AIE-active β-diketones containing pyridiniums: fluorogenic binding to cellulose and water-vapour-recoverable mechanochromic luminescence†
Tongqing
Xie
ab,
Baicheng
Zhang
a,
Xuepeng
Zhang
*a and
Guoqing
Zhang
*a
aHefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China. E-mail: gzhang@ustc.edu.cn
bCAS Key Laboratory of Soft Matter Chemistry, Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei, 230026 Anhui, China
First published on 10th October 2016
Abstract
A series of pyridinium ring substituted β-diketones were found to exhibit aggregation-induced emission (AIE) and water-vapour recoverable mechanochromic luminescence in the solid state. Certain water-soluble β-diketones could selectively bind to cellulose-based materials, where strong fluorescence similar to AIE was turned on.
Introduction
Aromatic β-diketones (BDKs) are frequently used as chelating ligands for Lewis acids and to produce complexes used in many applications such as catalysis,1 vapour deposition,2 and optoelectronics.3 For example, difluoroboron diketonates4–6 have lately received tremendous attention due to their mechanochromic luminescence (ML)7–9 and room-temperature phosphorescence properties.10–13 The BDK ligands by themselves are also important synthetic intermediates (e.g., quinoline synthesis)14 and UV-absorbing agents15,16 (e.g., avobenzone). However, reports of the fluorescence properties of the BDK ligands alone are rather scarce.17 Recently, Fraser et al.18 reported the unique aggregation-induced emission (AIE) and ML properties of an unsymmetrical aromatic diketone ligand, highlighting the possibility of employing BDK ligands alone as fluorescent materials when the molecular structure is properly designed. The lack of fluorescence for BDKs in solution is ascribed to intramolecular proton transfer followed by large conformational changes such as rotamerization, along with many other efficient non-radiative processes;19 in the solid or aggregated state, however, such conformational changes may be restricted or suppressed, which potentially allows for strong fluorescence and even RTP to emerge.20 Consequently, BDKs can be designed as a new class of AIE active materials.21–23To prevent self-quenching of fluorescent materials in the aggregated state, many strategies can be applied such as introducing spiro-structures,24 bulky side groups,25 donor–acceptor (D–A) moieties26 or permanent charges27 to the fluorophores. Spiro-structures or bulky side groups can effectively decrease the intermolecular electronic coupling in the solid state while the presence of D–A structures or charges may prevent the formation of usually non-emissive H-aggregate-like packing (face-to-face stacking). Both can suppress the presence of an optically forbidden state at the lowest electronic transition, which typically cannot compete with rapid thermal deactivation of the excited state at room temperature. In solution, however, strong D–A structures or the presence of charges may cause twisted ICT states or photo-induced electron transfer processes, both of which substantially quench the radiative states.
As for ML applications, Fraser et al. have already demonstrated that BDKs with different substituent groups can be suitable candidates.18 In our present investigation, we aim to extend the application scope to water-vapour-activated ML materials. In order to realize this particular application, the BDKs must be hydrophilic enough to interact with water molecules. Common strategies include adding long hydrophilic chains such as PEG polymers, which is not feasible in this case since long polymeric chains can disrupt the ordering of the BDK fluorophores and prevent the observation of ML phenomena. However, adding a positively charged pyridinium group may be a much better choice for water-vapour-recoverable ML since organic salts can maintain the hydrophilic properties of the molecules and at the same time can form ordered crystalline structures, which is a prerequisite for ML.
Herein, we show that when both D–A moieties and charges (pyridinium salts) are introduced into BDKs, AIE as well as water-vapour-recoverable ML can be achieved. In more detail, strong fluorescence (quantum yield ΦF = 0.17) can be observed in the aggregated state while little to no emission (ΦF < 0.01) is present in the solutions, and AIE-active BDKs can be thus obtained. In addition, some of the BDKs show strong binding affinity for cellulose-based materials such as paper or cotton fibres, where strong fluorescence similar to AIE was turned on. We also show that when the solid-state BDKs are sheared or rubbed, red-shifted ML is noted. When the smeared films are exposed to water vapour, rapid blue-shifted emissions can be observed. The process is reversible and reproducible. To the best of our knowledge, reports on ML that is water-vapour recoverable are rather limited28 and the present system may have important applications as both force- and water-activatable fluorescent paints.
Results and discussion
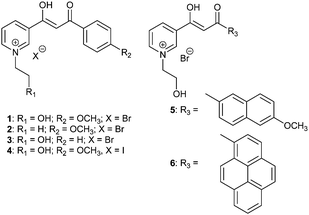
The β-diketones (BDKs) containing a pyridine ring were synthesized via routine Claisen condensation reactions between ethyl nicotinate and appropriate ketones using a previously reported method.10 The BDKs were then refluxed with halogenohydrin or haloalkanes, respectively, in toluene to obtain BDK pyridinium salts 1–6 as greenish yellow crystals with good yields (83–92%; Scheme 1).The solubility of 1–6 in common organic solvents was poor since the polarity of the quaternary ammonium salts is high. In water, however, these BDKs exhibit limited solubility. As shown in the UV-vis absorption spectra in Fig. 1a, the absorption peaks of 1–6 at 362 nm, 362 nm, 347 nm, 362 nm, 380 nm, and 410 nm are typical of the lowest transition state for BDKs, which is a combination of π–π* and n–π*. It has to be noted that there are two main transition states for 6, which is likely due to the large energy separation between the pyridinium and the pyrene moieties. For the rest of the BDKs, the transitions on the two rings are likely to be close in energy and thus become degenerate. In optically dilute aqueous solutions (10−5 M), BDKs 1–6 hardly show observable emissions. However, emission spectra of 1–6 could still be recorded using a sensitive spectrofluorometer. The steady-state emission spectra of the 1–6 aqueous solutions (Fig. 1b) peaked at 505 nm, 500 nm, 456 nm, 505 nm, 456 nm, and 467 nm and their quantum yields were measured to be all below 0.01, when using quinine sulphate (in 0.05 M H2SO4, ϕF = 0.54) as the standard. Intramolecular proton transfer and various molecular motions may be responsible for the low quantum yields of 1–6 in solution. The lifetimes of the 1–6 solutions were also measured but could not be reliably recorded given the extremely weak signal.
In the solid state, however, compounds 1–6 all exhibit intense visual emission under 365 nm UV light illumination. Fig. 1c presents the steady-state emission spectra of 1–6 powders with emission maxima centred at 473 nm, 480 nm, 430 nm, 480 nm, 540 nm, and 580 nm, and the lifetimes at these wavelengths were measured to be 1.4 ns, 0.5 ns, 0.9 ns, 0.8 ns, 0.7 ns and 1.6 ns, respectively. Compared to the emissions in solutions, the solid emissions of 1–4 are blue-shifted by 32 nm, 20 nm, 26 nm, and 25 nm, respectively. This may be due to the solvent effect that excited-state fluorophores can be stabilized by polar solvents and generate more red-shifted emissions in aqueous solutions.29 However, 5 and 6 exhibited largely red-shifted emissions in the solid state compared to the solution emissions. This may be a consequence of ground-state dimer or excimer formation in the solid states given the extended π conjugation of the naphthalene ring and pyrene ring in 5 and 6, respectively. The absolute quantum yields of the 1–6 powders were measured to be 0.17, 0.11, 0.03, 0.02, 0.10 and 0.05 respectively. The greatly enhanced emission efficiencies of 1–6 in the solid states compared to the solutions indicate they are AIE-active molecules. It was noted that the fluorescence emissions were much weaker for BDKs 3, 4, and 6. BDK 3 lacks an electron donating group so the internal rotation of the benzene ring is more prevalent. BDK 4, on the other hand, has a strongly quenching iodide counterion. In BDK 6, strong π–π stacking is likely to quench the fluorescence.
It was discovered that some of the BDK pyridiniums show strong binding affinity for cellulose-based materials on which the fluorescence of the pyridiniums can be “turned on”. As shown in Fig. 1e, when a cellulose-based material, such as a cotton swab, was immersed in the almost non-fluorescent aqueous solution of 1 (10−5 M), intense blue fluorescence of 1 can spread along the watermark on the cotton swab over time rapidly. When a filter paper, another cellulose-based material, was immersed into the aqueous solution of 1, the strong blue fluorescence of 1 was also “turned on” immediately on the filter paper. For other pyridiniums (2, 5 and 6), the same fluorescence turn-on phenomenon on cotton and filter paper was observed. To further demonstrate the application of the BDK pyridiniums, we filled a pen with the aqueous solution of 1 and then drew the chemical structure of cellulose on a piece of paper using the pen. Under ambient light, no legible marks could be noted on the paper; while under 365 nm UV light illumination, the drawn structure could be observed clearly, since it exhibits intense blue fluorescence (Fig. 1d). Therefore, the BDK pyridinium aqueous solutions can be used as a stealth ink and may find important applications in the field of data security.
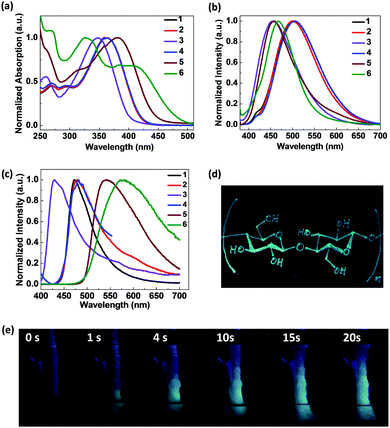
To examine whether these luminogens are mechanochromic luminescent (ML), their solid emission properties under external force stimuli were studied. While the as prepared solid powder of 1 exhibited bright blue emission at 472 nm under 365 nm UV light illumination, they show yellowish green emission with a maximum at 505 nm upon smearing with a cotton swab, demonstrating the ML characteristics of 1 (Fig. 2a and e). The emission colour could be recovered to the initial blue state after the smeared powder was fumed with water vapour for only a few seconds and the reversible ML process can be repeated multiple times. Similarly, the BDK pyridiniums 2, 5 and 6 also showed reversible and repeatable ML properties. The emission maxima of 2, 5 and 6 before and after smearing are 478/510 nm, 535/566 nm and 575/618 nm, respectively. The red shift of 1, 2, 5 and 6 solid emissions upon smearing all exceed 30 nm, indicating their potential as ML-based data storage materials. It is noted that, in all cases of 1, 2, 5 and 6, the emission spectra of the smeared powders become obviously broader than that of the as prepared powders in addition to the significant red shift. Even after careful water vapour annealing, the emission spectra remain broad as the smeared ones though the emission maximum wavelength could be totally restored to the initial states. Furthermore, common ML luminogens may show reversibility via fuming with various organic solvents. However, we demonstrated that the ML of the BDK pyridiniums could be recovered by fuming with water vapour instead of volatile organic solvents, which will be more environmentally friendly in practical applications. We also tried to investigate the ML properties of 3 and 4, however, the two salts were too hard in texture to be smeared or ground and thus we were not able to observe the ML properties.
Single crystal X-ray diffraction (XRD) measurements of 1 were conducted to shed light on the ML properties of the BDK pyridinium solids. Fig. 2f shows the crystal structure of 1, which indicates that the two aromatic rings and the enol ring stay coplanar within the molecule of 1. Neighbouring molecules are found to be stacked in the head-to-tail manner. Multiple intermolecular C–H⋯π interactions between neighbouring molecules can be observed. Smearing the as-prepared powder of 1 may lead to the breakage of the compact stacking and multiple C–H⋯π interactions between neighbouring molecules, resulting in a more amorphous state and the ML properties of 1. Since we failed to obtain single crystals of other BDK pyridiniums, we are not able to conduct single crystal XRD measurements and analyse them. However, we speculate that the mechanism of the ML behaviours of 2, 5 and 6 are similar to 1.
Conclusions
In summary, we have synthesized a series of water soluble BDK pyridiniums and found that they are AIE-active molecules. Though little to no emission was observed in the aqueous solution, the solid of the BDK pyridiniums exhibited bright emissions with absolute quantum yields up to 17%. Some of the pyridiniums in aqueous solution can selectively bind to cellulose-based materials after which intense fluorescence of the pyridiniums were turned on. In addition, water-vapour recoverable ML properties were also discovered for these pyridiniums. The combined multiple functions as well as cheap, facile synthesis of these BDK pyridiniums bestow on them great potential for preparing low-cost sensors, fibre brighteners and security inks.We are very thankful to the Key Research Fund from Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China (WK2340000068 to G. Z.) for financial support of this project. The authors also thank Mr Joshua D. Vaughn for taking the photos used in the figures.
Notes and references
- X. Zhang, M. Cui, R. Zhou, C. Chen and G. Zhang, Macromol. Rapid Commun., 2014, 35, 566–573 CrossRef CAS PubMed.
- R. Gardiner, D. W. Brown, P. S. Kirlin and A. L. Rheingold, Chem. Mater., 1991, 3, 1053–1059 CrossRef CAS.
- J. C.-H. Chan, W. H. Lam, H.-L. Wong, N. Zhu, W.-T. Wong and V. W.-W. Yam, J. Am. Chem. Soc., 2011, 133, 12690–12705 CrossRef CAS PubMed.
- S. Xu, R. E. Evans, T. Liu, G. Zhang, J. N. Demas, C. O. Trindle and C. L. Fraser, Inorg. Chem., 2013, 52, 3597–3610 CrossRef CAS PubMed.
- K. Tanaka, A. Hirose, K. Tamashima and Y. Chujo, Macromol. Rapid Commun., 2015, 36, 684–688 CrossRef CAS PubMed.
- R. Yoshii, A. Hirose, K. Tanaka and Y. Chujo, J. Am. Chem. Soc., 2014, 136, 18131–18139 CrossRef CAS PubMed.
- G. Zhang, J. Lu, M. Sabat and C. L. Fraser, J. Am. Chem. Soc., 2010, 132, 2160–2162 CrossRef CAS PubMed.
- X. Sun, X. Zhang, X. Li, S. Liu and G. Zhang, J. Mater. Chem., 2012, 22, 17332–17339 RSC.
- G. Zhang, J. P. Singer, S. E. Kooi, R. E. Evans, E. L. Thomas and C. L. Fraser, J. Mater. Chem., 2011, 21, 8295–8299 RSC.
- G. Zhang, G. M. Palmer, M. W. Dewhirst and C. L. Fraser, Nat. Mater., 2009, 8, 747–751 CrossRef CAS PubMed.
- J. Samonina-Kosicka, C. A. DeRosa, W. A. Morris, Z. Fan and C. L. Fraser, Macromolecules, 2014, 47, 3736–3746 CrossRef CAS PubMed.
- S. Mukherjee and P. Thilagar, Chem. Commun., 2015, 51, 10988–11003 RSC.
- P. Lehner, C. Staudinger, S. M. Borisov and I. Klimant, Nat. Commun., 2014, 5, 1–6 Search PubMed.
- I. Zumeta, T. Kluge, C. Mendicute-Fierro, C. Wagner, L. Ibarlucea, T. Rüffer, V. San Nacianceno, D. Steinborn and M. A. Garralda, Organometallics, 2014, 33, 788–795 CrossRef CAS.
- L. A. Baker, M. D. Horbury, S. E. Greenough, P. M. Coulter, T. N. V. Karsili, G. M. Roberts, A. J. Orr-Ewing, M. N. R. Ashfold and V. G. Stavros, J. Phys. Chem. Lett., 2015, 6, 1363–1368 CrossRef CAS PubMed.
- P. K. Verma, A. Steinbacher, F. Koch, P. Nuernberger and T. Brixner, Phys. Chem. Chem. Phys., 2015, 17, 8459–8466 RSC.
- G. Zhang, S. Kim, R. Evans, B. Kim, J. N. Demas and C. Fraser, J. Fluoresc., 2009, 19, 881–889 CrossRef CAS PubMed.
- T. Butler, W. A. Morris, J. Samonina-Kosicka and C. L. Fraser, Chem. Commun., 2015, 51, 3359–3362 RSC.
- P. K. Verma, F. Koch, A. Steinbacher, P. Nuernberger and T. Brixner, J. Am. Chem. Soc., 2014, 136, 14981–14989 CrossRef CAS PubMed.
- G. Zhang, R. E. Evans, K. A. Campbell and C. L. Fraser, Macromolecules, 2009, 42, 8627–8633 CrossRef CAS.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332–4353 RSC.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- K.-T. Wong, Y.-Y. Chien, R.-T. Chen, C.-F. Wang, Y.-T. Lin, H.-H. Chiang, P.-Y. Hsieh, C.-C. Wu, C. H. Chou, Y. O. Su, G.-H. Lee and S.-M. Peng, J. Am. Chem. Soc., 2002, 124, 11576–11577 CrossRef CAS PubMed.
- S.-H. Hwang, C. N. Moorefield and G. R. Newkome, Chem. Soc. Rev., 2008, 37, 2543–2557 RSC.
- L. Duan, J. Qiao, Y. Sun and Y. Qiu, Adv. Mater., 2011, 23, 1137–1144 CrossRef CAS PubMed.
- C.-X. Yuan, X.-T. Tao, Y. Ren, Y. Li, J.-X. Yang, W.-T. Yu, L. Wang and M.-H. Jiang, J. Phys. Chem. C, 2007, 111, 12811–12816 CAS.
- Y. Sagara, T. Komatsu, T. Ueno, K. Hanaoka, T. Kato and T. Nagano, Adv. Funct. Mater., 2013, 23, 5277–5284 CrossRef CAS.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, 3rd edn, 2006, ch. 6 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1500848. For ESI and crystallographic data in CIF or other electronic formats see DOI: 10.1039/c6qm00187d |
| This journal is © the Partner Organisations 2017 |