Light/temperature-enhanced emission characteristics of malononitrile-containing hexaphenyl-1,3-butadiene derivatives: the hotter, the brighter†
Yahui
Zhang
,
Lingwei
Kong
,
Huiling
Mao
,
Xiaoling
Pan
,
Jianbing
Shi
,
Bin
Tong
* and
Yuping
Dong
 *
*
Beijing Key Laboratory of Construction Tailorable Advanced Functional Materials and Green Applications, School of Materials Science and Engineering, Beijing Institute of Technology, 5 South Zhongguancun Street, Beijing, 100081, China. E-mail: bing@bit.edu.cn; chdongyp@bit.edu.cn
First published on 11th September 2017
Abstract
A Light/Temperature-Enhanced Emission (LTEE) based fluorescence turn-on probe was prepared via the photocyclization/crystallization of a malononitrile-containing hexaphenyl-1,3-butadiene derivative (HPB-CN) on filter paper. The HPB-CN-coated filter paper exhibited stronger PL intensity as the exposure time under UV light prolonged and the heating temperature increased, indicating the hotter, the brighter.
Fluorescent organic materials play a vital role in real-world applications and high-tech innovations. Most of the luminescent materials are used in solid films or aggregated states, such as those for lighting and display devices, or as bioimaging agents and bioprobes. However, their aggregation-caused quenching (ACQ) effect and temperature-caused quenching effect (TCQ) limit their applications. The fluorescence of organic materials can be significantly weakened or annihilated at high concentrations or in the aggregated state. To solve this problem, Tang et al. innovated a new type of fluorophore with aggregation-induced emission (AIE) properties.1 This inspiring phenomenon can alleviate the notorious ACQ effect of emitters, paving an alternative avenue to prepare efficient fluorescent organic materials in the solid state. To date, AIEgens have been used in a variety of fields, such as organic light-emitting diodes,2 chemosensors3 and biosensors.4
A high temperature can cause more vigorous motion (vibration and rotation) of molecules, enhance radiation channel decay and decrease the PL intensity, resulting in a TCQ effect. Therefore, most fluorescent materials can only be applied below specific temperatures in the “turn-off” form. The temperatures higher than such specific temperatures can completely quench the materials. So far, only a few fluorescence materials are available for application at high temperatures. It is well-known that temperature is an important parameter of pretreatment and an excellent accessory tool for the preparation and functions of fluorescent materials, such as tuning nanoparticle size,5 the color of fluorescence6 and so on. Therefore, it is impossible to study the fluorescent materials while bypassing the temperature problem. Researchers have made tremendous efforts to develop fluorescence materials with “turn-on” responses to temperature because, compared with fluorescence quenching, increasing fluorescence intensity is more favorable to detect sensitivity. Stuparu assembled larger supramolecular structures with orannulene in water by varying the solution temperature. The aqueous solution remained clear below 40 °C and became opaque and its PL intensity increased rapidly due to the gelation and the restriction of molecular motion as temperature increased to over 40 °C.7 Qian, et al. found that the air-stable tri(pyren-1-yl)phosphine oxide exhibited a prominent temperature-dependent feature in the temperature range between −40 °C and 100 °C due to its thermally populated emissive local excited states.8 Weder et al. designed a novel supramolecular polymer with cyano-substituted oligo(p-phenylenevinylene) and two ureido-4-pyrimidinone groups. The new material exhibited mechano/thermoresponsive luminescent behaviors with three emission colors in the solid state.9 However, its direct thermo response of PL intensity occurred in a narrow temperature range of 125–180 °C. So far, it is still unclear how temperature affects the PL intensity of fluorescent materials directly in the solid state, which will undoubtedly limit the scope of their applications. A novel strategy for the preparation of thermal “turn-on” fluorescent materials in the solid state is highly urgent. In our previous report, 1,1,4,4-tetraaryl-1,3-butadiene derivatives containing functional groups have been demonstrated as excellent candidates for AIE (AEE) luminophores with mechanochromic behaviors due to D–π–A structures with large dipole moments and intermolecular hydrogen bonding.10 The AIE properties of hexaphenyl-1,3-butadiene (HPB) without functional substitutes were first studied by Wong, et al.11
In previous work, we successfully detected volatile amine using an exaphenyl-1,3-butadiene derivative, 2,2′-((((1Z,3Z)-1,2,3,4-tetraphenylbuta-1,3-diene-1,4-diyl)bis(4,1-phenylene))bis(me-thanylylidene))dimalononitrile (HPB-CN), deposited on filter paper.12 We also found that HPB-CN is a typical AEEgen. To further explain and understand the above phenomenon, single crystals of HPB-CN were grown through a slow crystallization process. As shown in Fig. S1 (ESI†), HPB-CN adopted an extremely twisted conformation. The results indicated that all phenyl groups in HPB-CN can freely rotate in THF and consume the excitation energies, resulting in non-emission or little emission in solution. Upon aggregation, these intramolecular rotations are highly restricted, and this enhances the emissive efficiency (Chart 1).
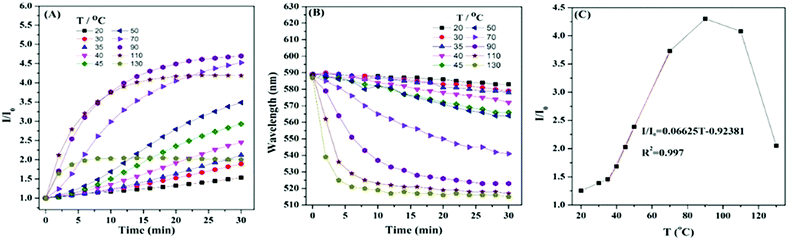
The filter paper strips were found to show “turn-on” response to temperature by accident. The PL intensity and the blue-shift of emission wavelength increased with temperature in the range from room temperature to 110 °C. Similar to other fluorescent materials, HPB-CN was quenched at temperatures over 110 °C. Further studies indicated that the crystallization induced by the paper fibers and the restriction of intramolecular motion caused by cyclization were the major contributors to the temperature-induced emission enhancement effect. In addition, both processes were indispensable (Fig. S2, ESI†). Our result provides a new strategy for the design of LTEE based light/thermo-sensitive turn-on probes to expand the related applications of fluorescent materials. HPB-CN with AEE features was prepared and well characterized in our previous report. Unlike the traditional fluorescent organic materials with TCQ behavior, the PL intensity of HPB-CN deposited on filter paper became stronger as temperature increased. As shown in Fig. 1A and Fig. S3 (ESI†), the fluorescence intensity of HPB-CN was slowly enhanced and the emission wavelength gradually blue-shifted with heating time at temperatures lower than 35 °C. The PL intensity was enhanced only ∼2.1 fold and the emission wavelength was blue-shifted 11 nm as the HPB-CN paper strip was heated at 35 °C for 30 min. Both PL intensity and emission wavelength blue-shift of the HPB-CN strip were significantly enhanced as the temperature increased from 35 °C to 90 °C. The PL intensity was enhanced ∼4.7 fold and the emission wavelength was blue-shifted 65 nm as the strip was heated at 90 °C for 30 min, suggesting its excellent LTEE performance. In addition, the PL intensity increased linearly with the increase of temperature from 35 °C to 70 °C after the strip was heated for 16 min (Fig. 1C). The differential calculations indicated that the PL intensity increased most rapidly as temperature varied between 35 and 40 °C, body temperature, indicating that HPB-CN could be used for bioimaging in physiological environments. The PL intensity decreased as the temperature was further increased to over 90 °C, although the equilibrium time of PL enhancement was shortened with the increase of heating time. The PL intensity of HPB-CN was enhanced as it was heated at 130 °C. However, it took only 6 min to reach a constant PL intensity and the maximum blue-shift of emission wavelength at 130 °C.
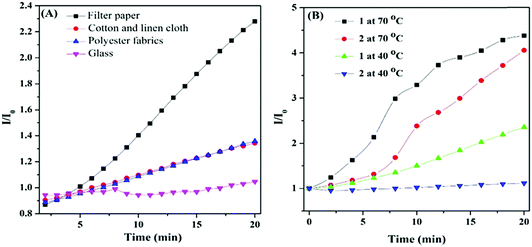
The enhanced PL indicates that the arrangement of HPB-CN molecules was more orderly and the intramolecular motions were more restricted with the increase of temperature, which restrained the radiation channel decay. To verify the hypothesis, we analyzed the aggregation states of HPB-CN on the surfaces of filter papers treated under different conditions (heating and irradiation) by powder X-ray diffraction (PXRD). HPB-CN powder exhibited many sharp diffraction peaks, indicating that it was a polycrystalline structure (Fig. S4A, ESI†). The HPB-CN deposited on filter paper showed a completely different crystalline form. The diffraction peak at 2θ = 23° that was attributed to the filter paper was set as the reference peak (Fig. S4 (ESI†), black line). Compared to the blank filter paper and HPB-CN powder, the HPB-CN deposited on filter paper exhibited two new diffraction peaks at 2θ = 18° (Fig. S4 (ESI†), peak 3 in red line) and 26° (Fig. S4 (ESI†), peak 4 in red line), indicating that the aggregation structure of HPB-CN on filter paper was different from that of its original powder. In addition, the diffraction peaks of the HPB-CN on filter paper exhibited no significant change after irradiation under UV light, suggesting that the aggregate and chemical structure were not affected by irradiation. However, as the HPB-CN filter paper strip was heated, the peak intensities of peaks 1 and 2 decreased, and peak 3 and 4 disappeared, suggesting that the transformation of the HPB-CN crystal structure on filter paper occurred. SEM was carried out to further confirm the aggregation changes caused by heating. The cuboid crystalline structure of HPB-CN powder became amorphous as it was coated on the filter paper (Fig. S5A and B, ESI†). After the HPB-CN coated filter paper was heated at 90 °C for 20 min, particles with smooth surfaces were observed, indicating that HPB-CN was induced to form new crystalline states by the crystalline paper fibers (Fig. S5C and D, ESI†). The SEM suggests that the filter paper was not a template, but acted as crystal seeds. The re-crystallized crystal exhibited a different crystalline structure from paper fibers (Fig. S5C and D, ESI†). To further confirm that the re-crystallization of HPB-CN was induced by paper fibers, cotton-linen cloth, polyester fabrics and glass with different degrees of crystallinity were tested. The HPB-CN deposited on these four materials exhibited different degrees of crystallinity (DC) (Fig. S6A–C, ESI†), among which HPB-CN on cotton-linen cloth and polyester fabrics showed similar degrees of crystallinity. As shown in Fig. 2A and Table 1, HPB-CN on filter paper exhibited stronger PL intensity than that on cotton-linen cloth and polyester fabrics due to the higher degree of crystallinity of HPB-CN on filter paper. In addition, the PL intensity of HPB-CN on cotton-linen cloth and polyester fabrics increased with temperature in similar trends. However, the PL intensity of the HPB-CN on glass was not affected by temperature due to the amorphous state of the glass. These results suggest that the degree of crystallinity of the substrate of HPB-CN can significantly affect its re-crystallization, and thus affect its PL intensity. To eliminate the re-crystallization induced by chemical composition, pristine filter paper with high crystallinity was pre-heated at 90 °C for 10 min, and then quenched in liquid nitrogen. The PL intensity of HPB-CN on the untreated filter paper was almost twice as strong as that on the treated filter paper as both were heated at 40 °C for 20 min because of the low crystallinity of the treated filter paper (Fig. S6A and D, ESI†). The PL intensity of HPB-CN on the treated filter paper became less than that on the untreated filter paper in a shorter period of time when heated at 70 °C and became similar when heated for longer than 20 min because the filter paper was restored to its original crystallinity as heating proceeded.
| Substrate | DC | Slope | I/I0 |
|---|---|---|---|
| Filter paper strips | 60.0 | 0.08549 | 2.27 |
| Cotton-linen cloth | 21.3 | 0.02552 | 1.34 |
| Polyester fabrics | 23.9 | 0.02689 | 1.35 |
| Glass | — | — | 1.00 |
The HPB-CN-coated filter paper became similar to the bare yellow filter paper when heated at 90 °C or irradiated under a UV lamp for 20 min, indicating that the structure of HPB-CN on filter paper remained unchanged. However, the HPB-CN-coated filter paper became blue under the simultaneous influences of UV light irradiation and heating (Fig. S2, ESI†) with a constant blue-shift in emission wavelength to 515 nm when heated at 130 °C for 6 min (Fig. 1B), suggesting a decreased degree of conjugation of HPB-CN molecules. HPB possesses a twisted conformation and can be cyclized into dihydronaphthalene because of the steric effect of its periphery phenyl. Photocyclization inherited the property of hexaphenyl-1,3-butadiene, no matter whether the substituent was presented or not. Bunz et al. found that HPB derivatives with a substituent such as –OCH3, 4-Tol and Pr could bring photoreaction to dihydronaphthalene and blue-shift the emission.13 It can be deduced that the polymer can exhibit similar properties.14 To determine whether HPB-CN was subject to photocyclization, the HPB-CN in solution and on filter paper strips was irradiated under UV light and analyzed by 1H NMR spectroscopy (Fig. S7, ESI†). A new resonance peak appeared at δ 4.30 due to the methine proton and partial resonance peaks of the phenyl protons shifted to high-field at 7.55 and 6.55, indicating the occurrence of the photocyclization of HPB-CN under UV light irradiation. In particular, the photocyclized HPB-CN in water/THF solution still exhibited typical AIEgen behaviors as exposed to UV light for 3 hours (Fig. 3 and Fig. S8, ESI†). Its emission peak at 513 nm was in agreement with that of the HPB-CN on filter paper (Fig. 1B). In addition, the PL intensity of HPB-CN in 90% water fraction solution increased almost 13 fold after UV light irradiation in Fig. S9 (ESI†), suggesting that the PL intensity of photocyclized HPB-CN was stronger. Based on these results, it can be concluded that UV light can drive photocyclization, and then enhance emission.
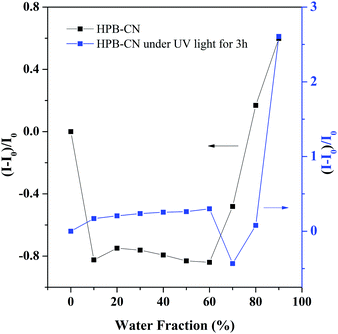 | ||
| Fig. 3 Correlation between the net change in PL intensity [(I − I0)/I0] with different water fractions in the THF/water mixtures before and after UV light for 3 hours. | ||
Unlike those of other fluorescent materials, the LTEE behavior of HPB-CN in the solid state is caused by both photocyclization and crystallization. The question is which occurs first. To answer this question, the photocyclization product of HPB-CN induced by UV light irradiation was coated on filter paper, and then measured for the PL change with heating time at 70 °C (Fig. S10, ESI†). The PL intensity gradually decreased with heating time, suggesting that the photocyclized HPB-CN could not show LTEE behavior. Therefore, it can be concluded that the crystallization occurred before photocyclization. Photocyclization only occurred in the solution state instead of the solid state, such as a powder or coating on glass (Fig. S11, ESI†). The intermolecular interaction in solid HPB-CN is stronger and the molecules are packed denser, which restricts the molecular motion. The crystallization induced by filter paper can overcome such restriction. The LTEE behavior indicates that the arrangement of HPB-CN molecules became more orderly and the intramolecular motions were more restricted at higher temperatures. Therefore, the radiation channel decay became restrained and PL emission was enhanced as temperature increased.
It is well-known that the photocyclization degree is directly proportional to the illumination time. In our work, HPB-CN-coated filter paper strips were irradiated with UV light for 20 s with an interval illumination time of 20 min, 10 min and 1 min, e.g. a cumulative illumination time of 20 s, 200 s and 400 s, respectively, at 70 °C (Fig. S12, ESI†). As shown in Fig. 4, the PL intensity significantly increased with the increase of illumination time.
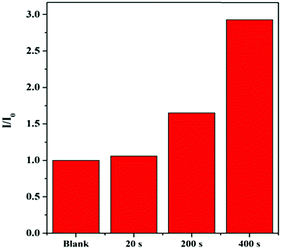 | ||
| Fig. 4 Fluorescence intensity of HPB-CN-coated filter paper strips exposed to UV light for different periods of time at 70 °C. UV light: handheld UV lamps of 365 nm. | ||
The results presented above verified our hypothesis of photocyclization. The relationship between the electronic structure and emissive properties of HPB-CN was also studied by Gaussian 09 calculations and the orbital distributions of the HOMO and LUMO energies before and after the photocyclization (Fig. S13, ESI†). The electron density of the HOMO orbital of HPB-CN before photocyclization was mainly localized on the electron-donating phenyl rings, with a calculated band gap of 2.690 eV that became 2.775 eV after photocyclization.
Conclusions
HPB-CN-coated filter paper was prepared and used as a light/thermo-sensitive fluorescent “turn-on” strip. The strip exhibited Light/Temperature-Enhanced Emission (LTEE) features and a fluorescent “turn-on” response to high temperature. The PL intensity of the HPB-CN-coated filter paper became stronger as the temperature increased. The light/thermo-sensitive mechanism was proposed based on the NMR, XRD and SEM characterization as follows. Filter paper causes the thermo-induced crystallization and UV light irradiation induces the photocyclization of HPB-CN, which can enhance the PL intensity of HPB-CN-coated paper strips. Our work provides a new perspective for the development of thermal-sensitive fluorescent organic materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Basic Research Program of China (973 Program; Grant No. 2013CB834704), and the National Natural Scientific Foundation of China (Grant No. 51673024, 21474009 and 51328302).References
- (a) J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, B. Z. Tang, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu and D. Zhu, Chem. Commun., 2001, 1740–1741 RSC; (b) J. Mei, N. L. Leung, R. T. Kwok, J. W. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- H. Shi, D. Xin, X. Gu, P. Zhang, H. Peng, S. Chen, G. Lin, Z. Zhao and B. Z. Tang, J. Mater. Chem. C, 2016, 4, 1228–1237 RSC.
- (a) X. Shi, H. Wang, T. Han, X. Feng, B. Tong, J. Shi, J. Zhi and Y. Dong, J. Mater. Chem., 2012, 22, 19296 RSC; (b) J. Mei, Y. Hong, J. W. Lam, A. Qin, Y. Tang and B. Z. Tang, Adv. Mater., 2014, 26, 5429–5479 CrossRef CAS PubMed; (c) Y. Gao, G. Feng, T. Jiang, C. Goh, L. Ng, B. Liu, B. Li, L. Yang, J. Hua and H. Tian, Adv. Funct. Mater., 2015, 25, 2857–2866 CrossRef CAS; (d) F. Wang, C. A. DeRosa, M. L. Daly, D. Song, M. Sabat and C. L. Fraser, Mater. Chem. Front., 2017, 1, 1866–1874 RSC; (e) A. Palma-Cando, D. Woitassek, G. Brunklaus and U. Scherf, Mater. Chem. Front., 2017, 1, 1118–1124 RSC.
- (a) D. Chen, H. Wang, L. Dong, P. Liu, Y. Zhang, J. Shi, X. Feng, J. Zhi, B. Tong and Y. Dong, Biomaterials, 2016, 103, 67–74 CrossRef CAS PubMed; (b) A. Shao, Y. Xie, S. Zhu, Z. Guo, S. Zhu, J. Guo, P. Shi, T. D. James, H. Tian and W. H. Zhu, Angew. Chem., Int. Ed., 2015, 54, 7275–7280 CrossRef CAS PubMed; (c) J. K. Jin, X. J. Chen, Y. Liu, A. J. Qin, J. Z. Sun and B. Z. Tang, Acta Polym. Sin., 2011, 9, 1079–1085 CrossRef; (d) M. Chen, J. Z. Sun, A. J. Qin and B. Z. Tang, Chin. Sci. Bull., 2016, 3, 304–314 Search PubMed.
- S. Turkdogan, F. Fan and C. Z. Ning, Adv. Funct. Mater., 2016, 26, 8521–8526 CrossRef CAS.
- Y. Sagara, K. Kubo, T. Nakamura, N. Tamaoki and C. Weder, Chem. Mater., 2017, 29, 1273–1278 CrossRef CAS.
- S. H. Mahadevegowda and M. C. Stuparu, Eur. J. Org. Chem., 2017, 570–576 CrossRef CAS.
- Q. Y. Fang, J. W. Li, S. Y. Li, R. H. Duan, S. Q. Wang, Y. P. Yi, X. D. Guo, Y. Qian, W. Huang and G. Q. Yang, Chem. Commun., 2017, 53, 5702–5705 RSC.
- A. Lavrenova, D. W. Balkenende, Y. Sagara, S. Schrettl, Y. C. Simon and C. Weder, J. Am. Chem. Soc., 2017, 139, 4302–4305 CrossRef CAS PubMed.
- (a) Y. J. Zhang, T. Han, S. Z. Gu, T. Y. Zhou, C. Zhao, Y. X. Guo, X. Feng, B. Tong, J. Bing, J. B. Shi, J. G. Zhi and Y. P. Dong, Chem. – Eur. J., 2014, 20, 8856–8861 CAS; (b) T. Han, Y. J. Zhang, X. Feng, Z. Lin, B. Tong, J. B. Shi, J. G. Zhi and Y. P. Dong, Chem. Commun., 2013, 49, 7049–7051 RSC.
- J. L. Banal, J. M. White, K. P. Ghiggino and W. H. Wong, Sci. Rep., 2014, 4, 4635–4639 CrossRef PubMed.
- L. W. Kong, Y. H. Zhang, H. L. Mao, X. L. Pan, Y. Tian, Z. L. Tian, X. K. Zeng, J. B. Shi, B. Tong and Y. P. Dong, Faraday Discuss., 2017, 196, 101–111 RSC.
- J. Freudenberg, A. C. Uptmoor, F. Rominger and U. H. F. Bunz, J. Org. Chem., 2014, 79, 11787–11791 CrossRef CAS PubMed.
- Y. J. Liu, J. W. Y. Lam, X. Y. Zheng, Q. Peng, R. T. K. Kwok, H. H. Y. Sung, I. D. Williams and B. Z. Tang, Macromolecules, 2016, 49, 5817–5830 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: UV, PL spectrograph, single crystal, XRD and SEM. CCDC 1535584. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7qm00304h |
| This journal is © the Partner Organisations 2017 |