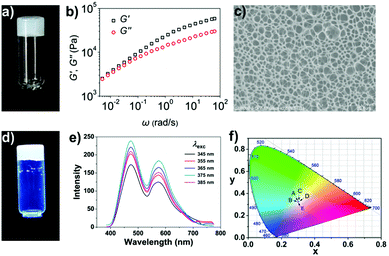
In chloroform, polymer 1 samples at appropriate concentrations were made to self-assemble into a supramolecular polymeric gel through the quadruple hydrogen bonding interactions between its polymer chains. A viscoelastic gel was obtained upon adding polymer 1 into the chloroform solution at a concentration of 60 mg mL−1 (Fig. 1a). Specifically, the rheological experimental results showed both the storage modulus, G′, and loss modulus, G′′, of the sample to increase with frequency, with G′ larger than G′′ throughout the range 0.005–100 rad s−1, indicating the sample to be a highly elastic gel (Fig. 1b). This highly viscoelastic gel was found to keep its shape over time: it did not flow down a bottle that was kept upside down for 24 h (Fig. 1a). The microstructure of the gel was characterized using scanning electron microscopy (SEM) (Fig. 1c); a sponge-like morphology was observed, resulting from the release of entrapped solvent during the drying process.Pyridinium salt 2, which is a donor–acceptor molecule, emits intense blue light in chloroform under UV light irradiation. As shown in Fig. S6 (ESI†), the pyridinium salt 2 in chloroform yielded the same fluorescence emission peak at a wavelength of about 538 nm whether the excitation wavelength was 345, 355, 365, 375, or 385 nm. On the other hand, the supramolecular polymer gel assembled from polymer 1 showed strong white light fluorescence under UV light (Fig. 1d). Polymer 1 yielded the same two fluorescence emission peaks at about 474 and 571 nm under different excitation wavelengths (Fig. 1e). We attributed the white light fluorescence here to the aggregation of the chromophore and the formation of a charge-transfer complex: this complex apparently gave an additional low-energy emission at 571 nm that combined with the non-quenched high-energy monomer emission at 474 nm to yield the white light. It is interesting that the dual emissions of polymer 1 exhibited an obvious excitation wavelength dependence. To clearly demonstrate the difference between the fluorescence intensity at 474 nm and that at 571 nm, we measured the intensities of each peak for various excitation wavelengths (Fig. S7 and S8, ESI†). After the intensity at 571 nm (low-energy emission) was normalized to 1, the intensity at 474 nm (high-energy emission) was found to be dependent on the excitation wavelength; similarly, when the intensity at 474 nm was normalized to 1, the intensity at 571 nm was also found to be dependent on the excitation wavelength. The excitation-wavelength-dependent emission indicated that the two emissions of the gel to be mutually isolated. Because the relative intensities of the two emissions changed with the excitation energy, the purity of the white light was analyzed according to the 1931 Commission Internationale de L'Eclairage (CIE) chromaticity diagram. The CIE coordinates of the fluorescent supramolecular gel was found to be (0.3008, 0.3266) when excited at 345 nm, (0.2983, 0.3232) at 355 nm, (0.3041, 0.3262) at 365 nm, (0.3064, 0.3254) at 375 nm, and (0.3005, 0.3243) at 385 nm (Fig. 1f). That is, the sample emitted white light for excitation wavelengths differing by over 40 nm. Thus, in contrast to traditional methods, we developed a pure white-light-emitting fluorescent gel by using a single fluorescent chromophore as the building block.5
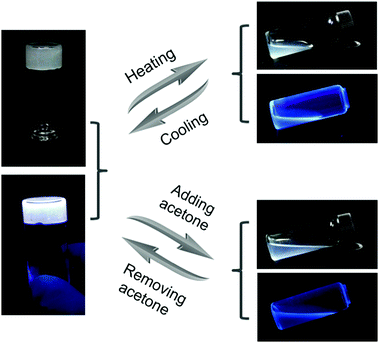
Due to the dynamic nature of the hydrogen bonds of UPy, which can be broken and formed again under external stimuli, the supramolecular gel showed reversible gel–sol transitions accompanied with the changes in fluorescence. As shown in Fig. 2, after being heated at 50 °C, the gel G was transformed to a sol state. At the same time, the charge-transfer complexes also disintegrated, so the fluorescence of the sample changed from white in the gel state to blue in the sol state. After cooling, the gel state and the white fluorescence of the sample recovered owing to the re-formation of the multiple hydrogen bonds. Based on a similar principle, the gel was also responsive to solvents with high polarity. When a volume of 0.5 mL of acetone was added to disrupt the hydrogen bonds, the white-light-emitting fluorescent gel G transformed to a sol state that exhibited blue fluorescence. Then, the solvent of the sample was allowed to evaporate completely and chloroform was added to reform the white-light-emitting fluorescent supramolecular gel G.
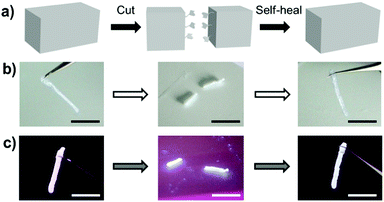
In addition, due to the reversibility of the hydrogen bonding, the fluorescent supramolecular gel showed a good healing ability. As shown in Fig. 3, a gel strip was cut into two pieces, and then the two fragments were simply placed next to each other. After adding a small drop of chloroform and waiting for about 70 s, the two fragments healed into one integrated gel, which could be lifted without breaking (Video S1, ESI†).
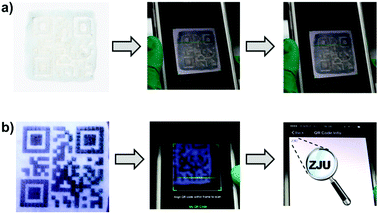
This white-light-emitting fluorescent supramolecular gel was used to construct the protected QR code. As shown in Fig. 4, the gel obtained from polymer 1 was coated onto a glass plate. The gel coating was fabricated into a QR code by laser carving according to a digital template. When the code was placed under natural light, there was no obvious difference between the color of the gel and that of the background, and hence the code could not be read by the QR code scanner of a cell phone. When the code was placed under 365 nm-wavelength UV light, however, the zebra pattern appeared because part of the gel showed white fluorescence and the carved location (i.e., the background) did not fluoresce and hence appeared black. In this condition, the code was easily read after one scan (Video S2, ESI†). These results indicated that the protected QR code made of the white-light-emitting fluorescent gel can hide and display the information as required.
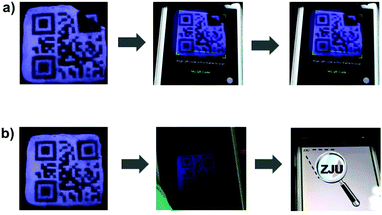
Due to the self-healing ability of the fluorescent supramolecular gel, the protected QR code should have improved resistance against mechanical damage. The damaged code was able to heal under a specific condition, which is important for applications. Fig. 5 shows an experiment attesting to the ability of the readability of this code to recover after damage. The tested code was damaged by cutting off a small corner, and then could not be read out under UV light due to the incomplete information. The fragment was then placed in its original location, and a small drop of chloroform was added to this region. After about 90 s, the fragment was observed to have re-joined the remaining part of the code, and no crack was evident in this region. Under UV light, the repaired QR code could be read out again (Video S3, ESI†). Therefore, it is possible to recover data from this fluorescent supramolecular QR code after being damaged.
Conclusions
In summary, we prepared a white-light-emitting fluorescent supramolecular gel, with a single chromophore aggregating to form a charge-transfer complex promoted by the self-assembly of polymer 1 through intermolecular quadruple hydrogen bonding. This advanced material is an ideal candidate for constructing intelligent information display/storage devices. A protected QR code was fabricated from this white-light-emitting fluorescent supramolecular gel, which was shown to hide information under natural light yet display it under UV light. Therefore, the code was found to be protected and only readable under a specific condition. Furthermore, due to the dynamic nature of hydrogen bonds, the supramolecular polymer gel and the resulting QR code were shown to have self-healing abilities, which is crucial for their applications. The information of an intentionally damaged QR code was incomplete and could not be read out under UV light irradiation, but we were able to mend the code, based on the interfacial self-assembly of the gels through multiple hydrogen bonding, and then recover the protected information.In conclusion, we developed a novel yet facile approach to prepare white-light-emitting fluorescent polymeric materials based on the aggregation of a single fluorescent chromophore. Furthermore, we fabricated a novel QR code with improved information security and durability against mechanical damage, which should find applications in a variety of fields.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We gratefully acknowledge the National Basic Research Program (2013CB834502), NSFC/China (91527301, 21434005, 51403184, 11302190 and 11432012), the Key Science Technology Innovation Team of Zhejiang Province (2013TD02), Open Project of State Key Laboratory of Supramolecular Structure and Materials (sklssm201611), and Fundamental Research Funds for the Central Universities for financial support.Notes and references
- (a) J. S. Pan, H. C. Huang, L. C. Jain and W. C. Fang, Intelligent Multimedia Data Hiding, 2007, vol. 2, pp. 461–464 Search PubMed; (b) J. S. Tan, Synth. J., 2008, 59–78 Search PubMed; (c) H. C. Huang and W. C. Fang, Simul. Model. Pract. Th., 2010, 18, 436–445 CrossRef; (d) H. C. Huang, F. C. Chang and W. C. Fang, IEEE Trans. Consum. Electron., 2011, 57, 779–787 CrossRef.
- (a) A. Kishimura, T. Yamashita, K. Yamaguchi and T. Aida, Nat. Mater., 2005, 4, 546–549 CrossRef CAS PubMed; (b) F. Huang, H. Wu and Y. Cao, Chem. Soc. Rev., 2010, 39, 2500–2521 RSC; (c) L. Zhu, C. Y. Ang, X. Li, K. T. Nguyen, S. Y. Tan, H. Ågren and Y. Zhao, Adv. Mater., 2012, 24, 4020–4024 CrossRef CAS PubMed; (d) C. Duan, K. Zhang, C. Zhong, F. Huang and Y. Cao, Chem. Soc. Rev., 2013, 42, 9071–9104 RSC; (e) Z. Zhao, J. W. Y. Lamb and B. Z. Tang, Soft Matter, 2013, 9, 4564–4579 RSC; (f) X. Ji, Y. Yao, J. Li, X. Yan and F. Huang, J. Am. Chem. Soc., 2013, 135, 74–77 CrossRef CAS PubMed; (g) L. Zhu, X. Li, Q. Zhang, X. Ma, M. Li, H. Zhang, Z. Luo, H. Ågren and Y. Zhao, J. Am. Chem. Soc., 2013, 135, 5175–5182 CrossRef CAS PubMed; (h) L.-L. Li, H.-L. Ma, G.-B. Qi, D. Zhang, F. Yu, Z. Hu and H. Wang, Adv. Mater., 2016, 28, 254–262 CrossRef CAS PubMed.
- (a) L. N. Thibos, A. Bradley, D. L. Still, X. Zhang and P. A. Howarth, Vision Res., 1990, 30, 33–49 CrossRef CAS PubMed; (b) P. Kruger, S. Mathews, K. Aggarwala and N. Sanchez, Vision Res., 1993, 33, 1397–1411 CrossRef CAS PubMed; (c) D. H. Marimont and B. A. Wandell, J. Opt. Soc. Am. A, 1994, 11, 3113–3122 CrossRef.
- (a) G. Tu, C. Mei, Q. Zhou, Y. Cheng, Y. Geng, L. Wang, D. Ma, X. Jing and F. Wang, Adv. Funct. Mater., 2006, 16, 101–106 CrossRef CAS; (b) Y. S. Zhao, H. Fu, F. Hu, A. Peng, W. Yang and J. Yao, Adv. Mater., 2008, 20, 79–83 CrossRef CAS; (c) S. H. Kim, S. Park, J. E. Kwon and S. Y. Park, Adv. Funct. Mater., 2011, 21, 644–651 CrossRef CAS; (d) K. P. Tseng, C. Fang, J. J. Shyue, K. T. Wong, G. Raffy, A. D. Guerzo and D. M. Bassani, Angew. Chem., Int. Ed., 2011, 50, 7032–7036 CrossRef CAS PubMed; (e) S. K. Samanta and S. Bhattacharya, Chem. – Eur. J., 2012, 18, 15875–15885 CrossRef CAS PubMed; (f) X.-H. Jin, C. Chen, C.-X. Ren, L.-X. Cai and J. Zhang, Chem. Commun., 2014, 50, 15878–15881 RSC.
- (a) P. Bairi, B. Roy, P. Chakraborty and A. K. Nandi, ACS Appl. Mater. Interfaces, 2013, 5, 5478–5485 CrossRef CAS PubMed; (b) R. Abbel, C. Grenier, M. J. Pouderoijen, J. W. Stouwdam, P. E. L. G. Leclère, R. P. Sijbesma, E. W. Meijer and A. P. H. J. Schenning, J. Am. Chem. Soc., 2009, 131, 833–843 CrossRef CAS PubMed.
- (a) H. W. Gibson, N. Yamaguchi and J. W. Jones, J. Am. Chem. Soc., 2003, 125, 3522–3533 CrossRef CAS PubMed; (b) Z. Niu and H. W. Gibson, Chem. Rev., 2009, 109, 6024–6046 CrossRef CAS PubMed; (c) Y. Li, T. Park, J. K. Quansah and S. C. Zimmerman, J. Am. Chem. Soc., 2011, 133, 17118–17121 CrossRef CAS PubMed; (d) D.-S. Guo and Y. Liu, Chem. Soc. Rev., 2012, 41, 5907–5921 RSC; (e) L. Chen, Y.-K. Tian, Y. Ding, Y.-J. Tian and F. Wang, Macromolecules, 2012, 45, 8412–8419 CrossRef CAS; (f) T. Xiao, X. Feng, S. Ye, Y. Guan, S.-L. Li, Q. Wang, Y. Ji, D. Zhu, X. Hu, C. Lin, Y. Pan and L. Wang, Macromolecules, 2012, 45, 9585–9594 CrossRef CAS; (g) S. Seiffert and J. Sprakel, Chem. Soc. Rev., 2012, 41, 909–930 RSC; (h) A. Noro, M. Hayashi and Y. Matsushita, Soft Matter, 2012, 8, 6416–6429 RSC; (i) Y. Han, Z. Meng, Y.-X. Ma and C.-F. Chen, Acc. Chem. Res., 2014, 47, 2026–2040 CrossRef CAS PubMed; (j) Z. Qi and C. A. Schalley, Acc. Chem. Res., 2014, 47, 2222–2233 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qm00164e |
| This journal is © the Partner Organisations 2017 |