Cyanopyridone flanked the tetraphenylethylene to generate an efficient, three-dimensional small molecule non-fullerene electron acceptor†
Anushri
Rananaware‡
a,
Akhil
Gupta‡
*b,
Gajanan
Kadam‡
c,
Duong
Duc La
a,
Ante
Bilic
d,
Wanchun
Xiang
 e,
Richard A.
Evans
f and
Sheshanath V.
Bhosale
e,
Richard A.
Evans
f and
Sheshanath V.
Bhosale
 *a
*a
aSchool of Science, RMIT University, GPO Box 2476, Melbourne, Victoria 3001, Australia. E-mail: sheshanath.bhosale@rmit.edu.au; Tel: +61 3 9925 2680
bInstitute for Frontier Materials, Deakin University, Waurn Ponds, Victoria 3216, Australia. E-mail: akhil.gupta@deakin.edu.au; Tel: +61 3 5247 9542
cDepartment of Organic Chemistry, School of Chemical Sciences, North Maharashtra University, Jalgaon 425001, Maharashtra, India
dData61 CSIRO, Molecular and Materials Modelling, Docklands, Victoria 8012, Australia
eState Key Laboratory of Silicate Materials for Architectures, Wuhan University of Technology, 122 Luoshi Rd, Wuhan, 430070, Hubei, P. R. China
fCSIRO Manufacturing, Bayview Avenue, Clayton South, Victoria 3169, Australia
First published on 6th September 2017
Abstract
Herein we report the design, synthesis and characterization of a novel material, (5Z,5′Z,5′′Z,5′′′E)-5,5′,5′′,5′′′-(((ethene-1,1,2,2-tetrayltetrakis(benzene-4,1-diyl))tetrakis(thiophene-5,2-diyl))tetrakis(methanylylidene))tetrakis(4-methyl-1-octyl-2,6-dioxo-1,2,5,6-tetrahydropyridine-3-carbonitrile) (TPE-CP4), which was generated using a combination of tetraphenylethylene and cyanopyridone functionalities. The tetraphenylethylene unit was terminally functionalized with cyanopyridone in order to generate a three-dimensional molecular architecture. The new material TPE-CP4 was produced via a Knoevenagel condensation reaction and was found to be highly soluble in a variety of common organic solvents, such as chloroform and o-dichlorobenzene. TPE-CP4 exhibited promising optoelectronic properties and energy levels complementing those of the conventional donor polymers poly(3-hexylthiophene) [P3HT] and Poly({4,8-bis[(2-ethylhexyl)oxy]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl}{3-fluoro-2-[(2-ethylhexyl)carbonyl]thieno[3,4-b]thiophenediyl}) [PTB7]. Due to its optoelectronic properties, solubility and good film forming capability, TPE-CP4 was incorporated as an n-type semiconducting component along with the commercially available P3HT and PTB7 as the p-type semiconductors in BHJ photovoltaic devices. TPE-CP4 performed very well with both of the donor polymers and afforded 6.02% and 6.72% power conversion efficiencies with P3HT and PTB7, respectively. Not only is TPE-CP4 the first reported example combining tetraphenylethylene and cyanopyridone structural units, but the device performance indicates that it is an efficient non-fullerene acceptor.
1. Introduction
In the 21st century, there is a pressing requirement for the development of renewable energy resources, not only for society's current needs but for the future generations to come. It is this need that has encouraged researchers around the globe to use natural resources, such as sunlight, to develop technologies such as solar cells, and organic solar cells in particular. Organic devices promise a number of advantages over their inorganic counterparts in terms of flexibility, light-weight, solution-processability and low-cost.1 The fabrication of organic solar cells, together with a different type of system called dye-sensitized solar cell,2 has been thoroughly researched over the past two decades. It has used the conventional donor–acceptor material combinations such as donor polymer poly(3-hexylthiophene) (P3HT) and soluble fullerene acceptors, [6,6]-phenyl-C61-butyric acid methyl ester (PC61BM) and its C71 analogue (PC71BM). It is notable to mention that fullerene derivatives have played an important role as efficient electron acceptor materials in the research field of organic solar cells, enabling power conversion efficiency (PCE) to around 11%.3 Despite the widespread usage of fullerene acceptors due to their excellent electron mobility (typically ranging 10−3–10−4 cm2 V−1 s−1), solubility and the ability to form a favourable nanoscale network with a diverse range of donor semiconducting components, they do suffer from a number of potential drawbacks such as weak absorption of visible light, poor chemical and electronic tunability, and high production cost. Moreover, a large electron affinity can result in low open-circuit voltage (Voc).4 Such disadvantages have slowed further advances in PCEs and are hindering the development of organic photovoltaic (OPV) technology, thus providing a strong incentive to researchers to seek alternative non-fullerene electron acceptors which can compete with fullerene acceptors.The design and development of an efficient non-fullerene acceptor is often considered challenging given its structural requirements, and especially when there are no set design rules. It is believed that not only the new structural formats should possess properties, such as strong accepting strength, solubility and thermal stability, and good electron mobility, but should be easily synthesizable with facile synthetic protocols and purification strategies. Moreover, such non-fullerene acceptor formats should be able to address the limitations of fullerene acceptors, for instance limited absorption in the visible region, structural diversity and electronic tunability. Given the recent literature precedence, for instance D. Meng et al.,5 Y. Lin et al.,6 Hwang et al.,7 Zhong et al.8 and A. Rananaware et al.,9 we understand that an efficient non-fullerene acceptor should be a highly-conjugated structure, mainly through a merger of a variety of building blocks such as donor and acceptor, and should carry lipophilic chains in order to demonstrate solution-processability. All of these reports not only have demonstrated encouraging efficiencies but have shown unique structural formats to be used as non-fullerene acceptors. Although the advances towards the design and development of non-fullerene acceptors have been impressive,10 there still remains a strong need to develop materials which not only will have better properties in terms of solubility, stability and strong accepting strength, but have energy levels complementing those of the conventional as well as conjugated polymeric and small molecular donor functionalities.
Compared to their polymeric counterparts, small molecule non-fullerene acceptors exhibit a number of advantages such as well-defined molecular structure, high purity, and good batch-to-batch reproducibility, to name a few. Learning from the work reported by us and others in the literature, and given the structural requirements of an efficient non-fullerene acceptor, we noticed that strong accepting functionalities, such as diketopyrrolopyrrole, naphthalenediimide and dicyanovinylidene, can be great allies when used as terminal acceptors in conjunction with central conjugated blocks.9,11 This prompted us to search for alternate functionalities which not only can be strong accepting blocks but can be fitted competently at the terminals. In 2008, Kronenberg et al. reported a series of merocyanine colorants based on a number of new accepting blocks, with varied accepting strengths, for fabricating bulk-heterojunction (BHJ) organic solar cells.12 One such block with strong accepting strength was cyanopyridone (CP), which attracted our attention, and in 2012, we expanded its use for generating oligothiophene dyes for solution-processable organic solar cells.13 Since then we have explored this acceptor in generating more examples for OPV applications,14 and found that it is a highly conjugated, aromatizable unit which offers its peripheral union with a variety of blocks, no matter donors or acceptors. This provided us a rationale to consider its union with a mild donating block, such as tetraphenylethylene (TPE), given the successful literature precedence,9,11,15 and to study the target chromophore as a non-fullerene acceptor.
Herein we report the very first use of CP functionality in generating a conjugated target, (5Z,5′Z,5′′Z,5′′′E)-5,5′,5′′,5′′′-(((ethene-1,1,2,2-tetrayltetrakis(benzene-4,1-diyl))tetrakis(thiophene-5,2-diyl))tetrakis(methanylylidene))tetrakis(4-methyl-1-octyl-2,6-dioxo-1,2,5,6-tetrahydropyridine-3-carbonitrile) [coded as TPE-CP4; see Fig. 1], which is a TPE-functionalized, three-dimensional, small molecule non-fullerene electron acceptor. TPE-CP4 was synthesized using straightforward and scalable synthetic protocols, purified through simple column chromatography and displayed good thermal stability with excellent solubility. It was further demonstrated that our idea of TPE-CP4 as a non-fullerene acceptor was validated as the solution-processable BHJ devices based on the blends of two different commercially available donor polymers, P3HT and PTB7, exhibited PCEs as high as 6.02% and 6.72%, respectively. Not only is TPE-CP4 the first reported example in the literature but the device outcome reported herein is among the highest efficiency numbers which has been achieved from simple devices using the conventional donor polymers, and without any special treatment. This work cements the idea of researching novel structural formats for their use as non-fullerene acceptors, and is a continuation of our efforts made in the design and development of small molecular chromophores for OPV applications.16
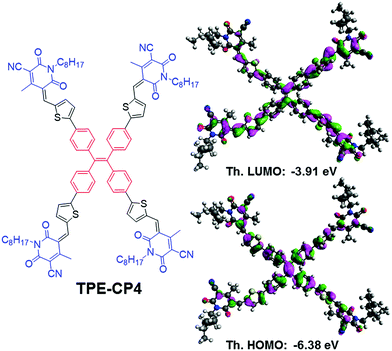 | ||
| Fig. 1 Molecular structure and calculated orbital densities of the HOMO and LUMO for the newly designed and synthesized target TPE-CP4. | ||
The target molecule, TPE-CP4, was synthesized via a Knoevenagel condensation reaction between 5,5′,5′′,5′′′-(ethene-1,1,2,2-tetrayltetrakis(benzene-4,1-diyl))tetrakis(thiophene-2-carbaldehyde) (intermediate 1) and CP (Scheme 1; Experimental section). The reaction was carried out in methanol at 70 °C for 16 h (Scheme 1), and the crude TPE-CP4 was purified via simple flash chromatography. The structure of TPE-CP4 was confirmed by elemental analysis as well as 1H and 13C NMR spectroscopies (for experimental spectra see ESI†). As per our expectation, TPE-CP4 was found to be highly soluble in a variety of commonly used solvents such as chloroform, dichlorobenzene and tetrahydrofuran, for instance 15 mg mL−1 in o-dichlorobenzene, thanks to the presence of nitrogen atom (CP unit) that allows a variety of alkyl groups to be located in order to refine solubility. In the present case, the octyl group was the substituent of choice for producing high solubility and excellent film forming properties. It is vital to mention that high solubility of a semiconducting material is crucial for fabricating solution-processable BHJ devices and TPE-CP4 fulfils this criterion.
2. Results and discussion
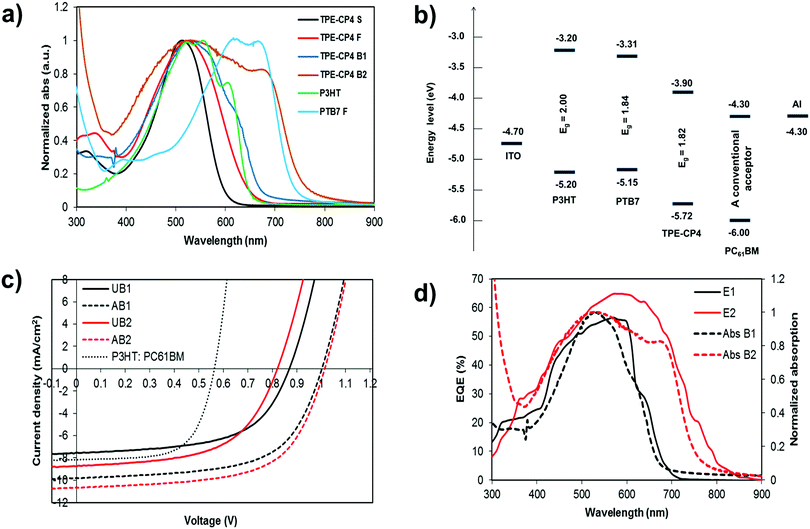
The normalized optical absorption spectra of TPE-CP4 in chloroform solution, as a thin solid film, and as a blend film with P3HT and PTB7 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 D
1.2 D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A) are shown in Fig. 2a. In solution, TPE-CP4 exhibited strong absorption with an absorption maximum (λmax) at 514 nm [(molar extinction coefficient = 78
A) are shown in Fig. 2a. In solution, TPE-CP4 exhibited strong absorption with an absorption maximum (λmax) at 514 nm [(molar extinction coefficient = 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L Mol−1 cm−1)] (see Fig. S1, ESI†). The thin and blend film spectra were red-shifted when compared with the solution spectrum. In fact, the λmax was red-shifted by 12 nm for pristine film. The red-shift of λmax and the broadness of blend film spectra suggest an effective π–π stacking in the solid state (absorption coefficient of pristine film = 396, 774 cm−1). The blend films of TPE-CP4 demonstrated strong light-harvesting ability, and in particular with PTB7, over most of the visible region, thus suggesting that there is an efficient charge transfer transition between donor and acceptor domains. According to the spectra shown in Fig. 2a, the thin-film absorption spectrum of TPE-CP4 (400–700 nm) complements excellently with the main absorption of PTB7 (580–810 nm), thus indicating that blend films can have more favourable optical absorption throughout the entire visible spectrum and tailing into the near infrared region. Therefore, the use of PTB7 in particular can indeed be helpful for controlling absorption profile, fine tuning energy levels and presumably enhancing BHJ performance. The photoluminescence (PL) quenching experiment was conducted with P3HT and indicated that the presence of P3HT in the blend film (P3HT
000 L Mol−1 cm−1)] (see Fig. S1, ESI†). The thin and blend film spectra were red-shifted when compared with the solution spectrum. In fact, the λmax was red-shifted by 12 nm for pristine film. The red-shift of λmax and the broadness of blend film spectra suggest an effective π–π stacking in the solid state (absorption coefficient of pristine film = 396, 774 cm−1). The blend films of TPE-CP4 demonstrated strong light-harvesting ability, and in particular with PTB7, over most of the visible region, thus suggesting that there is an efficient charge transfer transition between donor and acceptor domains. According to the spectra shown in Fig. 2a, the thin-film absorption spectrum of TPE-CP4 (400–700 nm) complements excellently with the main absorption of PTB7 (580–810 nm), thus indicating that blend films can have more favourable optical absorption throughout the entire visible spectrum and tailing into the near infrared region. Therefore, the use of PTB7 in particular can indeed be helpful for controlling absorption profile, fine tuning energy levels and presumably enhancing BHJ performance. The photoluminescence (PL) quenching experiment was conducted with P3HT and indicated that the presence of P3HT in the blend film (P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPE-CP4 1
TPE-CP4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 w/w) quenched PL, thus validating an efficient, photo-induced charge transfer transition between donor and acceptor domains, and a strong indication of favourable, well-plaited blend morphology (for PL spectra see Fig. S2, ESI†).
1.2 w/w) quenched PL, thus validating an efficient, photo-induced charge transfer transition between donor and acceptor domains, and a strong indication of favourable, well-plaited blend morphology (for PL spectra see Fig. S2, ESI†).
Density functional theory (DFT) calculations using the Gaussian 09 suite of programs and the B3LYP/6-311+G(d,p)//B3LYP/6-31G(d) level of theory17 indicated that the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) densities were uniformly distributed over the whole molecular backbone (Fig. 1), a recent finding that is quite usual with small molecule non-fullerene acceptors as is demonstrated by us and others.18 DFT calculations further indicated that the torsional angle between the central phenyl ring planes and the thiophene ring planes was around 23°, thus giving TPE-CP4 a non-planar structure overall (Fig. S3, ESI†). The non-planarity in small molecule non-fullerene electron acceptors is considered crucial over their planar counterparts as the non-planar arrangements can be helpful in preventing strong intermolecular aggregations, providing effective conjugation in multiple directions for systematic photon harvesting, and disclosing sufficient π-surfaces for electron transport. Experimental estimation of the HOMO and the LUMO energy levels was carried out using a combination of photoelectron spectroscopy in air (PESA) and UV-Vis spectroscopy on thin films. The analysis of the HOMO energy level of TPE-CP4 using pristine film indicated that the average HOMO level resides at around −5.72 eV. The LUMO energy level was calculated using the absorption onset (680 nm) of thin film of TPE-CP4. The energy level diagram represents these values and suggests that TPE-CP4 indeed has appropriate energy levels suiting those of the conventional donor polymers P3HT and PTB7 (Fig. 2b for energy level diagram, Fig. S4 (ESI†) for theoretical density distribution of additional (+/−) orbitals, and Fig. S5 (ESI†) for PESA curve). The theoretical optical absorption transitions of TPE-CP4 were also calculated and corroborated our experimental findings (Fig. S6, ESI†). We further realised that not only should organic semiconductors be highly soluble and chemically stable, they should exhibit thermal stability in order to prove that they can withstand adverse conditions or rough handling such as active surface annealing at a higher temperature, for instance ≥100 °C. We conducted thermogravimetric analysis (TGA) and found that TPE-CP4 indeed is a thermally stable chromophore, appearing stable up to 350 °C before the mass loss was observed. Hence, it can endure annealing conditions, if required (Fig. S7 for TGA curve, ESI†).
Because of its suitable optoelectronic properties, solubility and good film forming capability, TPE-CP4 was incorporated as an n-type semiconducting component along with two different commercially available donor polymers P3HT and PTB7 as the p-type semiconductors in BHJ photovoltaic devices. BHJ architectures typically deliver higher device power conversion efficiencies by maximising the surface area of the interface between donor and acceptor materials in the active layer. For TPE-CP4, the common device structure used was ITO/PEDOT:PSS (38 nm)/active layer/Ca (20 nm)/Al (100 nm) where the active layer was a solution-processed blend of either P3HT:TPE-CP4 or PTB7:TPE-CP4. This was done by spin coating a mixture of P3HT/PTB7 with TPE-CP4 in o-dichlorobenzene under ambient conditions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 weight ratio, respectively) with subsequent annealing. The optimum layer thickness was found to be in the range of 70 nm. It is important to mention that we chose a simple device architecture to start with, and to observe initial performance, stability, fabricating conditions and reproducibility of BHJ devices. Regarding the fabrication of small molecule non-fullerene acceptors, it is well established that thermal annealing of blend surfaces together with a high boiling point solvent is preferred for optimal performance, and to avoid formation of large-scale crystals on active surfaces. This principle has been demonstrated by Kim et al.,19 Lin et al.20 and Patil et al.21 Based on the literature findings and our own understanding of the BHJ device architecture, we used o-dichlorobenzene as the processing solvent and annealed our active blend surfaces at 110 °C for 5 min. The optimized donor
1.2 weight ratio, respectively) with subsequent annealing. The optimum layer thickness was found to be in the range of 70 nm. It is important to mention that we chose a simple device architecture to start with, and to observe initial performance, stability, fabricating conditions and reproducibility of BHJ devices. Regarding the fabrication of small molecule non-fullerene acceptors, it is well established that thermal annealing of blend surfaces together with a high boiling point solvent is preferred for optimal performance, and to avoid formation of large-scale crystals on active surfaces. This principle has been demonstrated by Kim et al.,19 Lin et al.20 and Patil et al.21 Based on the literature findings and our own understanding of the BHJ device architecture, we used o-dichlorobenzene as the processing solvent and annealed our active blend surfaces at 110 °C for 5 min. The optimized donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor weight ratio was found to be 1
acceptor weight ratio was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 where the BHJ devices gave encouraging performance. With the P3HT:TPE-CP4 combination, the photovoltaic cell parameters; Voc, short circuit current density (Jsc), fill factor (FF) and PCE, reached 0.99 V; 9.68 mA cm−2, 0.63, 6.02%, respectively, whereas with PTB7, we achieved higher device outcome and the cell parameters; Voc, Jsc, FF and PCE, reached 1.01 V; 10.61 mA cm−2, 0.63, 6.72%, respectively. It is important to mention that all of the P3HT and PTB7 devices yielded Voc nearing 1 V, a result consistent with the measured optical band gaps between the LUMO of TPE-CP4 and the HOMOs of donor polymers. Unannealed devices with a donor
1.2 where the BHJ devices gave encouraging performance. With the P3HT:TPE-CP4 combination, the photovoltaic cell parameters; Voc, short circuit current density (Jsc), fill factor (FF) and PCE, reached 0.99 V; 9.68 mA cm−2, 0.63, 6.02%, respectively, whereas with PTB7, we achieved higher device outcome and the cell parameters; Voc, Jsc, FF and PCE, reached 1.01 V; 10.61 mA cm−2, 0.63, 6.72%, respectively. It is important to mention that all of the P3HT and PTB7 devices yielded Voc nearing 1 V, a result consistent with the measured optical band gaps between the LUMO of TPE-CP4 and the HOMOs of donor polymers. Unannealed devices with a donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor weight ratio of 1
acceptor weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 afforded reasonable outcome too, and the PCE numbers reached to 3.81% and 4.06% with P3HT and PTB7, respectively. In contrast, the maximum PCE, Voc, and Jsc reached 3.02%, 0.57 V and 8.28 mA cm−2, respectively, for a device based on P3HT:PC61BM, when fabricated under similar annealing conditions. For current–voltage (J–V) curves, see Fig. 2c. Not only is TPE-CP4 the first reported example in the literature where CP unit has been terminally used to generate an efficient, non-planar, three-dimensional non-fullerene electron acceptor, but the device parameters reported herein are amongst the leading numbers that have been achieved using the conventional donor polymers, P3HT and PTB7, and especially without any special treatment to either blend solution or device architecture. Moreover, the results achieved in the current work validate the amalgamation of TPE and CP units and provide a strong incentive that TPE unit can certainly be replaced with a variety of building blocks, whether donors or acceptors, in order to generate a series of optimally performed, three-dimensional non-fullerene electron acceptors. The results with PTB7 in particular demonstrate that TPE-CP4 is an efficient non-fullerene acceptor that has a strong capability to plait well with a variety of donors. See Table 1 for detailed device parameters.
1.2 afforded reasonable outcome too, and the PCE numbers reached to 3.81% and 4.06% with P3HT and PTB7, respectively. In contrast, the maximum PCE, Voc, and Jsc reached 3.02%, 0.57 V and 8.28 mA cm−2, respectively, for a device based on P3HT:PC61BM, when fabricated under similar annealing conditions. For current–voltage (J–V) curves, see Fig. 2c. Not only is TPE-CP4 the first reported example in the literature where CP unit has been terminally used to generate an efficient, non-planar, three-dimensional non-fullerene electron acceptor, but the device parameters reported herein are amongst the leading numbers that have been achieved using the conventional donor polymers, P3HT and PTB7, and especially without any special treatment to either blend solution or device architecture. Moreover, the results achieved in the current work validate the amalgamation of TPE and CP units and provide a strong incentive that TPE unit can certainly be replaced with a variety of building blocks, whether donors or acceptors, in order to generate a series of optimally performed, three-dimensional non-fullerene electron acceptors. The results with PTB7 in particular demonstrate that TPE-CP4 is an efficient non-fullerene acceptor that has a strong capability to plait well with a variety of donors. See Table 1 for detailed device parameters.
| Acceptor material | Donor material | Testing conditions (donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor)a acceptor)a |
V oc (V) | J sc (mA cm−2) | FF | Best PCE (%) | Average PCE (%) (±std dev.)c |
|---|---|---|---|---|---|---|---|
| a BHJ devices with specified weight ratio. Device structure was ITO/PEDOT:PSS (38 nm)/active layer/Ca (20 nm)/Al (100 nm) with an active layer thickness of ∼70 nm. b A standard P3HT:PC61BM device afforded 3.02% efficiency, thus indicating the reliability of our fabrication strategy. c A total of ten devices were made for each combination; cell area = 0.1 cm2. d After repetitive testing. | |||||||
| TPE-CP4 | P3HT | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 (annealed) 1.2 (annealed) |
0.99 | 9.68 | 0.63 | 6.02 | 5.89 (±0.09) |
| TPE-CP4 | P3HT | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 (no annealing) 1.2 (no annealing) |
0.86 | 7.51 | 0.59 | 3.81 | 3.70 (±0.07) |
| TPE-CP4 | P3HT | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 (after a week's hold) 1.2 (after a week's hold) |
0.87 | 8.98 | 0.60 | 4.69 | 4.63 (±0.04)d |
| PC61BM | P3HT | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2b 1.2b |
0.57 | 8.28 | 0.64 | 3.02 | 2.95 (±0.05) |
| TPE-CP4 | PTB7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 (annealed) 1.2 (annealed) |
1.01 | 10.61 | 0.63 | 6.72 | 6.59 (±0.11) |
| TPE-CP4 | PTB7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 (no annealing) 1.2 (no annealing) |
0.83 | 8.76 | 0.56 | 4.06 | 3.92 (±0.09) |
The external quantum efficiency (EQE) of the P3HT:TPE-CP4- and PTB7:TPE-CP4-based devices showed wider and stronger photo-response with a maxima of ∼56% at 570 nm and ∼65% at 595 nm, respectively (Fig. 2d). In particular, the EQE response was stronger for PTB7:TPE-CP4 combination given its strong and broad absorption that spanned over most of the visible region (400–800 nm). This is an interesting outcome as it suggests that both donor and acceptor components in both of the blends made a considerable contribution to the EQE and Jsc. We realised that the better BHJ performance and the higher peak EQE in case of PTB7 can be attributed to the synergetic effect of better light-harvesting and the superior blending of donor and acceptor domains. Moreover, the photocurrents obtained from the EQE data were in close agreement with those of the current–voltage measurements conducted under the 1 Sun condition. To get an idea about initial stability and consistent outcome of the P3HT:TPE-CP4-based devices, we left the best performing device in open air under routine laboratory conditions (presence of unavoidable environment such as moisture, air, and contamination, to name a few). The device retained ∼80% of its original PCE after keeping it over a week's period (Table 1), thus suggesting that not only are the non-fullerene organic solar cells stable but the target chromophore TPE-CP4 is a type of acceptor which is thermally, environmentally and chemically stable.
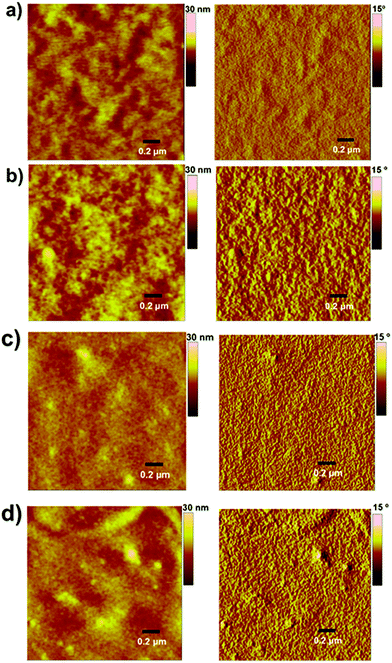
To examine the physical microstructure of the blend surfaces, we used atomic force microscopy (AFM) in tapping mode. The actual surface morphology of the blend films of P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPE-CP4 and PTB7
TPE-CP4 and PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPE-CP4 (1
TPE-CP4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
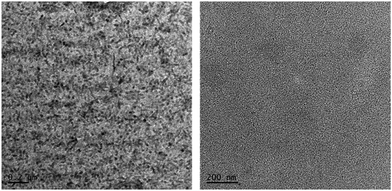
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 w/w) is shown in Fig. 3. The as-cast, unannealed blends appear to have smooth morphology, indicating rigorous mixing of donor and acceptor domains, which shifted to somewhat granular morphology upon annealing. It is notable to mention that morphologies such as granular, apart from others like worm and bush, are considered superior and often regarded for higher device performance when compared with morphologies such as amorphous and regular. The annealed blend surfaces showed surface roughnesses of 1.5 nm (P3HT:TPE-CP4) and 2.4 nm (PTB7:TPE-CP4), thus suggesting that TPE-CP4 is highly miscible with both donor polymers, a result that corroborates the broad blend film absorption due to well intermixing of donor and acceptor domains. The AFM analyses further indicated that the use of a high boiling point solvent is highly preferable as it generates smoother blend films that are free from perceptible projections, lumps, or indentations. It is important to point out that well intermixing of donor and acceptor domains was further confirmed by transmission electron microscopy (TEM) analysis where a fine, flat texture was observed for both the blends (Fig. 4), albeit the fineness of PTB7:TPE-CP4 blend was superior than P3HT combination. It is apparent that such featureless and uniform blend appearances usually result in relatively higher values of Jsc and FF, as is the case of PTB7:TPE-CP4 blend.
1.2 w/w) is shown in Fig. 3. The as-cast, unannealed blends appear to have smooth morphology, indicating rigorous mixing of donor and acceptor domains, which shifted to somewhat granular morphology upon annealing. It is notable to mention that morphologies such as granular, apart from others like worm and bush, are considered superior and often regarded for higher device performance when compared with morphologies such as amorphous and regular. The annealed blend surfaces showed surface roughnesses of 1.5 nm (P3HT:TPE-CP4) and 2.4 nm (PTB7:TPE-CP4), thus suggesting that TPE-CP4 is highly miscible with both donor polymers, a result that corroborates the broad blend film absorption due to well intermixing of donor and acceptor domains. The AFM analyses further indicated that the use of a high boiling point solvent is highly preferable as it generates smoother blend films that are free from perceptible projections, lumps, or indentations. It is important to point out that well intermixing of donor and acceptor domains was further confirmed by transmission electron microscopy (TEM) analysis where a fine, flat texture was observed for both the blends (Fig. 4), albeit the fineness of PTB7:TPE-CP4 blend was superior than P3HT combination. It is apparent that such featureless and uniform blend appearances usually result in relatively higher values of Jsc and FF, as is the case of PTB7:TPE-CP4 blend.
To gain an insight into the effective charge carrier mobilities, the space charge limited current (SCLC) method was applied to get information about charge transportation in P3HT devices. The electron-only devices, consisting of an active layer sandwiched between a ZnO coated ITO electrode and LiF/Al counter-electrode as the hole-blocking contact, were fabricated as per the sketch depicted in Fig. S8, ESI.† From the current density as a function of voltage data (Fig. S9, ESI†), the electron mobility in the trap-free SCLC region can be estimated using the Mott–Gurney equation, [J = 9(εμ)/8 × (V2/d3); where ε is the dielectric constant, μ is the charge-carrier mobility, d is the sample thickness, and V is the applied voltage]. Using this expression, an excellent electron mobility of the order of 10−3 was observed which is beneficial for higher Jsc and FF values of the resulting OPV devices.
3. Conclusions
In summary, a novel tetra-cyanopyridone substituted small molecule, TPE-CP4, was synthesized and its potential as an electron acceptor is demonstrated. The material is (1) structurally simple, (2) highly absorbing in the visible region, (3) thermally and chemically stable, and (4) can be synthesized via relatively few synthetic steps. These merits give TPE-CP4 an intrinsic advantage over fullerene acceptors, such as PC61BM, for which high cost, electronic and structural tuning, and lengthy purification techniques have been restrictive factors. TPE-CP4 possessed nonplanar molecular structure, and a higher lying LUMO energy relative to PC61BM in order to achieve a high Voc. TPE-CP4 was very effective with commercially available donor polymers, P3HT and PTB7, and the BHJ devices indicated that the present system is highly intermixed. The device parameters outlined herein are among the top numbers that have been achieved using the combination of a non-fullerene electron acceptor and the commercial donors. The results of this research further advocate that TPE-CP4 is an appropriately designed and highly promising non-fullerene acceptor which can be tested with other potential donors for future applications in BHJ devices.4. Experimental section
4.1 Materials and methods
All the reactions were carried out under nitrogen atmosphere, unless otherwise stated. Solvents used for various reactions were dried using a commercial solvent purification/drying system. Solvents used for extractions and column chromatography, and all other reagents were used as supplied by commercial vendors without further purifications or drying. 1,1,2,2-Tetrakis(4-(thiophen-2-yl)phenyl)ethene and 4-methyl-1-octyl-2,6-dioxo-1,2,5,6-tetrahydropyridine-3-carbonitrile were prepared according to the reported literature.13,22Thin layer chromatography (TLC) was performed using 0.25 mm thick plates pre-coated with Merck Kieselgel 60 F254 silica gel, and visualized using UV light (254 and 365 nm). Petroleum spirits with a boiling point range of 40–60 °C was used wherever indicated. Column chromatography was performed on either 40–60 or 20–40 μm silica gel. 1H NMR spectra were recorded at 300, 400 or 500 MHz, as indicated. The following abbreviations were used to explain multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad, dd = doublet of doublets, and dt = doublet of triplets. 13C NMR spectra were recorded at 75 or 101 MHz, as indicated. 1H and 13C chemical shifts were calibrated using residual non-deuterated solvent as an internal reference and are reported in parts per million (δ) relative to tetramethylsilane (δ = 0). Melting points were measured using a digital melting point apparatus. TGA experiments were carried out using Q-500 TGA instrument with nitrogen as a purging gas. Samples were heated to 800 °C at a rate of 10 °C per minute under nitrogen atmosphere. Details of spectroscopic measurements, and device fabrication and characterization of photovoltaic devices were reported previously.13,23
Atomic force microscopy topographic maps were directly performed on the active layers of P3HT:TPE-CP4 and PTB7:TPE-CP4 blends using an Asylum Research MFP-3D-SA instrument. The AFM was run in intermittent contact mode (tapping mode) using MicroMasch NSC18 tips (typical resonant frequency ∼100 kHz, typical probe radius ∼10 nm and typical aspect ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). TEM samples were prepared by solvent evaporation on a holey carbon grid and micrographs were produced using a JOEL 1010 100 kV TEM.
1). TEM samples were prepared by solvent evaporation on a holey carbon grid and micrographs were produced using a JOEL 1010 100 kV TEM.
4.2 Synthesis
Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. G. acknowledges Dr Gerry Wilson from CSIRO Manufacturing, Clayton, Victoria, Australia, for providing support through a visiting fellow position. A. G. is also thankful to the Alfred Deakin Fellowship Scheme at the Institute for Frontier Materials (IFM), Deakin University, Waurn Ponds, Victoria, Australia. S. V. B. acknowledges financial support from the Australian Research Council (ARC), Australia, under a Future Fellowship Scheme (FT110100152). A. G., A. R. and D. D. L. acknowledge various testing and analytical facilities at RMIT University, Deakin University, CSIRO Clayton and University of Melbourne, Victoria, Australia.Notes and references
- (a) G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 CAS; (b) C. J. Brabec, N. S. Sariciftci and J. C. Hummelen, Adv. Funct. Mater., 2001, 11, 15 CrossRef CAS; (c) F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2009, 93, 394 CrossRef CAS; (d) A. J. Heeger, Chem. Soc. Rev., 2010, 39, 2354 RSC; (e) L. T. Dou, J. B. You, Z. R. Hong, Z. Xu, G. Li, R. A. Street and Y. Yang, Adv. Mater., 2013, 25, 6642 CrossRef CAS PubMed.
- W. Xiang, A. Gupta, M. K. Kashif, N. Duffy, A. Bilic, R. A. Evans, L. Spiccia and U. Bach, ChemSusChem, 2013, 6, 256 CrossRef CAS PubMed.
- (a) Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS PubMed; (b) S.-H. Liao, H.-J. Jhuo, P.-N. Yeh, Y.-S. Cheng, Y.-L. Li, Y.-H. Lee, S. Sharma and S.-A. Chen, Sci. Rep., 2014, 4, 6813 CrossRef CAS PubMed; (c) C.-Z. Li, H.-L. Yip and A. K. Y. Jen, J. Mater. Chem., 2012, 22, 4161 RSC; (d) C.-Z. Li, C.-Y. Chang, Y. Zang, H.-X. Ju, C.-C. Chueh, P.-W. Liang, N. Cho, D. S. Ginger and A. K. Y. Jen, Adv. Mater., 2014, 26, 6262 CrossRef CAS PubMed; (e) B. Kan, Q. Zhang, M. Li, X. Wan, W. Ni, G. Long, Y. Wang, X. Yang, H. Feng and Y. Chen, J. Am. Chem. Soc., 2014, 136, 15529 CrossRef CAS PubMed.
- (a) J. Zhao, Y. Li, H. Lin, Y. Liu, K. Jiang, C. Mu, T. Ma, J. Y. Lin Lai, H. Hu, D. Yu and H. Yan, Energy Environ. Sci., 2015, 8, 520 RSC; (b) C.-Z. Li, H.-L. Yip and A. K. Y. Jen, J. Mater. Chem., 2012, 22, 4161 RSC.
- D. Meng, D. Sun, C. Zhong, T. Liu, B. Fan, L. Huo, Y. Li, W. Jiang, H. Choi, T. Kim, J. Y. Kim, Y. Sun, Z. Wang and A. J. Heeger, J. Am. Chem. Soc., 2016, 138, 375 CrossRef CAS PubMed.
- (a) Y. Lin, Q. He, F. Zhao, L. Huo, J. Mai, X. Lu, C.-J. Su, T. Li, J. Wang, J. Zhu, Y. Sun, C. Wang and X. Zhan, J. Am. Chem. Soc., 2016, 138, 2973 CrossRef CAS PubMed; (b) Y. Lin, F. Zhao, Q. He, L. Huo, Y. Wu, T. C. Parker, W. Ma, Y. Sun, C. Wang, D. Zhu, A. J. Heeger, S. R. Marder and X. Zhan, J. Am. Chem. Soc., 2016, 138, 4955 CrossRef CAS PubMed.
- Y.-J. Hwang, H. Li, B. A. E. Courtright, S. Subramaniyan and S. A. Jenekhe, Adv. Mater., 2016, 28, 124 CrossRef CAS PubMed.
- Y. Zhong, M. T. Trinh, R. Chen, G. E. Purdum, P. P. Khlyabich and M. Sezen, et al. , Nat. Commun., 2015, 6, 8241 CrossRef PubMed.
- A. Rananaware, A. Gupta, J. Li, A. Bilic, L. Jones, S. Bhargava and S. V. Bhosale, Chem. Commun., 2016, 52, 8522 RSC.
- (a) J. Zhao, Y. Li, H. Lin, Y. Liu, K. Jiang, C. Mu, T. Ma, J. Y. L. Lai, H. Hu, D. Yu and H. Yan, Energy Environ. Sci., 2015, 8, 520 RSC; (b) Y. Lin, Z.-G. Zhang, H. Bai, Y. Yao, Y. Li, D. Zhu and X. Zhan, Energy Environ. Sci., 2015, 8, 610 RSC; (c) Y. Lin, Z.-G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170 CrossRef CAS PubMed; (d) X. Liu, Y. Xie, H. Zhao, X. Cai, H. Wu, S.-J. Su and Y. Cao, New J. Chem., 2015, 39, 8771 RSC; (e) X. Liu, Y. Xie, X. Cai, Y. Li, H. Wu, S.-J. Su and Y. Cao, RSC Adv., 2015, 5, 107566 RSC; (f) S. Li, W. Liu, M. Shi, J. Mai, T.-K. Lau, J. Wan, X. Lu, C.-Z. Li and H. Chen, Energy Environ. Sci., 2016, 9, 604 RSC; (g) W. Chenab and Q. Zhang, J. Mater. Chem. C, 2017, 5, 1275 RSC; (h) A. Gupta, A. Rananaware, P. S. Rao, D. D. La, A. Bilic, W. Xiang, J. Li, R. A. Evans, S. V. Bhosale and S. V. Bhosale, Mater. Chem. Front., 2017, 1, 1600 RSC; (i) Y. Liu, C. Mu and K. Jiang, et al. , Adv. Mater., 2015, 27, 1015 CrossRef CAS PubMed; (j) D. Srivani, A. Gupta, S. V. Bhosale, A. L. Puyad, W. Xiang, J. Li, R. A. Evans and S. V. Bhosale, Chem. Commun., 2017, 53, 7080 RSC.
- (a) P. Sun, H. Sun, X. Li, Y. Wang, H. Shan, J. Xu, C. Zhang, Z. Xu, Z.-K. Chen and W. Huang, Dyes Pigm., 2017, 139, 412 CrossRef CAS; (b) S.-Y. Liu, W.-Q. Liu, C.-X. Yuan, A.-G. Zhong, D. Han, B. Wang, M. N. Shah, M.-M. Shi and H. Chen, Dyes Pigm., 2016, 134, 139 CrossRef CAS.
- N. M. Kronenberg, M. Deppisch, F. Wurthner, H. W. A. Lademann, K. Deing and K. Meerholz, Chem. Commun., 2008, 6489 RSC.
- A. Gupta, A. Ali, A. Billic, M. Gao, K. Hegedus, B. Singh, S. E. Watkins, G. J. Wilson, U. Bach and R. A. Evans, Chem. Commun., 2012, 48, 1889 RSC.
- (a) A. Gupta, A. Ali, T. B. Singh, A. Bilic, U. Bach and R. A. Evans, Tetrahedron, 2012, 68, 9440 CrossRef CAS; (b) R. V. Hangarge, A. Gupta, A. M. Raynor, D. Duc La, A. Bilic, J. Li, D. S. Dalal, R. A. Evans and S. V. Bhosale, Dyes Pigm., 2017, 137, 126 CrossRef CAS.
- N. Qiu, X. Yang, H. Zhang, X. Wan, C. Li, F. Liu, H. Zhang, T. P. Russell and Y. Chen, Chem. Mater., 2016, 28, 6770 CrossRef CAS.
- (a) A. Gupta, V. Armel, W. Xiang, G. Fanchini, S. E. Watkins, D. R. MacFarlane, U. Bach and R. A. Evans, Tetrahedron, 2013, 69, 3584 CrossRef CAS; (b) R. J. Kumar, Q. I. Churches, J. Subbiah, A. Gupta, A. Ali, R. A. Evans and A. B. Holmes, Chem. Commun., 2013, 49, 6552 RSC; (c) A. Gupta, A. Ali, T. B. Singh, A. Bilic, U. Bach and R. A. Evans, Tetrahedron, 2012, 68, 9440 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb and J. R. Cheeseman, et al., Gaussian 09 revision D.01, Gaussian Inc., Wallingford CT, 2013 Search PubMed.
- (a) H. Patil, W. X. Zu, A. Gupta, V. Chellappan, A. Bilic, P. Sonar, A. Rananaware, S. V. Bhosale and S. V. Bhosale, Phys. Chem. Chem. Phys., 2014, 16, 23837 RSC; (b) A. M. Raynor, A. Gupta, H. Patil, A. Bilic and S. V. Bhosale, RSC Adv., 2014, 4, 57635 RSC; (c) S. Li, W. Liu, M. Shi, J. Mai, T.-K. Lau, J. Wan, X. Lu, C.-Z. Li and H. Chen, Energy Environ. Sci., 2016, 9, 604 RSC.
- Y. Kim, C. E. Song, S.-J. Moon and E. Lim, Chem. Commun., 2014, 50, 8235 RSC.
- Y. Lin, P. Cheng, Y. Li and X. Zhan, Chem. Commun., 2012, 48, 4773 RSC.
- H. Patil, A. Gupta, A. Bilic, S. V. Bhosale and S. V. Bhosale, Tetrahedron Lett., 2014, 55, 4430 CrossRef CAS.
- Q. Chen, J.-X. Wang, F. Yang, D. Zhou, N. Bian, X.-J. Zhang, C.-G. Yan and B.-H. Han, J. Mater. Chem., 2011, 21, 13554 RSC.
- P. S. Rao, A. Gupta, S. V. Bhosale, A. Bilic, W. Xiang, R. A. Evans and S. V. Bhosale, Dyes Pigm., 2017, 146, 502 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: PL spectra, DFT calculations, and PESA, theoretical optical absorption, TGA, SCLC and experimental spectra. See DOI: 10.1039/c7qm00355b |
| ‡ Denotes equal contribution. |
| This journal is © the Partner Organisations 2017 |