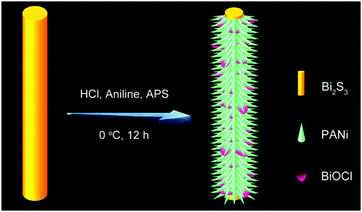
One-dimensional polyaniline thorn/BiOCl chip heterostructures: self-sacrificial template-induced synthesis and electrochemical performance†
Guangdi
Nie
a,
Xiaofeng
Lu
*a,
Wei
Wang
b,
Maoqiang
Chi
a,
Yanzhou
Jiang
a and
Ce
Wang
*a
aAlan G. MacDiarmid Institute, College of Chemistry, Jilin University, Changchun, 130012, P. R. China. E-mail: xflu@jlu.edu.cn; cwang@jlu.edu.cn; Fax: +86-431-85168292; Tel: +86-431-85168292
bState Key Laboratory of Urban Water Resource and Environment (SKLUWRE), School of Municipal and Environmental Engineering, Harbin Institute of Technology, Harbin, 150090, P. R. China
First published on 23rd November 2016
Abstract
A simple and green strategy was proposed for the first time to fabricate uniform polyaniline (PANi) thorn/BiOCl chip (BPB) heterostructures at a low temperature without the assistance of any surfactant. During the synthetic process, Bi2S3 nanowires acted as both the sacrificial template and the Bi source for BiOCl, affording the synchronous formation of HCl-doped PANi conductive arrays and BiOCl chips supported on residual Bi2S3 nanowires. As expected, when utilized as electrode materials for supercapacitors, the obtained BPB nanocomposite exhibited a better electrochemical performance in neutral media with enhanced specific capacitance, a more acceptable rate capability and a smaller charge-transfer resistance in comparison with the individual PANi nanofibers due to the unique hierarchical nanostructure of BPB and the synergistic effect between the ionizable BiOCl and the PANi chain.
Introduction
Nowadays, the rational design and construction of advanced nanostructures for high-performance supercapacitors (SCs) which can fill the void between conventional capacitors and batteries are absolutely essential to meet the urgent demand for clean and efficient alternative power sources due to the excessive depletion of fossil fuels and ever-aggravating environmental contamination.1 Conducting polymers, particularly polyaniline (PANi), as one of the most frequently-used electrode active materials for SCs, possess large theoretical specific capacitance, relatively high conductivity, ease of synthesis, good environmental stability and low cost, and have thus attracted tremendous attention over the past few decades.2 As is generally known, the electrochemical performance of PANi is significantly dependent on its morphology and dimension.3 Among various shaped materials in nanoscale, one-dimensional (1D) nanowires, nanotubes, nanobelts and nanorods are emerging as promising building components in energy storage devices on account of their increased surface area and short diffusion path for ions.4 In fact, it is tough to manipulate the morphology of conducting polymers compared with that of inorganic species.5 Recently, a template method which is simple, versatile and controllable has been developed by Martin et al. and has been widely utilized in the preparation of nanostructured PANi.6 However, usually only in acidic solution, ordinary PANi-based materials can deliver superior electroactivity, which restricts their functions in neutral electrolytes.7 According to previous reports, the protons doped on the nitrogen atoms of the PANi chain retained by the large counter ions or the inserted ionogenic substitutes are responsible for the maintenance of conductivity and redox activity in high pH media.8Bismuth oxychloride (BiOCl), an important V–VI–VII main group multicomponent semiconductor, presents a tetragonal lamellar structure in which the adjacent [Cl–Bi–O–Bi–Cl] sheets are stacked together by the nonbonding van der Waals interaction through the Cl atoms along the c-axis with [Bi2O2]2+ layers sandwiched between two slabs of Cl−.9 It is also a simple member of the Sillén family, which is partially expressed as [M2O2][Clm] (m = 1–3).10 Song et al. demonstrated that BiOCl could decompose into bismuth monoxide (BiO) and Cl2 under heat treatment in a N2 flow.11 Furthermore, BiOCl can be precisely cleaved parallel to the (001) plane, facilitating the formation of a flake-like morphology with an exposed surface of the Cl layer, which may serve as the counter anion for H+ doping in conducting polymers to improve their electrochemical properties.12 Two-dimensional (2D) BiOCl nanoplates as assembling blocks for complex architectures have found numerous applications in optical, electrical, magnetic, catalytic and biomedical fields owing to their unique geometric anisotropy, excellent photostability, perfect crystallinity and nontoxicity.13 Nevertheless, to the best of our knowledge, it is still an enormous challenge to fabricate the hierarchical self-assemblies of BiOCl-conducting polymer hybrids with well-defined nanostructures via facile, controllable, eco-friendly and surfactant-free approaches.
Herein, for the first time, a novel one-pot strategy is proposed for the synthesis of uniform PANi thorn/BiOCl chip (BPB) heterostructures at a low temperature using Bi2S3 nanowires as both the sacrificial template and the Bi source, during which process hydrochloric acid (HCl) also plays a crucial role in providing the Cl element for BiOCl and the dopant for PANi. As expected, when utilized as electrode materials for SCs, the obtained sample BPB exhibited enhanced specific capacitance, more acceptable rate capability and better conductivity compared with the individual PANi nanofibers, implying that the trapped BiOCl could in turn hold H+ in the vicinity of PANi and then improve its electroactivity in neutral media.
Experimental
Synthesis of materials
Bi2S3 nanowires were initially obtained from the direct hydrothermal reaction consulting Ma's work as published elsewhere.14 In detail, 3.0 g of thioacetamide (C2H5NS, TAA, Sinopharm Chemical Reagent Co., Ltd) and 0.97 g of bismuth nitrate pentahydrate (Bi(NO3)3·5H2O, Xilong Chemical Co., Ltd) were successively dissolved into 30 mL of deionized water under vigorous ultrasonic irradiation. After that, the brown mixture was transferred into a 40 mL Teflon-lined autoclave and kept at 180 °C for 12 h. Finally, the black precipitates were collected via centrifugation, washed thoroughly with deionized water, and dried in a vacuum freeze drier for further use.For the preparation of the BPB nanocomposite, in a typical procedure, 70 μL of the distilled aniline monomer (Xilong Chemical Co., Ltd) was added to 16 mL of a 1 M HCl (Beijing Chemical Works) aqueous solution containing 20 mg of the resulting Bi2S3 nanowires, followed by ultrasonication for more than 0.5 h to form a homogeneous dispersion. Subsequently, 4 mL of the pre-cooled HCl solution (1 M) with 175 mg of ammonium persulfate ((NH4)2S2O8, APS, Beijing Chemical Works) was quickly poured into the above system, which was kept under constant stirring at about 0 °C for another 12 h. The blackish green products were centrifuged, washed and dried in sequence.
In contrast, pure PANi nanofibers were synthesized using a similar method to that of the preparation of BPB, except without the addition of the Bi2S3 template. BiOCl nanoflakes were also obtained via a facile route; that is, 75.6 mg of Bi(NO3)3·5H2O and 175 mg of APS were first dissolved in 20 mL of a 1 M HCl aqueous solution. Second, 3 mL of a sodium hydroxide (NaOH, 0.8 g, Beijing Chemical Works) solution was instilled under magnetic agitation at room temperature. Eventually, the white floccules were separated, rinsed with deionized water several times, and then dried in a vacuum freeze drier.
Characterization
Field-emission scanning electron microscopy (FESEM, FEI Nova NanoSEM) and transmission electron microscopy (TEM, JEOL JEM-2000EX) operated at 15 and 100 kV, respectively, were applied to observe the morphologies of the as-prepared samples. The high-resolution TEM (HRTEM) images, selected area electron diffraction (SAED) pattern and energy dispersive X-ray (EDX) analysis of BPB were acquired using a FEI Tecnai G2 F20 electron microscope at an acceleration voltage of 200 kV, which was installed with a high-angle annular dark-field (HAADF) detector in the scanning TEM (STEM) system. The crystallographic structures were confirmed via X-ray diffraction (XRD, PANalytical B.V. Empyrean) under Cu Kα radiation. Fourier-transform infrared (FTIR) spectra and Raman characterization were implemented separately on a Bruker Vector 22 spectrometer and a Horiba LabRAM HR Evolution apparatus with a 633 nm laser as the excitation source. Ultraviolet-visible (UV-vis) absorption spectra were recorded on a Shimadzu UV-2501 PC spectrometer. For the BPB sample, the chemical composition and the mass percentage of BiOCl were investigated using an X-ray photoelectron spectroscope (XPS, Thermo Scientific ESCALAB250) and an inductively coupled plasma emission spectrometer (ICP, Agilent 725).Electrochemical measurement
Electrochemical measurements were mainly carried out on a CHI 660E electrochemical workstation (Shanghai Chenhua instrument Co., Ltd) in 0.5 M of Na2SO4 (Beijing Chemical Works) aqueous electrolyte using a traditional three-electrode cell, which was composed of a sample-modified working electrode, a saturated calomel reference electrode (SCE) and a platinum foil counter electrode. The working electrode was fabricated as in our early work.15 Briefly, powders of the active materials (total mass: 8 mg), acetylene black (commercially available) and polytetrafluoroethylene (PTFE, Aladdin) with a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were ground well and blended together in a few drops of ethanol. Afterwards, this slurry was coated and pressed onto the cleansed nickel foam (geometrical area: ∼1 × 1 cm2) and dried at 40 °C overnight. The exact loading amount of the active material on the electrode was ∼7.6 mg cm−2, determined by the weight difference of the current collector before and after the coating of the mixture above, using a microbalance with an accuracy of 10 μg (Mettler Toledo, Switzerland). A potential window of −0.2 to 0.8 V was set in all the cyclic voltammetry (CV) experiments at multiple scan rates of 3, 5, 10, 20 and 50 mV s−1, and galvanostatic charge–discharge (GCD) tests at different current densities of 0.5, 1.0, 2.0, 3.0 and 4.0 A g−1 unless otherwise stated. The electrochemical impedance spectra (EIS) were recorded over the frequency range from 105 to 0.01 Hz with an alternating current (AC) amplitude of 5 mV.
1 were ground well and blended together in a few drops of ethanol. Afterwards, this slurry was coated and pressed onto the cleansed nickel foam (geometrical area: ∼1 × 1 cm2) and dried at 40 °C overnight. The exact loading amount of the active material on the electrode was ∼7.6 mg cm−2, determined by the weight difference of the current collector before and after the coating of the mixture above, using a microbalance with an accuracy of 10 μg (Mettler Toledo, Switzerland). A potential window of −0.2 to 0.8 V was set in all the cyclic voltammetry (CV) experiments at multiple scan rates of 3, 5, 10, 20 and 50 mV s−1, and galvanostatic charge–discharge (GCD) tests at different current densities of 0.5, 1.0, 2.0, 3.0 and 4.0 A g−1 unless otherwise stated. The electrochemical impedance spectra (EIS) were recorded over the frequency range from 105 to 0.01 Hz with an alternating current (AC) amplitude of 5 mV.
Results and discussion
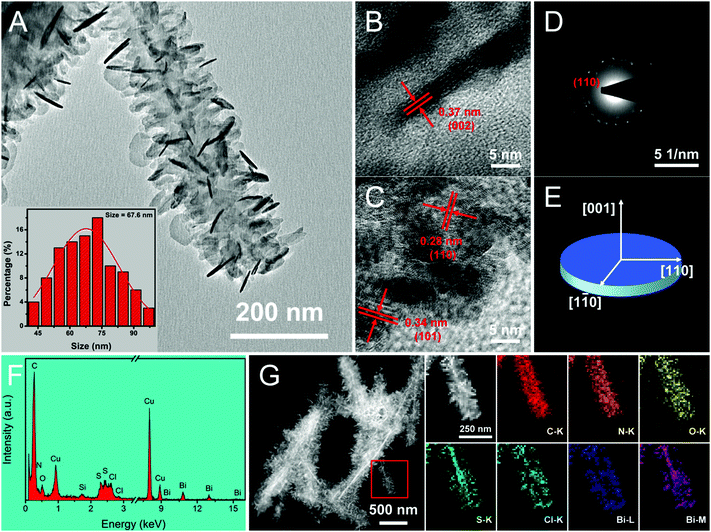
The convenient synthetic route of hierarchical BPB nanostructures is briefly delineated in Scheme 1, during which process the formation of PANi and BiOCl was speculated to take place at the surface of the Bi2S3 sacrificial template. Commonly, HCl (1 M) can gradually corrode Bi2S3 nanowires to release Bi3+ and H2S, and simultaneously as the doping agent of PANi, the concentration of H+ decreased along with the polymerization of aniline, hence resulting in the precipitation of BiOCl nanoflakes based on the following equation: Bi2S3 + 2HCl + 2H2O → 2BiOCl + 3H2S↑, even though the solubility of Bi2S3 (Ksp = 1.0 × 10−97) is rather lower than that of BiOCl (Ksp = 1.8 × 10−31).16The representative scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of the Bi2S3 nanowires and BPB heterostructures are shown in Fig. 1 to characterize their morphologies. We can clearly see from Fig. 1A and B that large-scale Bi2S3 nanowires in high quality are randomly oriented with a length up to several microns and a diameter ranging from 15 to 40 nm. For the obtained BPB composite (Fig. 1C and D), the uniform BiOCl nanoflakes (average transverse size: 67.6 nm, inset in Fig. 2A) are interspersed among the hierarchical PANi thorn-like arrays that surround the residual Bi2S3 buttressing skeleton to maintain a 1D configuration with empty space between adjacent PANi thorns, which could accelerate the propagation of the electrolyte, thus contributing to the optimization of the electrochemical performance.17
 | ||
| Fig. 1 (A and C) SEM and (B and D) TEM images of (A and B) pure Bi2S3 nanowires and (C and D) the resultant BPB heterostructures. | ||
As illustrated in the high-resolution TEM (HRTEM) images of BPB (Fig. 2B and C), the spacing of 0.37 nm between two adjacent lattice fringes on the side of a BiOCl chip (Fig. 2B) corresponds to the characteristic d002 of the tetragonal BiOCl phase, suggesting that the [001] orientation is the slowest growth direction due to weak c-axis bonding.12 Two types of interplanar distances of 0.28 and 0.34 nm belonging to d110 and d101 are observed at the edge of a BiOCl nanoflake (Fig. 2C). The existence of different facets may be attributed to the damage and partial reorientation of BiOCl crystals derived from electron beam irradiation with a high energy.18 The distinct spots displayed in the selected area electron diffraction (SAED) pattern (Fig. 2D) can be lightly indexed to the (110) plane of the BiOCl monocrystal. Fig. 2E shows a schematic model with crystal axes for the BiOCl chip. The energy dispersive X-ray (EDX) spectrum (Fig. 2F) of the prepared BPB sample exhibits the signals of C, N, O, Cu, Si, S, Cl and Bi elements, among which the Cu and Si atoms are supposed to originate from the carbon-coated copper grid and the instrument substrate, respectively. The relevant elemental mapping (Fig. 2G) provides potent evidence that the S atoms are primarily distributed at the core of the BPB heterostructures, indicating that some unreacted Bi2S3 still remain in the final product. Meanwhile, the S element at the edge of the nanostructure should be imputed to SO42− on the PANi arrays stemming from the reduction of ammonium persulfate during the polymerization of aniline.19 Individual PANi and BiOCl were also fabricated for comparison. It is obvious that both the PANi short nanofibers (Fig. S1A and B, ESI†) and the larger BiOCl nanoflakes (Fig. S1C and D, ESI†) with serious self-agglomeration are homogeneous in shape.
To validate the crystallographic natures of the samples, X-ray diffraction (XRD) patterns are shown in Fig. 3, from which we can discover that HCl-doped PANi possesses partial crystallinity with three weak peaks centered at 2θ = 14.8, 20.3 and 25.1°, which are assigned to the (010), (100) and (110) reflections of the emeraldine salt in a pseudo-orthorhombic unit cell, respectively.20 As for the pristine Bi2S3 nanowires, all the sharp peaks can be readily attributed to the orthorhombic phase of bismuthinite (JCPDS card No. 17-0320) without any other impurities. By comparison with individual BiOCl, it seems that the obtained BPB composite exhibits a similar tetragonal structure to BiOCl (JCPDS card No. 06-0249) except it has trace amounts of Bi2S3 remained. What should be noted is that the enhanced relative intensity of the (110) peak reveals a highly preferred [110] orientation in the BiOCl chip, matching perfectly with the HRTEM results.
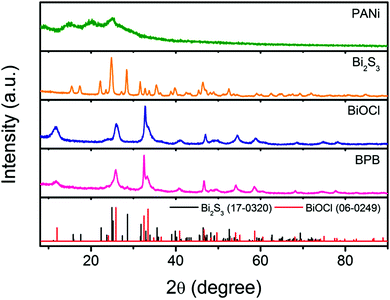 | ||
| Fig. 3 XRD patterns of the PANi nanofibers, Bi2S3 nanowires, BiOCl nanoflakes and hierarchical BPB nanostructures. | ||
The molecular structures of the samples were investigated with the assistance of Fourier-transform infrared (FTIR) measurements. As depicted in Fig. S2A (ESI†), the overlapping band at about 3444 cm−1 is caused by the stretching vibration of the O–H bond resulting from the adventitious water, and no other patent absorption peaks are observed for the Bi2S3 template. With regard to the pure BiOCl nanoflakes, the peak in the region of 534 cm−1 can be ascribed to the Bi–O stretching mode.21 The FTIR spectrum of the BPB composite is almost the same as that of the single PANi nanofibers. The characteristic peaks located at around 1574 and 1490 cm−1 are identified as the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of the quinoid and benzenoid rings, respectively.22 The C–N and C
C stretching vibration of the quinoid and benzenoid rings, respectively.22 The C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching modes of PANi appear at 1300 and 1243 cm−1.23 The strong peaks at 1126, 802 and 508 cm−1 are associated with the in-plane and out-of-plane bending of C–H in a 1,4-disubstituted aromatic ring, implying a linear structure of PANi in both the samples.24 A parallel conclusion can be drawn from the normalized Raman spectra (Fig. S2B, ESI†). The typical bands of PANi chains are observed at 415, 522 (the C–N–C out-of-plane torsion), 1164 (the C–H in-plane bending deformation in the 1,4-disubstituted aromatic rings), 1220 (the C–N stretching vibration of amines), 1326 (the C–N˙+ stretching species), 1467 and 1586 cm−1 (C
N stretching modes of PANi appear at 1300 and 1243 cm−1.23 The strong peaks at 1126, 802 and 508 cm−1 are associated with the in-plane and out-of-plane bending of C–H in a 1,4-disubstituted aromatic ring, implying a linear structure of PANi in both the samples.24 A parallel conclusion can be drawn from the normalized Raman spectra (Fig. S2B, ESI†). The typical bands of PANi chains are observed at 415, 522 (the C–N–C out-of-plane torsion), 1164 (the C–H in-plane bending deformation in the 1,4-disubstituted aromatic rings), 1220 (the C–N stretching vibration of amines), 1326 (the C–N˙+ stretching species), 1467 and 1586 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching modes of quinonoid rings) for the individual PANi nanofibers as well as the obtained BPB composite.25 Besides, the peaks at 576, 1408 and 1640 cm−1 pertain to the cross-linked phenazine units, demonstrating the structural imperfection of the PANi phase.26 The signals of the pristine Bi2S3 are positioned at 110, 180, 235 (Ag) and 253 cm−1 (B1g), which are separately related to the transverse and longitudinal optical phonon modes.27 For the pure BiOCl, the bands at 144 and 198 cm−1 are assigned to the A1g and Eg internal Bi–Cl stretching vibrations, respectively.28
C stretching modes of quinonoid rings) for the individual PANi nanofibers as well as the obtained BPB composite.25 Besides, the peaks at 576, 1408 and 1640 cm−1 pertain to the cross-linked phenazine units, demonstrating the structural imperfection of the PANi phase.26 The signals of the pristine Bi2S3 are positioned at 110, 180, 235 (Ag) and 253 cm−1 (B1g), which are separately related to the transverse and longitudinal optical phonon modes.27 For the pure BiOCl, the bands at 144 and 198 cm−1 are assigned to the A1g and Eg internal Bi–Cl stretching vibrations, respectively.28
Detailed information on the chemical composition and surface electronic state of BPB is supplemented by X-ray photoelectron spectroscopy (XPS) shown in Fig. 4. The annotated XPS full spectrum (Fig. 4A) indicates the presence of C, N, O, S, Cl and Bi elements, which is in complete agreement with the EDX analysis. The C 1s region (Fig. 4B) of PANi in the BPB heterostructures could be briefly deconvoluted into two Gaussian peaks corresponding to C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–H (284.6 eV) and C–N/C
C/C–H (284.6 eV) and C–N/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (285.4 eV).29 The fitted peaks in the fine-scan N 1s spectrum (Fig. 4C) correspond to the benzenoid amine (–NH–) at 399.4 eV and the positively charged nitrogen (N+) at 400.8 eV.30 The O 1s envelope (Fig. 4D) is a superposition of the oxygen moieties like O2− in a bismuth–oxygen bond of BiOCl (530.4 eV) and the absorbed water (H2O/–OH) or C
N (285.4 eV).29 The fitted peaks in the fine-scan N 1s spectrum (Fig. 4C) correspond to the benzenoid amine (–NH–) at 399.4 eV and the positively charged nitrogen (N+) at 400.8 eV.30 The O 1s envelope (Fig. 4D) is a superposition of the oxygen moieties like O2− in a bismuth–oxygen bond of BiOCl (530.4 eV) and the absorbed water (H2O/–OH) or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups (531.5 eV).21,31 The dual peaks with binding energies of 198.3 and 199.9 eV displayed in Fig. 4E can be resolved into Cl 2p3/2 and Cl 2p1/2, respectively, which is characteristic of Cl− in BiOCl. Fig. 4F represents the high-resolution XPS spectrum of the Bi 4f and S 2p regions. It is perspicuous that the predominant doublet peaks at 159.5 and 164.8 eV indexed to the normal Bi3+ in BiOCl are accompanied by a pair of weak shoulder peaks at 158.1 and 163.5 eV attributed to the lower valence state of Bi or the Bi(III) in Bi2S3.32 Additionally, the wide band at about 168.9 eV is caused by the counter anion SO42− on the PANi chain.33 The S 2s signal (the inset in Fig. 4F) at 228.3 eV is consistent with the S8 species, further proving the existence of Bi2S3 in the BPB composite.34
O groups (531.5 eV).21,31 The dual peaks with binding energies of 198.3 and 199.9 eV displayed in Fig. 4E can be resolved into Cl 2p3/2 and Cl 2p1/2, respectively, which is characteristic of Cl− in BiOCl. Fig. 4F represents the high-resolution XPS spectrum of the Bi 4f and S 2p regions. It is perspicuous that the predominant doublet peaks at 159.5 and 164.8 eV indexed to the normal Bi3+ in BiOCl are accompanied by a pair of weak shoulder peaks at 158.1 and 163.5 eV attributed to the lower valence state of Bi or the Bi(III) in Bi2S3.32 Additionally, the wide band at about 168.9 eV is caused by the counter anion SO42− on the PANi chain.33 The S 2s signal (the inset in Fig. 4F) at 228.3 eV is consistent with the S8 species, further proving the existence of Bi2S3 in the BPB composite.34
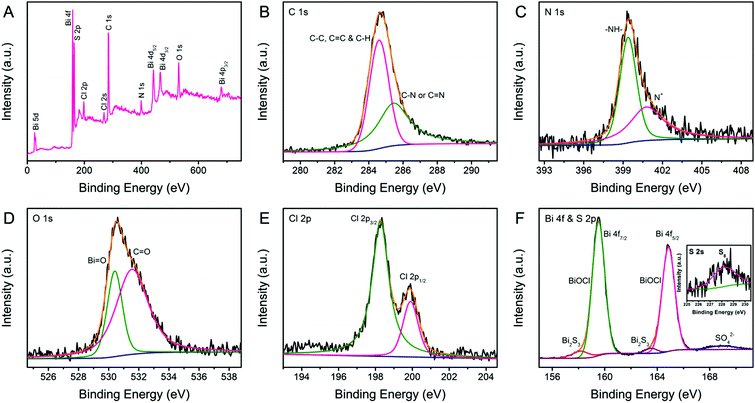 | ||
| Fig. 4 XPS spectra of BPB: (A) full survey spectra, (B) C 1s, (C) N 1s, (D) O 1s, (E) Cl 2p, and (F) Bi 4f and S 2p regions. Inset: S 2s region. | ||
The ultraviolet-visible (UV-vis) spectra of the prepared Bi2S3, BiOCl, PANi and BPB samples are presented in Fig. S3A (ESI†). The pristine Bi2S3 exhibits a continuous absorption band nearly over the whole visible light range, while the pure BiOCl only shows noticeable absorption in the ultraviolet region without a legible absorption edge. With respect to the individual PANi nanofibers and BPB composite, the absorption bands at 350–375, 425–450 and >800 nm are attributed to the benzenoid π–π*, polaron–π* and π–localized polaron transition, respectively, which indicates that these PANi are in their emeraldine salt form.23,35 The plausible conjecture for the structural formula of HCl- and BiOCl-doped PANi is illustrated in Fig. S3B (ESI†). However, more studies should be implemented to corroborate the point that the exposed Cl layer in BiOCl can serve as the counter anion for H+ doping in PANi.
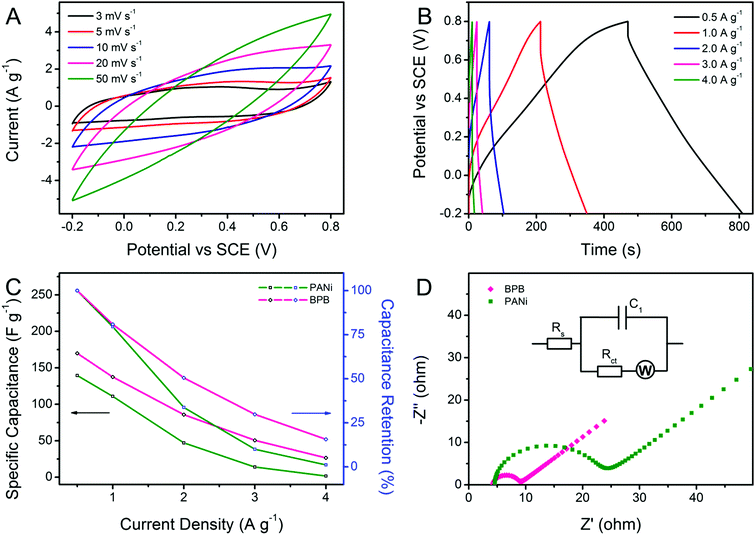
Cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD) measurements were carried out for a better understanding of the electrochemical performance of the obtained Bi2S3, BiOCl, PANi and BPB electrodes. As shown in Fig. 5A, the contributions of bare Bi2S3 and BiOCl to the capacitance can be neglected. There is only a pair of redox responses observed for the PANi electrode, demonstrating that the individual PANi shows weak electroactivity in a neutral electrolyte, which is coincident with the previously published results.36 Apparently, the CV curve of the BPB electrode with a wide anodic peak and two cathodic peaks is quite different from that of the PANi electrode, suggesting the electric double-layer capacitance (EDLC) and Faradaic pseudocapacitance behaviors of the BPB electrode with the reaction rate controlled by mass transfer at a scan rate of 5 mV s−1.36 Based on the integral area of the CV curves, which is proportional to the specific capacitance, we can infer that the electrode modified with the BPB sample possesses a larger capacitance value than the pure PANi electrode. Likewise, an analogous conclusion is extracted from the associated galvanostatic discharge branches with a smaller voltage drop for the BPB electrode (Fig. 5B). The gravimetric specific capacitance is determined according to the following equation: C (F g−1) = (I × Δt)/(m × ΔV), where I (A) is the applied current, Δt (s) is the discharge time, m (g) is the mass of electrode materials and ΔV (V) is the potential window. At a current density of 0.5 A g−1, it is calculated that the attained BPB electrode shows a higher specific capacitance of 169.9 F g−1 in comparison with the individual PANi electrode (139.4 F g−1) based on the total weight of materials. The neat Bi2S3 and BiOCl almost have no capability to store charges in 0.5 M of Na2SO4 solution. Therefore, when only considering the PANi (∼61.4 wt%) in the BPB heterostructures, the gravimetric specific capacitance of the BPB electrode is converted into 276.7 F g−1. Such a good electrochemical performance of the BPB composite may be due to its unique hierarchical structure with a short pathway for ion penetration and the ionizable BiOCl connected to the PANi chain.
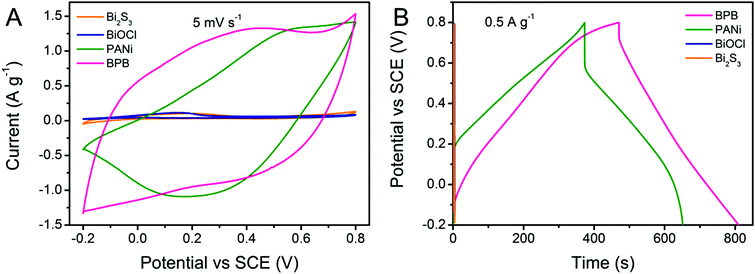 | ||
| Fig. 5 (A) CV curves recorded at a scan rate of 5 mV s−1 and (B) GCD profiles at a current density of 0.5 A g−1 for PANi, Bi2S3, BiOCl and BPB electrodes. | ||
Taking the BPB electrode as an example, the shape of the CV curves (Fig. 6A) that gradually deviates from a regular rectangle because of the electrode polarization remains essentially constant along with the increase in scan rate, indicating the good rate capability of the BPB heterostructures.37 However, it should be emphasized that no evident redox peaks are detected at very high scan rates, which manifests in a little contribution of pseudocapacitance to the total capacitance value of the nanocomposite relative to EDLC. Fig. 6B exhibits the corresponding current density-dependent GCD profiles. All discharge segments are nearly symmetrical to their charge counterparts, testifying the good capacitive property of the BPB electrode. The attenuation of capacitance at a higher current density is common for PANi-based nanomaterials since only the outer pores or active sites of the electrode are accessible to electrolyte ions under this circumstance.38 As plotted in Fig. 6C, the specific capacitance of the BPB hybrid decreases to 26.6 F g−1 (15.6% of the value at 0.5 A g−1) at a current density of 4.0 A g−1, which is much higher than that of pristine PANi (1.6 F g−1, 1.2% of the capacitance at 0.5 A g−1), thus revealing the better rate capability of the BPB hybrid. In order to further evaluate the electrochemical performance of the as-prepared BPB and PANi electrodes, the electrochemical impedance spectra (EIS, Fig. 6D) were measured and fitted using an equivalent circuit (inset in Fig. 6D) composed of Rs, the intrinsic series resistance of the electrode and bulk solution, Rct, the interfacial charge-transfer resistance, W, the Warburg impedance, and C1, the double-layer capacitance. Both of the Nyquist plots contain a semicircle at high frequency and a straight line in the low-frequency area. By contrast, it is apparent that the tail sections at low frequency for the two electrodes are almost parallel to each other, demonstrating the equal diffusion resistance in such two systems. In the high-frequency range, the intersection point of the semicircle on the real axis stands for Rs, which is nearly the same for both the BPB and PANi electrodes, implying that the introduction of BiOCl has no obvious effect upon increasing the internal resistance of the nanocomposite. The value of Rct for the BPB electrode calculated from the semicircle diameter is much lower than that for the PANi electrode owing to the hierarchical structure of the BPB hybrid and the ionizable BiOCl connected to the PANi chain. We believe that the combination of fast ion diffusion and more effective electron transfer is responsible for the enhanced electrochemical property of the BPB electrode.
Conclusions
In summary, a novel hierarchical nanostructure of BPB with BiOCl nanoflakes interspersed among the uniform PANi thorn-like arrays on the residual Bi2S3 nanowires was synthesized via a facile one-pot approach at low temperature for the first time, during which process Bi2S3 acted as both the sacrificial template and the Bi source for BiOCl. As anticipated, when used as an active material for SCs, the BPB-modified electrode exhibited an improved specific capacitance of 169.9 F g−1 at 0.5 A g−1, a more acceptable rate capability of 15.6% at 4.0 A g−1 and a smaller charge-transfer resistance in comparison with the individual PANi electrode, highlighting that the trapped BiOCl could enhance the electroactivity of the nanocomposite in a neutral electrolyte. It is believed that this unique composite nanostructure might be extended to applications in photocatalysis, photoelectric devices and chloride ion batteries in the future.Acknowledgements
This work was financially supported by the research grants from the National Natural Science Foundation of China (51273075, 21274052, 51473065 and 21474043), and the Graduate Innovation Fund of Jilin University (2015011).Notes and references
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28 CrossRef CAS PubMed; S. W. Pan, J. Ren, X. Fang and H. S. Peng, Adv. Energy Mater., 2016, 6, 1501867 CrossRef; P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845 CrossRef PubMed; L. Hao, X. L. Li and L. J. Zhi, Adv. Mater., 2013, 25, 3899 CrossRef PubMed.
- J. P. Li, Y. Q. Ren, Z. H. Ren, S. G. Wang, Y. J. Qiu and J. Yu, J. Mater. Chem. A, 2015, 3, 23307 Search PubMed; A. Khosrozadeh, M. A. Darabi, M. Xing and Q. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 11379 Search PubMed; C. L. Hu, S. Y. Chen, Y. Wang, X. H. Peng, W. H. Zhang and J. Chen, J. Power Sources, 2016, 321, 94 CrossRef CAS; L. J. Pan, G. H. Yu, D. Y. Zhai, H. R. Lee, W. T. Zhao, N. Liu, H. L. Wang, B. C.-K. Tee, Y. Shi, Y. Cui and Z. N. Bao, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 9287 CrossRef PubMed; Y. Zhao, B. R. Liu, L. J. Pan and G. H. Yu, Energy Environ. Sci., 2013, 6, 2856 Search PubMed.
- K. Wang, H. P. Wu, Y. N. Meng and Z. X. Wei, Small, 2014, 10, 14 CrossRef CAS PubMed.
- Z. G. Yin and Q. D. Zheng, Adv. Energy Mater., 2012, 2, 179 CrossRef CAS; W. Xiong, X. Hu, X. Wu, Y. Zeng, B. Wang, G. H. He and Z. H. Zhu, J. Mater. Chem. A, 2015, 3, 17209 Search PubMed.
- L. J. Ren, G. N. Zhang, J. F. Wang, L. P. Kang, Z. B. Lei, Z. W. Liu, Z. T. Liu, Z. P. Hao and Z. H. Liu, Electrochim. Acta, 2014, 145, 99 CrossRef CAS.
- C. R. Martin, Science, 1994, 266, 1961 Search PubMed; W. J. Hou, Y. M. Xiao, G. Y. Han, D. Y. Fu and R. F. Wu, J. Power Sources, 2016, 322, 155 CrossRef CAS; I. Y. Choi, J. Lee, H. Ahn, J. Lee, H. C. Choi and M. J. Park, Angew. Chem., Int. Ed., 2015, 54, 10497 CrossRef PubMed; R. X. Yuan, H. Y. Wang, T. Ji, L. W. Mu, L. Chen, Y. J. Zhu and J. H. Zhu, J. Mater. Chem. A, 2015, 3, 19299 Search PubMed.
- H. R. Ghenaatian, M. F. Mousavi and M. S. Rahmanifar, Electrochim. Acta, 2012, 78, 212 CrossRef CAS; H. G. Wei, H. B. Gu, J. Guo, S. Y. Wei, J. R. Liu and Z. H. Guo, J. Phys. Chem. C, 2013, 117, 13000 Search PubMed.
- L. J. Bian, F. Luan, S. S. Liu and X. X. Liu, Electrochim. Acta, 2012, 64, 17 CrossRef CAS; J. Luo, X. H. Wang, J. Li, X. J. Zhao and F. S. Wang, Electrochem. Commun., 2007, 9, 1175 CrossRef.
- M. L. Guan, C. Xiao, J. Zhang, S. J. Fan, R. An, Q. M. Cheng, J. F. Xie, M. Zhou, B. J. Ye and Y. Xie, J. Am. Chem. Soc., 2013, 135, 10411 CrossRef CAS PubMed; J. Li, Y. Yu and L. Z. Zhang, Nanoscale, 2014, 6, 8473 RSC; M. Li, J. Y. Zhang, H. Gao, F. Li, S. E. Lindquist, N. Q. Wu and R. M. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 6662 Search PubMed.
- X. Zhang, Z. H. Ai, F. L. Jia and L. Z. Zhang, J. Phys. Chem. C, 2008, 112, 747 Search PubMed; J. Y. Xiong, G. Cheng, G. F. Li, F. Qin and R. Chen, RSC Adv., 2011, 1, 1542 RSC.
- J. M. Song, C. J. Mao, H. L. Niu, Y. H. Shen and S. Y. Zhang, CrystEngComm, 2010, 12, 3875 RSC.
- X. Y. Zhao, Z. R. Zhao-Karger, D. Wang and M. Fichtner, Angew. Chem., Int. Ed., 2013, 52, 13621 CrossRef CAS PubMed.
- M. Guerrero, S. Pané, B. J. Nelson, M. D. Baró, M. Roldán, J. Sort and E. Pellicer, Nanoscale, 2013, 5, 12542 RSC; Y. Myung, F. Wu, S. Banerjee, J. Park and P. Banerjee, Chem. Commun., 2015, 51, 2629 RSC; Y. H. Wu, B. Yuan, M. R. Li, W. H. Zhang, Y. Liu and C. Li, Chem. Sci., 2015, 6, 1873 RSC; X. Zhang, X. B. Wang, L. W. Wang, W. K. Wang, L. L. Long, W. W. Li and H. Q. Yu, ACS Appl. Mater. Interfaces, 2014, 6, 7766 Search PubMed.
- J. M. Ma, J. Q. Yang, L. F. Jiao, T. H. Wang, J. B. Lian, X. C. Duan and W. J. Zheng, Dalton Trans., 2011, 40, 10100 RSC.
- G. D. Nie, X. F. Lu, M. Q. Chi, Y. Z. Jiang and C. Wang, RSC Adv., 2016, 6, 54693 RSC; G. D. Nie, X. F. Lu, J. Y. Lei, L. Yang and C. Wang, Electrochim. Acta, 2015, 154, 24 CrossRef CAS.
- H. F. Cheng, B. B. Huang, X. Y. Qin, X. Y. Zhang and Y. Dai, Chem. Commun., 2012, 48, 97 RSC.
- G. Q. Zhang and X. W. (D.) Lou, Sci. Rep., 2013, 3, 1470 Search PubMed.
- H. B. Wu and W. Chen, Nanoscale, 2011, 3, 5096 RSC; W. Xiao, D. L. Wang and X. W. Lou, J. Phys. Chem. C, 2010, 114, 1694 Search PubMed.
- M. Chakraborty, D. C. Mukherjee and B. M. Mandal, Langmuir, 2000, 16, 2482 CrossRef CAS.
- U. Bogdanović, V. Vodnik, M. Mitrić, S. Dimitrijević, S. D. Škapin, V. Žunič, M. Budimir and M. Stoiljković, ACS Appl. Mater. Interfaces, 2015, 7, 1955 Search PubMed; H. W. Park, T. Kim, J. Huh, M. Kang, J. E. Lee and H. Yoon, ACS Nano, 2012, 6, 7624 CrossRef CAS PubMed.
- G. Cheng, J. Y. Xiong and F. J. Stadler, New J. Chem., 2013, 37, 3207 RSC.
- W. Chen, R. B. Rakhi and H. N. Alshareef, J. Mater. Chem. A, 2013, 1, 3315 CAS.
- Y. Z. Li, X. Zhao, Q. Xu, Q. H. Zhang and D. J. Chen, Langmuir, 2011, 27, 6458 CrossRef CAS PubMed.
- M. M. Sk, C. Y. Yue and R. K. Jena, Polymer, 2014, 55, 798 CrossRef CAS.
- H. R. Tantawy, A. T. Weakley and D. E. Aston, J. Phys. Chem. C, 2014, 118, 1294 CAS.
- N. A. Ogurtsov, Y. V. Noskov, V. N. Bliznyuk, V. G. Ilyin, J. L. Wojkiewicz, E. A. Fedorenko and A. A. Pud, J. Phys. Chem. C, 2016, 120, 230 CAS.
- I. Zumeta-Dubé, J. L. Ortiz-Quiñonez, D. Díaz, C. Trallero-Giner and V. F. Ruiz-Ruiz, J. Phys. Chem. C, 2014, 118, 30244 Search PubMed.
- F. Dong, Y. J. Sun, M. Fu, Z. B. Wu and S. C. Lee, J. Hazard. Mater., 2012, 219–220, 26 CrossRef CAS PubMed.
- H. L. Li, Y. He, V. Pavlinek, Q. L. Cheng, P. Saha and C. Z. Li, J. Mater. Chem. A, 2015, 3, 17165 CAS.
- M. Kim, C. Lee and J. Jang, Adv. Funct. Mater., 2014, 24, 2489 CrossRef CAS.
- G. D. Nie, X. F. Lu, J. Y. Lei, L. Yang, X. J. Bian, Y. Tong and C. Wang, Electrochim. Acta, 2013, 99, 145 CrossRef CAS.
- Y. Yu, C. Y. Cao, H. Liu, P. Li, F. F. Wei, Y. Jiang and W. G. Song, J. Mater. Chem. A, 2014, 2, 1677 CAS.
- G. D. Nie, L. Zhang, X. F. Lu, X. J. Bian, W. N. Sun and C. Wang, Dalton Trans., 2013, 42, 14006 RSC.
- J. W. Thomson, L. Cademartiri, M. MacDonald, S. Petrov, G. Calestani, P. Zhang and G. A. Ozin, J. Am. Chem. Soc., 2010, 132, 9058 CrossRef CAS PubMed.
- X. F. Lu, X. J. Bian, Z. C. Li, D. M. Chao and C. Wang, Sci. Rep., 2013, 3, 2955 Search PubMed.
- C. M. Li and S. L. Mu, Synth. Met., 2005, 149, 143 CrossRef CAS.
- C. Z. Yuan, J. Y. Li, L. R. Hou, X. G. Zhang, L. F. Shen and X. W. (D.) Lou, Adv. Funct. Mater., 2012, 22, 4592 CrossRef CAS.
- L. Q. Mai, F. Yang, Y. L. Zhao, X. Xu, L. Xu and Y. Z. Luo, Nat. Commun., 2011, 2, 381 CrossRef CAS PubMed; S. K. Meher, P. Justin and G. R. Rao, ACS Appl. Mater. Interfaces, 2011, 3, 2063 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qm00232c |
| This journal is © the Partner Organisations 2017 |