Investigation of supramolecular interactions between liquid crystals and PCBM for improved morphological stability in solar cells†
Weihua
Zhou
ab,
Kunxing
Hu
a,
Xingxing
Shen
c,
Yuanpeng
Xie
a,
Lin
Zhang
a,
Qingyun
Ai
a,
Jingping
Yin
b and
Yiwang
Chen
 *ab
*ab
aSchool of Material Science and Engineering/Institute of Polymers, Nanchang University, 999 Xuefu Avenue, Nanchang 330031, China. E-mail: ywchen@ncu.edu.cn; Fax: +86 791 83969561; Tel: +86 791 83968703
bCollege of Chemistry/Jiangxi Provincial Key Laboratory of New Energy Chemistry, Nanchang University, 999 Xuefu Avenue, Nanchang 330031, China
cInstitute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
First published on 7th October 2016
Abstract
Supramolecular interactions between liquid crystals (LCs) of different chemical structures and PC61BM molecules have been studied, showing that 4-cyano-4′-pentylterphenyl (5CT) containing electron-withdrawing cyano substituents on the phenyl ring exhibited the strongest interactions with PC61BM, as revealed via density functional theory (DFT) calculations and experimental analysis based on Fourier transform infrared spectrometry (FTIR), differential scanning calorimetry (DSC) and polarized optical microscopy (POM). In contrast, 4-octyloxy-4′-cyanobiphenyl (8OCB) comprising an electro-donating octyloxyl group and dioctylterthiophene (8TTP8) with thiophene rings and long alkyl groups showed weaker interactions with PC61BM. After electric field treatment at 600 V mm−1 in an air environment, the P3HT:PC61BM:8OCB specimen showed a higher power conversion efficiency (PCE) of 2.9% and a more stable morphology than P3HT:PC61BM:8TTP8 with a PCE of 2.7%. Upon annealing at 150 °C for 1 h, 5CT is most effective in restricting the aggregation and crystallization of PC61BM molecules, thus stabilizing the morphology of P3HT:PC61BM. Moreover, the supramolecular interaction between LCs and PCBM could also influence the thermal stability in the narrow bandgap system of PTB7-Th:PC71BM, with 8TTP8 showing the highest ability to restrict the depression of the PCE value.
Introduction
Organic photovoltaics (OPVs) have made substantial progress recently and have shown their potential in low-cost, flexible, and lightweight solar energy conversion devices.1,2 Power conversion efficiencies (PCEs) over 10% have been achieved for polymer-based OPVs with bulk heterojunction (BHJ) architectures.3,4 Nevertheless, a further increase of their PCE is needed for future applications, and the long-term stability still remains to be a great challenge.In order to solve the above problems, various approaches including chemical methods such as synthesis of novel donor and acceptor materials via molecular design and development of novel interfacial materials,5–7 and physical methods such as thermal and solvent annealing,8,9 and the addition of third components have been demonstrated to improve the PCE and thermal stability of OPVs. It is noted that the third components such as light-absorbing small molecules10 or polymers,11 fullerene12 or non-fullerene acceptors,13 metal- or carbon-based nanomaterials,14,15 quantum dots16 and polymer or small molecule nonvolatile additives17,18 can improve the absorption behavior or optimize the phase separation of the active layers. Among the different types of third additives, liquid crystalline molecules such as 2,3,6,7,10,11-hexaacetoxytriphenylene discotic liquid crystals (DLCs) can improve the PCE from 3.03% to 3.97% at a weight ratio of 3 wt% in the poly(3-hexyl thiophene) (P3HT) and [6,6]-phenyl-C61-butyric acid methyl ester (PC61BM) system.19 Meanwhile, the nematic liquid crystals (NLCs) were also demonstrated to improve the performance of P3HT:PC61BM solar cells.20 It is well accepted that liquid crystals (LCs) can facilitate the crystallization of P3HT, leading to an improvement in absorption and charge transportation. However, the interactions between LCs and PC61BM molecules are not well understood. Jeong et al.21 speculated that the incorporation of nematic liquid crystals into the P3HT:PC61BM system may lead to the formation of slightly larger PC61BM domains according to the X-ray diffraction spectrum. However, they did not have more evidence to confirm their speculations. In our previous study,22 we found that there existed specific interactions between the 4-cyano-4′-pentylterphenyl (5CT) and PC61BM molecules, leading to the disappearance of PC61BM melting peaks at the 5CT weight ratio above 6 wt% in PC61BM:5CT binary blends as revealed by differential scanning calorimetry (DSC). Additionally, 5CT and PC61BM could form rod-like complexes as observed using transmission electron microscopy (TEM). The reason why noncovalent interactions exist between 5CT and PC61BM molecules is not known. We believe that that the complexes might originate from the supramolecular interactions between liquid crystals and PC61BM molecules, depending on the chemical structure of the liquid crystals.
The supramolecular concept was first proposed by Lehn in 1978,23,24 and has been extensively studied by scientists, as it plays a vital role in the aggregation and self-assembly of molecules. As one of the supramolecular interactions, the π–π interaction widely exists in systems containing aromatic rings.25 Nakamura26 found that there was a favorable face-to-face interaction between a perfluoroaromatic ring and the π surface of fullerene. Moreover, Cheng27 found that the supramolecular interaction between [6,6]-phenyl-C61 butyric acid pentafluorophenyl ester (PC61BPF) and the C60 cores of PC61BM can effectively suppress the PC61BM materials from extensive thermal-driven aggregation to overcome morphological stability.
In this article, we aimed to demonstrate the influence of the chemical structure of LCs on the strength of the supramolecular interactions between LCs and PC61BM. Additionally, the effect of LCs on the morphology, photovoltaic performance and morphological stability of solar cells was extensively investigated. It is revealed that the 5CT containing electron-withdrawing substituents of the cyano group (CN) on the benzene ring is the most effective in restricting the aggregation and crystallization of the PC61BM molecules, thus stabilizing the morphology of the active layer and improving the stability of the solar cells. The P3HT:PC61BM specimen containing 4-octyloxy-4′-cyanobiphenyls (8OCB) exhibited a higher short circuit current (Jsc) and PCE than those of dioctylterthiophene (8TTP8) after electric field treatment, showing a stronger interaction of 8OCB with PC61BM in contrast to 8TTP8. However, in the narrow bandgap system of PTB7-Th:PC71BM, 8TTP8 was found to be the most effective in enhancing the thermal stability. Besides the supramolecular interaction between the LCs and PCBM, the interaction between the liquid crystals and the donor polymer should also be taken into consideration to improve the thermal stability of the solar cells.
Results and discussion
In our previous study, the 5CT liquid crystal was found to induce the crystallization of P3HT and restrict the crystallization of the PC61BM molecules.22 It is well known that PC61BM contains phenyl rings in the molecular structure. Based on Cheng's findings,27 there exist supramolecular perfluorophenyl–C60 interactions in the active layer. Moreover, Sherrill28 found that the dimmers containing electron-withdrawing and electron-donating substituents bind more strongly than the benzene dimmer, based on the second-order perturbation theory (MP2). The electron-withdrawing substituents stabilize the transition state by decreasing the repulsion between the π electrons on each aryl ring, while electron-donating substituents destabilize the transition state by increasing the repulsion between the two π systems. Thus, the benzene containing electron-withdrawing substituents should have stronger interactions with benzene, due to the electrostatic interactions. The benzene containing electron-donating substituents should bind more weakly with benzene due to the electrostatic repulsions. However, the interactions may be slightly stronger than the benzene–benzene interactions when considering the dispersion interactions. In this article, as shown in Fig. 1, the 5CT molecule contains one CN group, one pentyl group and three benzene rings, in contrast to the 8OCB molecule containing one CN group, one octyloxyl group and two benzene rings. It is noted that the 5CT molecule contains more phenyl rings than the 8OCB molecule, in addition to the shorter alkyl group and the absence of electron-donating substituents. The electron-donating octyloxyl group in 8OCB may depress the electron-withdrawing effect of the CN group. Therefore, 5CT is believed to have stronger interactions with the PC61BM molecules than 8OCB. Where 8TTP8 is concerned, the thiophene ring exhibiting a π56 structure with a higher electron density shows a stronger electrostatic repulsion than the phenyl ring. The relatively longer alkyl group may expand the distance between the thiophene ring and the phenyl ring. It is speculated that the interaction between 8TTP8 and PCBM should be weaker than that of 5CT and 8OCB.In order to confirm the above speculation, we performed theoretical calculations based on the density functional theory (DFT).29 The initial configurations of the three complexes including PC61BM:5CT, PC61BM:8OCB and PC61BM:8TTP8 are shown in Fig. 2, where a pentagon of PC61BM is parallel to 5CT, 8OCB and 8TTP8, while the intermolecular distances were set to be 3.5 Å. The configurations of the complexes were then optimized using DFT at the B3LYP/6-31G** level. In order to calculate the binding energies between the LCs and PC61BM, the single point energies of these optimized complexes were computed considering the Basis Set Superposition Error (BSSE), and the corresponding result is shown in Table 1. The binding energy of PC61BM:5CT, PC61BM:8OCB and PC61BM:8TTP8 is calculated to be −6.19 eV, −3.98 eV and −4.20 eV, respectively. It is concluded that the interaction between 5CT and PCBM should be the highest. The binding energy between PC61BM:8OCB and PC61BM:8TTP8 is close to each other. However, by taking into account the alkyl groups in 8TTP8, the relatively longer alkyl groups in 8TTP8 may depress the interaction with PC61BM, thus showing the weakest ability to restrict the crystallization of PC61BM. The theoretical calculation result is consistent with the above analysis.
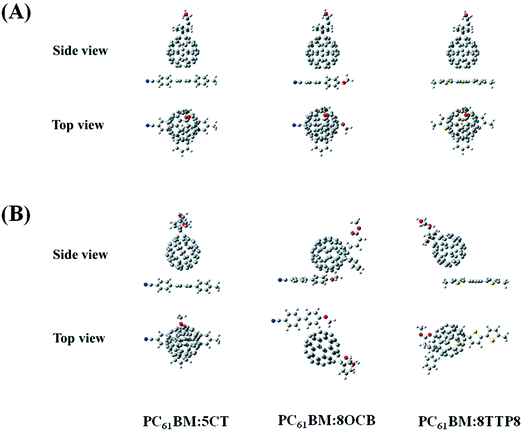 | ||
| Fig. 2 (A) Initial configurations and (B) optimized configurations of the PC61BM:5CT, PC61BM:8OCB and PC61BM:8TTP8 complexes. | ||
| PC61BM:5CT | PC61BM:8OCB | PC61BM:8TTP8 | |
|---|---|---|---|
| E b (kcal mol−1) | −6.19 | −3.98 | −4.20 |
Furthermore, we performed experimental investigations on the supramolecular interactions between the liquid crystals and the PC61BM molecules. The Fourier transform infrared spectroscopy (FTIR) spectra (Fig. S1, ESI†) of the binary blends based on PC61BM:8TTP8, PC61BM:8OCB and PC61BM:5CT present a novel absorption peak at 752 cm−1, ascribed to the supramolecular interaction between the liquid crystals and the PC61BM molecules. However, the influence of the chemical structure of the liquid crystals on the strength of the supramolecular interactions is unobvious based on the FTIR analysis.
The interactions between the LCs and PC61BM were then investigated via differential scanning calorimetry (DSC) analysis. According to the previous result, the melting peak of PC61BM almost disappeared at the 5CT weight ratio above 6 wt%, illustrating the strong supramolecular interaction.22 In the PC61BM:8TTP8 blend as shown in Fig. 3a, the liquid crystal phase transition temperature for 8TTP8 remained almost unchanged after blending with PC61BM, indicating that 8TTP8 could crystallize in its own region. The melting peak of PC61BM remains obvious, shifting gradually to lower temperatures as the 8TTP8 weight ratio is increased to 20 wt%. In addition, the melting peaks of 8OCB change from three sharp peaks to one major round peak after blending with PC61BM in the PC61BM:8OCB blend (Fig. 3b). The melting peak of PC61BM becomes less obvious after the incorporation of 8OCB, in contrast to the disappearance of the melting peak of PC61BM in the PC61BM:5CT blends. In order to further confirm the interactions between the liquid crystals and the PC61BM molecules, the DSC cooling curves are shown in Fig. 3c and d. In the PC61BM:5CT blend, no discernible crystallization peak contributing to PC61BM could be observed at the 5CT weight ratio of 10 wt%.22 In the PC61BM:8TTP8 blend, the crystallization peak temperature of PC61BM shifts from 240.6 °C to a lower temperature of 176.8 °C as the 8TTP8 weight ratio is increased to 20 wt%. Additionally, the crystallization peaks of the PC61BM molecules are obvious even at high contents of 8TTP8. In contrast, the crystallization peak of PC61BM in the PC61BM:8OCB blends almost disappears at the 8OCB weight ratio of 10 wt%, indicating that the crystallization of the PC61BM molecules becomes very difficult in the presence of 8OCB liquid crystals.
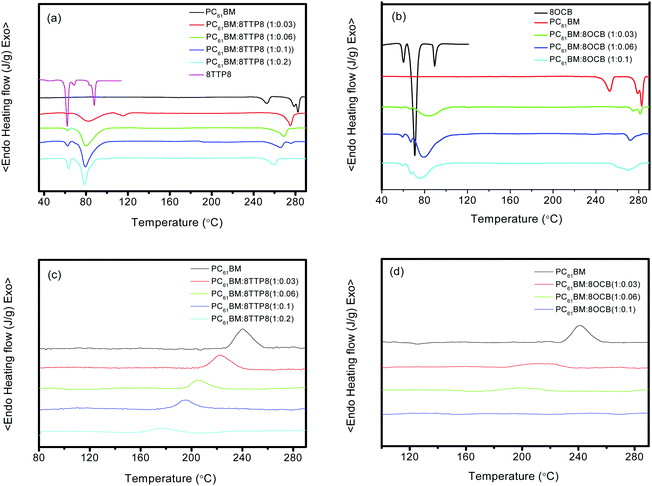 | ||
| Fig. 3 DSC heating curves for (a) PC61BM:8TTP8 and (b) PC61BM:8OCB, and cooling curves for the (c) PC61BM:8TTP8 and (d) PC61BM:8OCB blends at different weight ratios of liquid crystals. | ||
To further explore the effect of liquid crystals on the crystallization of the PC61BM molecules, we investigated the morphology of the P3HT:PC61BM:LC (0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06) films after annealing at 200 °C for 10 min via polarized optical microscopy (POM) (Fig. S2, ESI†). The P3HT:PC61BM (0.05
0.06) films after annealing at 200 °C for 10 min via polarized optical microscopy (POM) (Fig. S2, ESI†). The P3HT:PC61BM (0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) specimen forms of large spherulites contributing to the PC61BM crystals with clear interfaces. By the incorporation of 6 wt% 8TTP8, PC61BM develops into fibrillar bundles of crystals. 8OCB could induce the formation of relatively small PC61BM spherulites as compared with the P3HT:PC61BM (0.05
1) specimen forms of large spherulites contributing to the PC61BM crystals with clear interfaces. By the incorporation of 6 wt% 8TTP8, PC61BM develops into fibrillar bundles of crystals. 8OCB could induce the formation of relatively small PC61BM spherulites as compared with the P3HT:PC61BM (0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) specimen. Moreover, 5CT could seriously restrict the cold crystallization of PC61BM showing only a few undeveloped PCBM spherulites.
1) specimen. Moreover, 5CT could seriously restrict the cold crystallization of PC61BM showing only a few undeveloped PCBM spherulites.
Based on the above experimental data, it is concluded that there exists supramolecular interactions between the liquid crystals and PC61BM molecules, depending on the chemical structure of the liquid crystals. On the DSC heating curves, the melting peak of PC61BM almost disappeared at the 5CT weight ratio above 6 wt%.22 In contrast, the melting peak of PC61BM decreased after the incorporation of 8OCB, while the melting peak of PC61BM was still obvious even at the 8TTP8 weight ratio of 20 wt%. Similarly, on the DSC cooling curves, the crystallization peak of PC61BM could not be discerned at a 5CT and 8OCB weight ratio above 6 wt%, while the crystallization peak of PC61BM was still obvious in the PC61BM:8TTP8 blend. It is believed that 5CT exhibits the strongest supramolecular interactions with PC61BM, and 8TTP8 shows the weakest supramolecular interaction with PC61BM, depending on the type of aromatic rings, electron-donating or electron-withdrawing substituents. According to the POM images, the diameter and shape of the PC61BM crystallites are dependent on the nucleating density and the migration of the PC61BM molecules. The existence of liquid crystals facilitates the nucleation of PC61BM. Due to the relatively weak supramolecular interactions between 8TTP8 and PC61BM, the PC61BM molecules could migrate to the crystallization growth front of the PC61BM crystals more easily, leading to the appearance of fibrillar bundles in a higher density. In the PC61BM:8OCB film, the supramolecular interaction between 8OCB and PC61BM should be stronger, the migration of PC61BM molecules onto the crystallization growth front becomes difficult, leading to the appearance of spherulites with a smaller size. Due to the strongest interaction between 5CT and PC61BM, the growth of the PC61BM spherulites is seriously restricted by 5CT.
Taking the supramolecular interaction into consideration, the incorporation of liquid crystals into P3HT:PC61BM is supposed to enhance the photovoltaic properties. In our previous study, the P3HT:PC61BM:5CT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
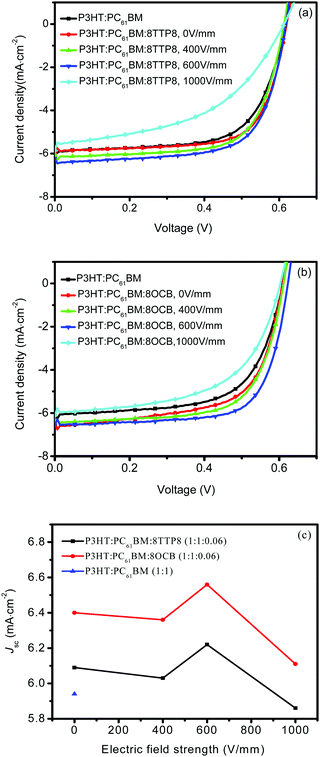
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06) system showed the highest photovoltaic performance with a PCE of 3.5%, a short-circuit current (Jsc) of 9.87 mA cm−2, an open circuit voltage (Voc) of 0.621 V, and a fill factor (FF) of 56.6% under an external electric field strength of 600 V mm−1.22Fig. 4 presents the current–voltage (J–V) characteristics of the solar cells based on the P3HT:PC61BM:8TTP8 and P3HT:PC61BM:8OCB blends after electric field treatment of different strengths in an air environment, and the corresponding parameters are shown in Table 2. The device based on pristine P3HT:PC61BM exhibits a PCE of 2.4%, with a Jsc of 5.94 mA cm−2, Voc of 0.618 V, and FF of 65.5%, respectively. When 6 wt% 8TTP8 and 8OCB were added into the P3HT:PC61BM system, the PCE value slightly increases to 2.5%. Where the P3HT:PC61BM:8TTP8 specimen is concerned, the PCE value reaches the maximal value of 2.7% as the electric field strength is increased to 600 V mm−1. Then, the PCE significantly drops to 1.7% at the electric field strength of 1000 V mm−1. Moreover, the influence of the electric field strength on the photovoltaic performance of the P3HT:PC61BM:8OCB specimen is similar to that of the P3HT:PC61BM:8TTP8 specimen, showing the highest PCE of 2.9% at an electric field strength of 600 V mm−1. In general, the electric field treatment seems to play a vital role in improving the photovoltaic performance of the ternary blend system. The appropriate electric field strength is found to be 600 V mm−1, which is the same as in our previous study.22 However, the photovoltaic performance of the devices seems to be dependent on the chemical structure of the liquid crystals. The specimen containing 8OCB exhibits a higher PCE value than the specimen containing 8TTP8 at an electric field strength of 600 V mm−1. According to the relationship of short-circuit current versus electric field strength, it is noted that the incorporation of liquid crystals into P3HT:PC61BM could enhance the Jsc value. Moreover, the Jsc value reached the highest at an electric field strength of 600 V mm−1. The specimen containing 8OCB showed a higher Jsc value as compared to the specimen containing 8TTP8.
0.06) system showed the highest photovoltaic performance with a PCE of 3.5%, a short-circuit current (Jsc) of 9.87 mA cm−2, an open circuit voltage (Voc) of 0.621 V, and a fill factor (FF) of 56.6% under an external electric field strength of 600 V mm−1.22Fig. 4 presents the current–voltage (J–V) characteristics of the solar cells based on the P3HT:PC61BM:8TTP8 and P3HT:PC61BM:8OCB blends after electric field treatment of different strengths in an air environment, and the corresponding parameters are shown in Table 2. The device based on pristine P3HT:PC61BM exhibits a PCE of 2.4%, with a Jsc of 5.94 mA cm−2, Voc of 0.618 V, and FF of 65.5%, respectively. When 6 wt% 8TTP8 and 8OCB were added into the P3HT:PC61BM system, the PCE value slightly increases to 2.5%. Where the P3HT:PC61BM:8TTP8 specimen is concerned, the PCE value reaches the maximal value of 2.7% as the electric field strength is increased to 600 V mm−1. Then, the PCE significantly drops to 1.7% at the electric field strength of 1000 V mm−1. Moreover, the influence of the electric field strength on the photovoltaic performance of the P3HT:PC61BM:8OCB specimen is similar to that of the P3HT:PC61BM:8TTP8 specimen, showing the highest PCE of 2.9% at an electric field strength of 600 V mm−1. In general, the electric field treatment seems to play a vital role in improving the photovoltaic performance of the ternary blend system. The appropriate electric field strength is found to be 600 V mm−1, which is the same as in our previous study.22 However, the photovoltaic performance of the devices seems to be dependent on the chemical structure of the liquid crystals. The specimen containing 8OCB exhibits a higher PCE value than the specimen containing 8TTP8 at an electric field strength of 600 V mm−1. According to the relationship of short-circuit current versus electric field strength, it is noted that the incorporation of liquid crystals into P3HT:PC61BM could enhance the Jsc value. Moreover, the Jsc value reached the highest at an electric field strength of 600 V mm−1. The specimen containing 8OCB showed a higher Jsc value as compared to the specimen containing 8TTP8.
| Specimens | Electric field strength (V mm−1) | J sc (mA mm−2) | V oc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
P3HT:PCBM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0 | 5.94 | 0.618 | 65.5 | 2.4 |
P3HT:PCBM:8TTP8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06) 0.06) |
0 | 6.09 | 0.619 | 67.5 | 2.5 |
| 400 | 6.03 | 0.612 | 69.7 | 2.6 | |
| 600 | 6.22 | 0.617 | 70.0 | 2.7 | |
| 1000 | 5.86 | 0.612 | 46.2 | 1.7 | |
P3HT:PCBM:8OCB (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06) 0.06) |
0 | 6.40 | 0.608 | 65.3 | 2.5 |
| 400 | 6.36 | 0.612 | 68.0 | 2.6 | |
| 600 | 6.56 | 0.623 | 70.6 | 2.9 | |
| 1000 | 6.11 | 0.602 | 55.8 | 2.1 | |
The morphology of the active layer before and after the electric field treatment was analyzed via transmission electron microscopy (TEM) and atomic force microscopy (AFM). As shown in Fig. 5, the morphology of the films displays a strong dependence on the electric field strength. Where the P3HT:PCBM:8TTP8 specimen is concerned, the black rod-like aggregates should contribute to the 8TTP8 crystals, due to the relatively weak interaction between 8TTP8 and PC61BM. The width distribution of the nanofibrils is centered at around 50–70 nm. After the electric field assisted treatment at a strength of 400 V mm−1, the morphology of the film shows no significant change as compared with the specimen without any electric field assisted treatment, showing that the width of the nanofibrils reduced to 30–50 nm. At an electric field strength of 600 V mm−1, the black dots with a diameter of about 20 nm distribute homogeneously in the matrix. Upon increasing the electric field strength to 1000 V mm−1, some black aggregates in the diameter range of about 80–140 nm could be observed, implying that the nanorods could orient to the direction of the electric field. In contrast, the P3HT:PC61BM:8OCB specimen exhibits an obviously different morphology showing no bundles in the matrix. The black dots ascribed to 8OCB and the PC61BM complexes distribute homogeneously in the matrix with sizes ranging from 40 to 60 nm. Upon treatment with an electric field of 400 V mm−1, the diameter of the black dots slightly reduces and they are distributed more homogeneously in the matrix. At an electric field strength of 600 V mm−1, the diameter of the black dots significantly reduces to about 20 nm. As the electric field strength continuously increases to 1000 V mm−1, the diameter of the black dots shows no serious change. Based on the above analysis, we can conclude that 8OCB exhibits stronger supramolecular interactions with PC61BM. Thus, the 8TTP8 molecules tend to self-aggregate into crystalline phases, while 8OCB tends to form complexes with PC61BM due to supramolecular interactions, facilitating the morphological stability in the presence of an external force. Even at an electric field strength of 1000 V mm−1, the morphology of the specimen containing 8OCB remains the same without significant change. The specimen containing 8TTP8 tends to form larger black dots, resulting in much lower values of Jsc and PCE.
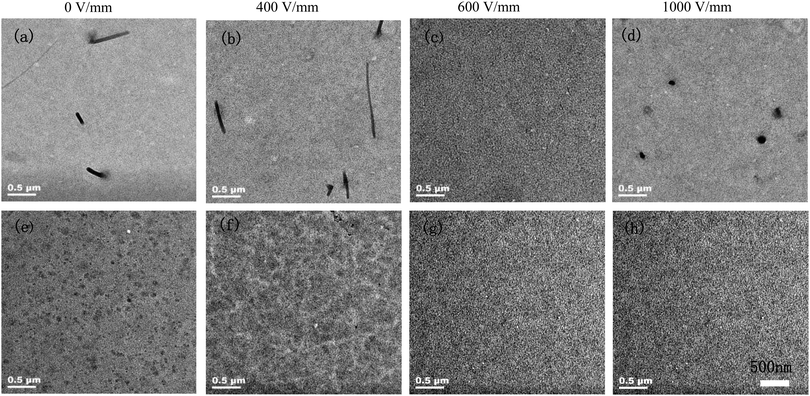 | ||
Fig. 5 TEM images of the (a–d) P3HT:PC61BM:8TTP8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06) and (e–h) P3HT:PC61BM:8OCB (1 0.06) and (e–h) P3HT:PC61BM:8OCB (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06) specimens after electric field treatment of different strengths. 0.06) specimens after electric field treatment of different strengths. | ||
In order to further explore the meso-scale film morphology in the lateral direction of films before and after electric field (600 V mm−1) assisted treatment, the AFM images are presented in Fig. S3 (ESI†). The pristine P3HT:PC61BM film shows a relatively large phase-separated morphology with a root mean square (RMS) surface roughness of 7.18 nm. Where the specimens containing 8TTP8 and 8OCB are concerned, the phase domain size significantly reduces in contrast to the pristine P3HT:PC61BM specimen. After electric field assisted treatment, the phase domain size of the films further decreases, in addition to the reduction of the RMS surface roughness of the films. For example, the RMS value of the specimen containing 8TTP8 decreases from 2.01 nm to 0.74 nm. Moreover, the RMS value of the specimen containing 8OCB decreases from 2.49 nm to 2.03 nm. It is believed that 8TTP8 tends to distribute more homogeneously in the matrix upon treatment with an electric field, leading to a reduction in the RMS value. However, due to stronger supramolecular interactions between 8OCB and PC61BM, the morphology seems to be more stable, accompanied by less reduction in the RMS value. It may lead to the formation of PC61BM clusters in appropriate sizes, facilitating the electron transport.
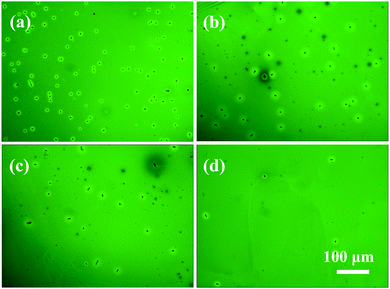
According to the above data, it is recognized that the supramolecular interactions between the liquid crystals and PC61BM may stabilize the morphology of active layers. Fig. 6 shows the POM images of the P3HT:PC61BM:LC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06) films after thermal annealing at 150 °C for 1 h. For comparison, the POM images of specimens prior to thermal annealing are presented in Fig. S4 (ESI†). The pristine P3HT:PC61BM film shows no significant structure in the whole image, indicating mixing of P3HT and PC61BM at the molecular level. After the incorporation of 8TTP8, bright dots can be noticed, attributed to the 8TTP8 crystals. In contrast, the specimen containing 8OCB shows smaller bright dots. However, the morphology of the specimen containing 5CT is similar to that of the pristine P3HT:PC61BM film, showing a few bright dots. The above result indicates that 8TTP8 tends to self-assemble into the liquid crystalline phase while 5CT tends to form complexes with PC61BM, demonstrating that 5CT exhibits the strongest supramolecular interaction with the PC61BM molecules. In Fig. 6, it is observed that some needle-like crystals ascribed to PC61BM distributes in the whole image of the P3HT:PC61BM specimen. By the incorporation of 6 wt% 8TTP8, the density of the PC61BM crystals slightly reduces, showing that the presence of 8TTP8 liquid crystals could restrict the crystallization of PC61BM. As the P3HT:PC61BM:8OCB specimen is concerned, the density of the PC61BM crystals is much lower than that of pristine P3HT:PC61BM and the P3HT:PC61BM:8TTP8 specimens. In the P3HT:PC61BM:5CT specimen, only a few PC61BM crystals could be discerned, confirming that 5CT has the strongest supramolecular interaction with the PC61BM molecules. It is speculated that 5CT could enhance the morphological stability of the active layer under thermal treatment.
0.06) films after thermal annealing at 150 °C for 1 h. For comparison, the POM images of specimens prior to thermal annealing are presented in Fig. S4 (ESI†). The pristine P3HT:PC61BM film shows no significant structure in the whole image, indicating mixing of P3HT and PC61BM at the molecular level. After the incorporation of 8TTP8, bright dots can be noticed, attributed to the 8TTP8 crystals. In contrast, the specimen containing 8OCB shows smaller bright dots. However, the morphology of the specimen containing 5CT is similar to that of the pristine P3HT:PC61BM film, showing a few bright dots. The above result indicates that 8TTP8 tends to self-assemble into the liquid crystalline phase while 5CT tends to form complexes with PC61BM, demonstrating that 5CT exhibits the strongest supramolecular interaction with the PC61BM molecules. In Fig. 6, it is observed that some needle-like crystals ascribed to PC61BM distributes in the whole image of the P3HT:PC61BM specimen. By the incorporation of 6 wt% 8TTP8, the density of the PC61BM crystals slightly reduces, showing that the presence of 8TTP8 liquid crystals could restrict the crystallization of PC61BM. As the P3HT:PC61BM:8OCB specimen is concerned, the density of the PC61BM crystals is much lower than that of pristine P3HT:PC61BM and the P3HT:PC61BM:8TTP8 specimens. In the P3HT:PC61BM:5CT specimen, only a few PC61BM crystals could be discerned, confirming that 5CT has the strongest supramolecular interaction with the PC61BM molecules. It is speculated that 5CT could enhance the morphological stability of the active layer under thermal treatment.
To further explore the effect of liquid crystals on the thermal stability of devices based on the narrow bandgap poly[4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b′]dithiophene-co-3-fluorothieno[3,4-b]thiophene-2-carboxylate] (PTB7-Th):[6,6]-phenyl-C71-butyric acid methyl ester (PTB7-Th:PC71BM) system, 8TTP8, 8OCB and 5CT were incorporated into PTB7-Th:PC71BM and the corresponding parameters are illustrated in Table 3 and Fig. S5 (ESI†). It is noted that the PCE value of all the devices reduced significantly upon thermal treatment. The pristine PTB7-Th:PC71BM exhibits a PCE value of 7.3%, which sharply decreases to 4.0% after heating at 150 °C for 20 min. In PTB7-Th:PC71BM:8TTP8, the PCE value reduces from 7.0% to 4.4% upon thermal treatment, showing that the incorporation of 8TTP8 could slightly improve the thermal stability of PTB7-Th:PC71BM. In PTB7-Th:PC71BM:8OCB, the PCE value decreases from 7.5% to 3.3%. In contrast, the PCE value decreases from 6.8% to 3.6% in PTB7-Th:PC71BM:5CT. It is believed that 8OCB and 5CT are not beneficial to the thermal stability of PTB7-Th:PC71BM, especially for 8OCB. It should be pointed out that the change in Voc upon heating is dependent on the chemical structure of the liquid crystals. It is observed that the Voc continuously decreases due to heating time in the pristine PTB7-Th:PC71BM and PTB7-Th:PC71BM:8OCB systems. However, the reduction in Voc upon heating time in PTB7-Th:PC71BM:5CT seems to be slower. Surprisingly, the Voc value in PTB7-Th:PC71BM:8TTP8 remains constant irrespective of heating time. It is well accepted that the change in Voc should be related to the morphology evolution of the active layer. The corresponding TEM images of the active layer before and after thermal treatment are shown in Fig. S6 and S7 (ESI†). It is noted that the specimens before thermal treatment exhibit an optimized phase-separated morphology, especially for pristine PTB7-Th:PC71BM and specimens containing 8OCB and 8TTP8. PTB7-Th:PC71BM:5CT shows obvious black regions contributing to the 5CT phase, which exhibit a relatively lower PCE. After thermal treatment, the phase domain size of PTB7-Th:PC71BM:8OCB and PTB7-Th:PC71BM:8TTP8 reduces, showing less obvious phase separation. However, the phase domain size of PTB7-Th:PC71BM:5CT increases.
| Systems | Time (min) | V oc (V) | J sc (mA cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
PTB7-Th:PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) 1.5) |
0 | 0.781 | 16.34 | 57.0 | 7.3 |
| 5 | 0.758 | 11.04 | 49.3 | 4.1 | |
| 10 | 0.751 | 12.00 | 47.0 | 4.2 | |
| 20 | 0.726 | 12.04 | 46.1 | 4.0 | |
PTB7-Th:PC71BM:8TTP8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06) 0.06) |
0 | 0.775 | 15.53 | 58.3 | 7.0 |
| 5 | 0.788 | 10.12 | 49.5 | 4.0 | |
| 10 | 0.791 | 11.14 | 49.5 | 4.4 | |
| 20 | 0.787 | 10.99 | 50.4 | 4.4 | |
PTB7-Th:PC71BM:8OCB (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06) 0.06) |
0 | 0.773 | 15.60 | 61.8 | 7.5 |
| 5 | 0.732 | 10.48 | 48.6 | 3.7 | |
| 10 | 0.714 | 10.09 | 50.0 | 3.6 | |
| 20 | 0.699 | 9.29 | 51.9 | 3.3 | |
PTB7-Th:PC71BM:5CT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06) 0.06) |
0 | 0.780 | 14.00 | 62.0 | 6.8 |
| 5 | 0.764 | 10.52 | 47.0 | 3.8 | |
| 10 | 0.744 | 10.20 | 47.6 | 3.6 | |
| 20 | 0.745 | 10.14 | 47.7 | 3.6 | |
In the PTB7-Th:PC71BM system, the phase separation between PTB7-Th and PC71BM should be the main factor in determining the morphological stability upon thermal treatment. Due to the similarity in chemical structure between 8OCB and 5CT, they tend to reside in the PC71BM phase. During the heating process, the stronger supramolecular interaction between 5CT and PC71BM could restrict the serious aggregation of PC71BM, showing better thermal stability than the specimen containing 8OCB. In contrast, 8TTP8 may distribute in the interface between PTB7-Th and PC71BM, which could reduce the interfacial force and thus stabilize the morphology upon thermal treatment. It is concluded that the use of molecules to restrict the aggregation and crystallization of PCBM should be the major route to stabilizing the morphology of the active layer, enhancing the thermal stability in the systems containing semicrystalline polymers such as P3HT. In the systems containing narrow band gap polymers, how to reduce the interfacial force in order to improve the morphology stability as well as the thermal stability should be taken into consideration. The selection of appropriate molecules which could interact well with both the donor and acceptor molecules through π–π or hydrogen bond interactions should be important. Researchers should pay more attention to the supramolecular interaction between the third additive and the donor and acceptor to explore how to stabilize the morphology of the active layer upon thermal treatment, enhancing the thermal stability of organic solar cells.
Conclusions
The supramolecular interactions between liquid crystals and PCBM molecules have been investigated via theoretical calculations and experimental analyses, with the strength depending on the chemical structure of the liquid crystals. The DFT calculation results demonstrate that the binding energy between 5CT and PCBM was the highest. Based on the FTIR, DSC and POM analyses, 5CT exhibited the strongest interaction to restrict the crystallization of PC61BM, while 8TTP8 seemed to show the weakest interaction to restrict the crystallization of PC61BM. In the P3HT:PC61BM:LC system, the specimen containing 8OCB showed a higher Jsc and PCE than that of 8TTP8 after annealing at different electric field strengths. The highest PCE of 2.9% was achieved for the P3HT:PC61BM:8OCB specimen at an electric field strength of 600 V mm−1, showing higher morphological stability under an external force due to the stronger interaction between 8OCB and PC61BM. 5CT was found to seriously restrict the crystallization of PCBM after thermal annealing at 150 °C in the P3HT:PC61BM specimen, attributing to the strongest supramolecular interactions. In the narrow bandgap PTB7-Th:PC71BM system, 8TTP8 could slightly improve the thermal stability while 5CT and 8OCB are less effective. Besides the supramolecular interaction between the liquid crystals and PCBM, the interaction between the liquid crystals and donor polymers should also be taken into consideration. Corresponding research should be further carried out to explore the relationship between the chemical structure of the third additive and the thermal stability of other systems showing high PCE values.Experimental
Materials
Regioregular P3HT (Mw = 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 g mol−1, head-to-tail regioregularity >90%) and poly[4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b′]dithiophene-co-3-fluorothieno[3,4-b]thiophene-2-carboxylate] (PTB7-Th) used in this study were purchased from Rieke Metals, Inc. and 1-Materail, respectively. PC61BM (99.5% purity) and PC71BM were supplied by Nano-C, whereas the indium tin oxide (ITO) glass was purchased from Delta Technologies Limited. Zinc acetate dihydrate (Zn(CH3COO)2·2H2O) and ethanolamine (NH2CH2CH2OH) were purchased from Sigma Aldrich. 4-Octyloxy-4′-cyanobiphenyls (8OCB), dioctylterthiophene (8-TTP-8) and 4-cyano-4′-pentylterphenyl (5CT) were supplied by Energy Chemical Co. Chlorobenzene (CB) and dichlorobenzene (DCB) were purchased from Aldrich. All the reagents were used directly as received without further purification.
300 g mol−1, head-to-tail regioregularity >90%) and poly[4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b′]dithiophene-co-3-fluorothieno[3,4-b]thiophene-2-carboxylate] (PTB7-Th) used in this study were purchased from Rieke Metals, Inc. and 1-Materail, respectively. PC61BM (99.5% purity) and PC71BM were supplied by Nano-C, whereas the indium tin oxide (ITO) glass was purchased from Delta Technologies Limited. Zinc acetate dihydrate (Zn(CH3COO)2·2H2O) and ethanolamine (NH2CH2CH2OH) were purchased from Sigma Aldrich. 4-Octyloxy-4′-cyanobiphenyls (8OCB), dioctylterthiophene (8-TTP-8) and 4-cyano-4′-pentylterphenyl (5CT) were supplied by Energy Chemical Co. Chlorobenzene (CB) and dichlorobenzene (DCB) were purchased from Aldrich. All the reagents were used directly as received without further purification.
Device fabrication
Solution-processed solar cells with the conventional device architecture of ITO/ZnO/P3HT:PC61BM:LCs/MoO3/Ag or ITO/ZnO/PTB7-Th:PC71BM:LCs/MoO3/Ag were fabricated according to the following procedure. The ITO glass was cleaned through sequential ultrasonic treatment in acetone, detergent, deionized water, and isopropanol, and then treated in an ultraviolet-ozone chamber for 10 min. After treating with UV/ozone for 20 min, the ZnO precursor solution was spin coated at 4000 rpm and the ZnO layer was generated at 220 °C in an ambient atmosphere. The active layers were spun at 800 rpm from solutions of P3HT:PC61BM:LCs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06) and at 1000 rpm from solutions of PTB7-Th:PC71BM:LCs (1
0.06) and at 1000 rpm from solutions of PTB7-Th:PC71BM:LCs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06) containing DIO (0.3 v%). The electric field assisted treatment for the P3HT:PC61BM:LCs (1
0.06) containing DIO (0.3 v%). The electric field assisted treatment for the P3HT:PC61BM:LCs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06) specimens was then performed by applying various magnitudes of voltage during the solvent drying process for 30 min in air. After evaporation of the solvents, MoO3 was deposited via sequential thermal evaporation of 7 nm followed by 90 nm of Ag.
0.06) specimens was then performed by applying various magnitudes of voltage during the solvent drying process for 30 min in air. After evaporation of the solvents, MoO3 was deposited via sequential thermal evaporation of 7 nm followed by 90 nm of Ag.
Measurements
Theoretical calculations
The configurations of the PC61BM:8TTP8, PC61BM:8OCB and PC61BM:5CT complexes were optimized using density functional theory (DFT) at the B3LYP/6-31G** level. The single point energies of these optimized complexes were computed considering Basis Set Superposition Error (BSSE) to compute the binding energies between the three small molecules and PC61BM. All the calculations were carried out using the Gaussian-09 program.30Author contributions
W. Zhou and K. Hu contributed equally to this work.Conflicts of interest
The authous declare no competing financial interest.Acknowledgements
This work was financially supported by the National Science Fund for Distinguished Young Scholars (51425304) and the National Natural Science Foundation of China (51303077 and 51563016).References
- N. Espinosa, M. Hösel, D. Angmo and F. C. Krebs, Solar cells with one-day energy payback for the factories of the future, Energy Environ. Sci., 2012, 5, 5117–5132 CAS.
- A. C. Arias, J. D. MacKenzie, I. McCulloch, J. Rivnay and A. Salleo, Materials and applications for large area electronics: Solution-based approaches, Chem. Rev., 2010, 110, 3–24 CrossRef CAS PubMed.
- Z. He, B. Xiao, F. Liu, H. Wu, Y. Yang, S. Xiao, C. Wang, T. P. Russell and Y. Cao, Single-Junction Polymer Solar Cells with High Efficiency and Photovoltage, Nat. Photonics, 2015, 9, 174–179 CrossRef CAS.
- B. Kan, M. Li, Q. Zhang, F. Liu, X. Wan, Y. Wang, W. Ni, G. Long, X. Yang and H. Feng, A series of simple oligomer-like small molecules based on oligothiophenes for solution-processed solar cells with high efficiency, J. Am. Chem. Soc., 2015, 137, 3886–3893 CrossRef CAS PubMed.
- R. Tao, T. Umeyama, T. Higashino, T. Koganezawa and H. Imahori, Synthesis and isolation of cis-2 regiospecific ethylene-tethered indene dimer–[70] fullerene adduct for polymer solar cell applications, ACS Appl. Mater. Interfaces, 2015, 7, 16676–16685 CAS.
- H. Hu, K. Jiang, G. Yang, J. Liu, Z. Li, H. Lin, Y. Liu, J. Zhao, J. Zhang and F. Huang, Terthiophene-based D–A polymer with an asymmetric arrangement of alkyl chains that enables efficient polymer solar cells, J. Am. Chem. Soc., 2015, 137, 14149–14157 CrossRef CAS PubMed.
- H. L. Yip and A. K. Y. Jen, Recent advances in solution-processed interfacial materials for efficient and stable polymer solar cells, Energy Environ. Sci., 2012, 5, 5994–6011 CAS.
- O. Oklobia and T. Shafai, A quantitative study of the formation of PCBM clusters upon thermal annealing of P3HT/PCBM bulk heterojunction solar cell, Sol. Energy Mater. Sol. Cells, 2013, 117, 1–8 CrossRef CAS.
- S. Nam, S. Woo, J. Seo, W. H. Kim, H. Kim, C. R. McNeill, T. J. Shin, D. D. Bradley and Y. Kim, Pronounced cosolvent effects in polymer: Polymer bulk heterojunction solar cells with sulfur-rich electron-donating and imide-containing electron-accepting polymers, ACS Appl. Mater. Interfaces, 2015, 7, 15995–16002 CAS.
- H. Cha, D. S. Chung, S. Y. Bae, M. J. Lee, T. K. An, J. Hwang, K. H. Kim, Y. H. Kim, D. H. Choi and C. E. Park, Complementary absorbing star-shaped small molecules for the preparation of ternary cascade energy structures in organic photovoltaic cells, Adv. Funct. Mater., 2013, 23, 1556–1565 CrossRef CAS.
- M. Koppe, H. J. Egelhaaf, G. Dennler, M. C. Scharber, C. J. Brabec, P. Schilinsky and C. N. Hoth, Near IR sensitization of organic bulk heterojunction solar cells: Towards optimization of the spectral response of organic solar cells, Adv. Funct. Mater., 2010, 20, 338–346 CrossRef CAS.
- K. Sivula, Z. T. Ball, N. Watanabe and J. M. Fréchet, Amphiphilic diblock copolymer compatibilizers and their effect on the morphology and performance of polythiophene: Fullerene solar cells, Adv. Mater., 2006, 18, 206–210 CrossRef CAS.
- Y. Lin, P. Cheng, Y. Li and X. Zhan, A 3D star-shaped non-fullerene acceptor for solution-processed organic solar cells with a high open-circuit voltage of 1.18 V, Chem. Commun., 2012, 48, 4773–4775 RSC.
- C. H. Kim, S. H. Cha, S. C. Kim, M. Song, J. Lee, W. S. Shin, S. J. Moon, J. H. Bahng, N. A. Kotov and S. H. Jin, Silver nanowire embedded in P3HT:PCBM for high-efficiency hybrid photovoltaic device applications, ACS Nano, 2011, 5, 3319–3325 CrossRef CAS PubMed.
- C. Y. Nam, Q. Wu, D. Su, C. Chiu, N. J. Tremblay, C. Nuckolls and C. T. Black, Nanostructured electrodes for organic bulk heterojunction solar cells: Model study using carbon nanotube dispersed polythiophene-fullerene blend devices, J. Appl. Phys., 2011, 110, 064307 CrossRef.
- C. Liu, J. Li, X. Zhang, Y. He, Z. Li, H. Li, W. Guo, L. Shen and S. Ruan, Improving the efficiency of inverted polymer solar cells by introducing inorganic dopants, Phys. Chem. Chem. Phys., 2015, 17, 7960–7965 RSC.
- M. Han, H. Kim, H. Seo, B. Ma and J. W. Park, Photovoltaic efficiency enhancement by the generation of an embedded silica-like passivation layer along the P3HT/PCBM interface using an symmetric block copolymer additive, Adv. Mater., 2012, 24, 6311–6317 CrossRef CAS PubMed.
- A. Sharenko, N. D. Treat, J. A. Love, M. F. Toney, N. Stingelin and T. Q. Nguyen, Use of a commercially available nucleating agent to control the morphological development of solution-processed small molecule bulk heterojunction organic solar cells, J. Mater. Chem. A, 2014, 2, 15717–15721 CAS.
- S. Jeong, Y. Kwon, B. D. Choi, H. Ade and Y. S. Han, Improved efficiency of bulk heterojunction poly(3-hexylthiophene):[6,6]-phenyl-C61-butyric acid methyl ester photovoltaic devices using discotic liquid crystal additives, Appl. Phys. Lett., 2010, 96, 183305 CrossRef.
- S. W. Heo, K. H. Baek, H. J. Song, T. H. Lee and D. K. Moon, Improved performance of P3HT:PCBM based solar cells using nematic liquid crystals as a processing additive under low processing temperature conditions, Macromol. Mater. Eng., 2014, 299, 353–360 CrossRef CAS.
- S. Jeong, Y. Kwon, B. D. Choi, G. Kwak and Y. S. Han, Effects of nematic liquid crystal additives on the performance of polymer solar cells, Macromol. Chem. Phys., 2010, 211, 2474–2479 CrossRef CAS.
- W. Zhou, J. Shi, L. Lv, L. Chen and Y. Chen, A mechanistic investigation of morphology evolution in P3HT-PCBM films induced by liquid crystalline molecules under external electric field, Phys. Chem. Chem. Phys., 2015, 17, 387–397 RSC.
- J. M. Lehn, Cryptates: The chemistry of macropolycyclic inclusion complexes, Acc. Chem. Res., 1978, 11, 49–57 CrossRef CAS.
- J. M. Lehn, In supramolecular chemistry, Proc. - Indian Acad. Sci., Chem. Sci., 1994, 915–922 CAS.
- L. M. Salonen, M. Ellermann and F. Diederich, Aromatic rings in chemical and biological recognition: Energetics and structures, Angew. Chem., Int. Ed., 2011, 50, 4808–4842 CrossRef CAS PubMed.
- C. Z. Li, Y. Matsuo, T. Niinomi, Y. Sato and E. Nakamura, Face-to-face C6F5-[60] fullerene interaction for ordering fullerene molecules and application to thin-film organic photovoltaics, Chem. Commun., 2010, 46, 8582–8584 RSC.
- M. H. Liao, C. E. Tsai, Y. Y. Lai, F. Y. Cao, J. S. Wu, C. L. Wang, C. S. Hsu, I. Liau and Y. J. Cheng, Morphological stabilization by supramolecular perfluorophenyl-C60 interactions leading to efficient and thermally stable organic photovoltaics, Adv. Funct. Mater., 2014, 24, 1418–1429 CrossRef CAS.
- M. O. Sinnokrot and C. D. Sherrill, Unexpected substituent effects in face-to-face π-stacking interactions, J. Phys. Chem. A, 2003, 107, 8377–8379 CrossRef CAS.
- X. Ye, Z.-H. Li, W. Wang, K. Fan, W. Xu and Z. Hua, The parallel π–π stacking: A model study with MP2 and DFT methods, Chem. Phys. Lett., 2004, 397, 56–61 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford, CT, USA, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: FTIR spectra, POM images, AFM images, DSC curves, UV-vis absorption spectra, and photoluminescence spectra. See DOI: 10.1039/c6qm00199h |
| This journal is © the Partner Organisations 2017 |