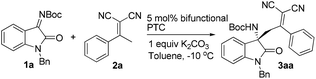
Effective asymmetric vinylogous Mannich reaction of isatin imines with α,α-dicyanoolefins in the presence of a simple chiral amide phosphonium bifunctional phase transfer catalyst†
Cang
Cheng
a,
Xuehe
Lu
a,
Luo
Ge
a,
Jie
Chen
a,
Weiguo
Cao
*a,
Xiaoyu
Wu
*ab and
Gang
Zhao
b
aDepartment of Chemistry, Shanghai University, 99 Shangda Rd, Shanghai 200444, China. E-mail: wgcao@shu.edu.cn; wuxy@shu.edu.cn
bKey Laboratory of Synthetic Chemistry of Natural Substances, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Lu, Shanghai 200032, China
First published on 20th October 2016
Abstract
An asymmetric vinylogous Mannich reaction between α,α-dicyanoolefins and N-Boc isatin imines has been developed by employing 1 mol% bifunctional amide phosphonium salt as a phase transfer catalyst and K2CO3 as a base. When α,α-dicyanoolefins derived from aryl methyl ketones were used as substrates, the normal Mannich adducts of 3,3-disubstituted aminooxindoles were obtained in good to excellent yields with high ee values, while α,α-dicyanoolefins derived from tetralone, propiophenone and methyl tert-butyl ketone led to cyclized products through a cascade sequence of Mannich reaction and intramolecular cyclization. The α,α-dicyanoolefin moiety in the Mannich adduct may be used as an aryl methyl ketone surrogate and the corresponding 3,3-disubstituted aminooxindole ketones may be effectively obtained after removal of the malonic nitrile by KMnO4 oxidation on a gram scale.
Introduction
3-Substituted 3-aminooxindoles bearing a C3-tetrasubstituted stereogenic center are present in a variety of natural products and biologically active molecules.1 Thus it is not surprising that tremendous efforts have been devoted to the asymmetric construction of this structural motif in the last decade.2 Among the methodologies developed targeting 3-substituted 3-aminooxindoles, direct addition of nucleophiles to isatin imines at the C-3 position is probably one of the most straightforward and efficient strategies. However, studies on asymmetric catalytic reactions involving isatin imines as reaction components remained inactive until 2012,3 when Shibata et al. and Wang et al. independently reported the application of N-Boc isatin imine and related compounds as electrophiles to react with malonic acid half thioester and 1,3-dicarbonyl compounds respectively.4N-Alkoxycarbonyl isatin imines possess higher reactivity compared with N-aryl imines due to the enhancement of electrophilicity induced by the electron-withdrawing alkoxycarbonyl groups.4 The higher reactivity and the easy access to N-Boc isatin imines via the aza-Wittig reaction,4b as well as relatively facile removal of the Boc protecting group allowing further elaboration of the adducts have made this type of imine attractive reaction components in the design of asymmetric catalytic reactions targeting enantioenriched 3-substituted 3-aminooxindoles.2e Since the seminal work of Shibata and Wang groups,4N-alkoxycarbonyl isatin imines, particularly N-Boc isatin imines, have been recruited by quite a number of research groups as reaction components in asymmetric catalytic reactions,5–11 such as the aza-Friedel–Crafts reaction,5 the aza-Morita–Baylis–Hillman reaction,6 the Mannich reaction,7 the aza-Henry reaction,8 the Strecker reaction,9 cycloaddition reaction,10etc., through either organocatalysis or Lewis acid catalysis. As far as the organocatalytic Mannich reaction is concerned, the research in this area mainly focused on the addition of active methylene or methine nucleophiles to N-alkoxycarbonyl isatin imines promoted by cinchona alkaloid-derived amine catalysts or tertiary amines tethered with H-bond donor(s).7 To our surprise, the asymmetric catalytic addition of simple ketones, such as aryl methyl ketones, to N-alkoxycarbonyl isatin imine has not yet been recorded, possibly due to the lower reactivity of aryl methyl ketones compared with 1,3-dicarbonyl nucleophiles.12 To circumvent this issue, we envisioned that α,α-dicyanoolefins, which can be prepared readily by the Knoevenagel condensation of aryl methyl ketones with malonic nitrile, could be employed as surrogates of the corresponding aryl methyl ketones in the nucleophilic addition to N-alkoxycarbonyl isatin imines because the C(CN)2 group in the Mannich adducts could be oxidatively cleaved by treating with KMnO4.14g,i,n
α,α-Dicyanoolefins have been recognized as versatile vinylogous nucleophiles in reactions with various electrophiles, such as α,β-unsaturated carbonyl compounds,14c–h nitroolefins,14i–j Morita–Baylis–Hillman carbonates,14k–l aldimines,14m–qetc., since the first introduction of this type of nucleophile in organocatalytic reactions by Chen and Jørgensen groups in 2005.13,14 To our knowledge, the Mannich reactions between α,α-dicyanoolefins and aldimines have been successfully realized in high stereoselectivity by employing cinchona alkaloid-derived bifunctional catalysts14m or pyrrolidium salt-based phase-transfer catalysts (PTCs).14n Nevertheless, the asymmetric Mannich reaction of α,α-dicyanoolefins with ketimines has not yet been reported until very recently. During our studies in progress, Li and co-workers reported the cinchona alkaloid-derived squaramide catalyzed Mannich reaction of α,α-dicyanoolefins with N-Boc isatin imines to provide the Mannich adducts in good to excellent yields and enantioselectivities.15 Despite being efficient, Li and co-workers’ catalytic system involved high catalyst loading and quite long reaction times, usually three to eight days to achieve completion. Herein, we present our investigation of this Mannich reaction catalyzed by 1 mol% of amino alcohol-derived amide phosphonium PTCs with excellent yields and good to excellent ees in the reaction time ranging from 12 to 48 h.16,17 In addition, the yields of the oxidative cleavage of the malonic nitrile moiety in the Mannich adducts were much improved by using a modified protocol from the literature.14g
Results and discussion
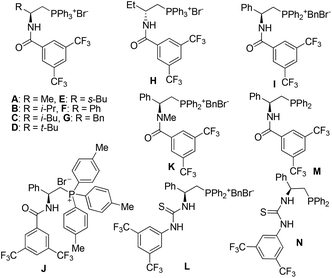
We commenced our investigation with the reaction between N-Boc isatin imine 1a and α,α-dicyanoolefin 2a derived from phenylethanone in the presence of bifunctional PTCs A–K (Fig. 1) in toluene with solid K2CO3 as the base. The results are listed in Table 1. Firstly, the background reaction was carried out, and no formation of the racemic 3aa was observed (entry 1). Then, various amide-phosphonium salts derived from natural amino acids were tested. Interestingly, catalysts with substituents of varied steric hindrance uniformly performed well to deliver the Mannich adduct 3aa in excellent yields and ees after a reaction time of 2 h (entries 2–8). This phenomenon is somewhat unusual as the reactivity and enantioselectivity seem to be independent of the bulkiness of the R group of catalysts. Catalyst H prepared from inexpensive (S)-2-amino-1-butanol led to the formation of ent-3aa in excellent yield and ee uneventfully (entry 9). Next, the effect of the substituents on the phosphonium center on the performance of the catalysts was surveyed (entries 10–11). When catalyst I with a benzyl group replacing one of three phenyl groups on the phosphonium moiety of F and catalyst J with p-tolyl groups replacing the phenyl groups on the phosphonium moiety of F were employed, longer reaction times were required to achieve complete conversion of the starting material, and the desired 3aa was obtained in lower yields and ee's. Furthermore N-methylated phosphonium salt K was also examined in this reaction, delivering 3aa in 94% yield after 12 h, albeit in a very low ee of 7% (entry 12). This result indicates that the presence of NH hydrogen in the secondary amide moiety as an H-bonding donor is important to both reactivity and stereoselectivity. Catalyst L with the thiourea moiety replacing the amide moiety of catalyst I led to 3aa in a comparable yield of 88% and lower ee (entry 13).| Entry | Cat. | Time (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|
| a General conditions: 1a (0.2 mmol), 2a (0.22 mmol), bifunctional PTC (5 mol%), and K2CO3 (1 equiv.) in toluene (1 mL) at −10 °C. b Yield referred to isolated pure 3aa. c The ee of 3aa was determined by chiral HPLC analysis. d Background reaction without PTC. e Conditions: 1a (0.2 mmol), 2a (0.22 mmol), phosphine catalyst (5 mol%) and methyl acrylate (6 mol%) in toluene (1 mL) at −10 °C. f M or N employed as a catalyst replacing bifunctional PTC under general conditions. | ||||
| 1d | — | 24 | 0 | — |
| 2 | A | 2 | 97 | 91 |
| 3 | B | 2 | 99 | 92 |
| 4 | C | 2 | 99 | 92 |
| 5 | D | 2 | 99 | 92 |
| 6 | E | 2 | 98 | 92 |
| 7 | F | 2 | 99 | 90 |
| 8 | G | 2 | 99 | 90 |
| 9 | H | 2 | 99 | −93 |
| 10 | I | 10 | 93 | 86 |
| 11 | J | 4 | 90 | 83 |
| 12 | K | 12 | 94 | 7 |
| 13 | L | 12 | 88 | 56 |
| 14e | M | 24 | 80 | 85 |
| 15e | N | 24 | 90 | 73 |
| 16f | M | 24 | 5 | 1 |
| 17f | N | 24 | 6 | 2 |
Previously, Zhao et al. and Lu et al. independently reported that the in situ generated amide phosphonium species from the corresponding amide phosphines are excellent catalysts for asymmetric addition reactions.18 By adopting similar protocols, we next tried the combination of phenylglycine derived phosphine and methyl acrylate as the catalyst in the reaction between 1a and 2a (entries 14–15) and amide phosphine M and thiourea phosphine N showed comparable performance, affording 3aa in high yields with good ees. Both of them required 24 h to ensure complete conversion of the starting material. Recently, Yao et al. described an asymmetric 1,6-conjugate addition of the α,α-dicyanoolefins to para-quinone methides catalyzed by an amino acid-derived thiourea phosphine in the presence of Et3N.14t However, in the presence of either Et3N or K2CO3 the reactions between 1a and 2a with either amide phosphine M or thiourea phosphine N as the catalyst all proceeded sluggishly, leading to almost racemic 3aa with less than 10% conversion rate after 24 h (entries 16 and 17). Thus, amide-phosphonium salt proved to be the best choice of the catalyst in the current system. Catalyst H provided ent-3aa with higher ee (entry 9) and was chosen for further optimization.
A brief screening of solvents revealed toluene to be the best one in terms of yield and enantioselectivity (Table 2, entries 1–6). Phenyl chloride provided 3aa with the performance comparable to that of toluene (entry 3). When ortho-xylene was employed as a solvent, 92% ee was achieved, albeit in a lower yield of 83% (entry 2). Other solvents, such as DCM, THF and acetonitrile, all resulted in lower enantioselectivity (entries 4–6). Next, the effect of temperature on the outcome of the reaction was surveyed (entries 7–9). Lowering the temperature to −20 °C led to an increase in the enantioselectivity from 93% to 96% ee in almost quantitative yield (entry 7). Further lowering the temperature to −40 °C did not provide higher ee, but resulted in a lower reaction rate (entry 8). When the reaction was carried out at rt, the reaction completed in about 10 min, affording 3aa with 90% ee, albeit in a relatively lower yield of 85% due to the formation of unidentified side products (entry 9). To our delight, the loading of the catalyst H could be reduced to 1 mol% without obvious detriment to the ee or the yield, only at the expense of an extended reaction time of 12 h (entry 10). Further optimization of the reaction conditions by screening of bases was unsuccessful (not shown), as no better results were obtained.
| Entry | Solvent | Temp (°C) | Time (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a General conditions: 1a (0.2 mmol), 2a (0.22 mmol), H (5 mol%), and K2CO3 (1 equiv.) in toluene (1 mL). b Yield referred to isolated pure ent-3aa. c The ee of ent-3aa was determined by chiral HPLC analysis. d Reaction performed with 1 mol% H in 0.5 mL toluene. | |||||
| 1 | Toluene | −10 | 2 | 99 | 93 |
| 2 | o-Xylene | −10 | 4 | 83 | 92 |
| 3 | PhCl | −10 | 4 | 98 | 91 |
| 4 | DCM | −10 | 6 | 79 | 82 |
| 5 | THF | −10 | 5 | 83 | 59 |
| 6 | CH3CN | −10 | 5 | 94 | 22 |
| 7 | Toluene | −20 | 6 | 99 | 96 |
| 8 | Toluene | −40 | 48 | 88 | 89 |
| 9 | Toluene | 20 | 0.2 | 85 | 90 |
| 16d | Toluene | −20 | 12 | 99 | 96 |
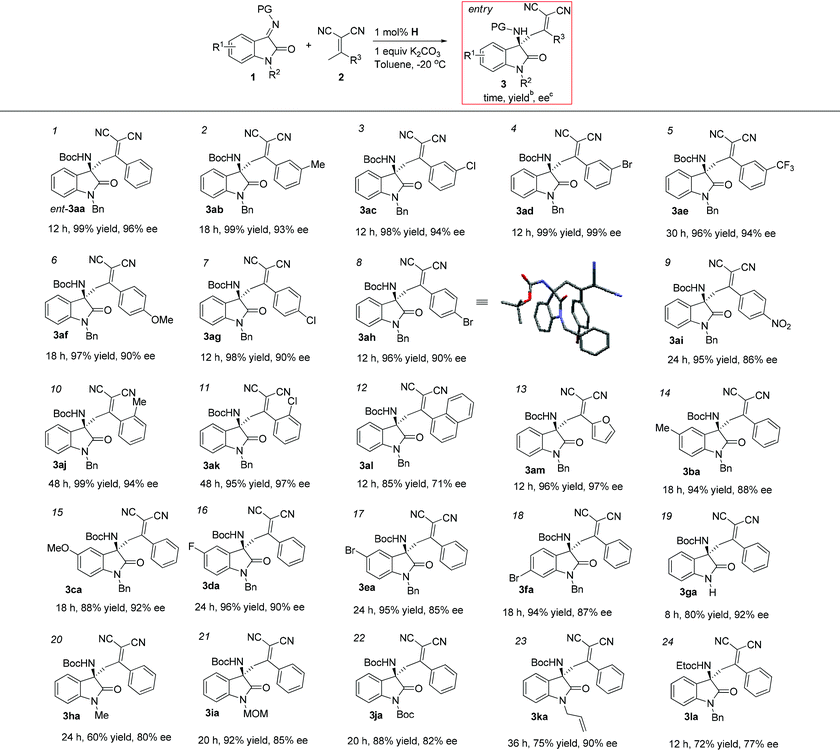
With the optimized reaction conditions in hand (Table 2, entry 7), we set out to explore the scope of the reaction with regard to α,α-dicyanoolefins bearing phenyl groups with different substituents (Table 3). To our delight, phenyl substituted α,α-dicyanoolefins with either electron-donating or -withdrawing substituents all performed well to deliver the Mannich adducts in excellent yields (95–99%) with good to excellent enantioselectivities (86–99% ee) (entries 1–11). The yields and enantioselectivities are independent of the position of the substituent on the phenyl ring of α,α-dicyanoolefins, whereas ortho-substituted substrates, such as 2j and 2k, exhibited attenuated reactivity as a longer reaction time of 48 h was necessary to give a satisfactory conversion rate compared with that of meta- and para-substituted ones (entries 10–11). Presumably, the repulsion between vicinal substituents on the phenyl ring in 2j and 2k would force the phenyl ring out of the plane of dicyanoolefin, therefore preventing the approach of in situ generated vinylogous anion species to the isatin imines. Substrate 2l derived from α-naphthylethanone, which is formally a 2,3-disubstituted phenylethanone, only gave a modest ee of 71% (entry 12). Substrate 2m bearing a 2-furanyl group was also tolerated in this reaction, leading to 3am in excellent yield and ee (entry 13).
Next, isatin imines of different substitution patterns were evaluated in the reaction with 2a under the optimal reaction conditions. We first examined N-Boc isatin imines prepared from various substituted N-benzyl isatins, and were delighted to find that all the reactions proceeded smoothly to provide the desired products in excellent yields and ees regardless of the electronic nature of the substituents (entries 14–18). Notably, substrate 1g prepared from isatin without a benzyl group on the nitrogen atom also reacted well with 2a to afford the Mannich adduct in 80% yield with 92% ee (entry 19). Furthermore, we were pleased to find that various substituents at the N1 position of the isatin imines were also tolerated (entries 20–23). The desired products were obtained with greater than 80% ees, albeit in the cases of substrates 1h and 1k, bearing methyl and allyl groups respectively at the N1 position, only modest yields of 60% and 75% were achieved. However, when the imino-nitrogen was protected with an ethoxycarbonyl group, the corresponding product 3la was obtained with an obvious drop in both yield and ee (entry 24). The obtained Mannich adducts bearing multiple functional groups are valuable synthetic intermediates for potential transformations. For example, as shown in Li and co-workers’ report, 3aa could be converted into a spirocyclic oxindole type α-lactam, the backbone of which is present in a bioactive molecule with anticancer efficacy.15
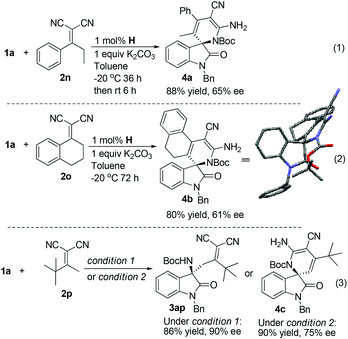
To further expand the scope of the reaction, we next evaluated α,α-dicyanoolefins derived from ketones other than substituted phenylethanones (Scheme 1). Interestingly, under the same conditions as above, the reaction of α,α-dicyanoolefin 2n prepared from propiophenone with 1a showed two new spots on TLC, and the less polar one gradually converted to the more polar one, which was characterized to be a cyclized product 4a (88% yield and 65% ee). Similarly, the reaction between cyclic α,α-dicyanoolefin 2o and 1a proceeded smoothly to give 4b in 80% yield with 61% ee. The structure of 4b was determined by single crystal X-ray diffraction analysis.19 The formation of 4a and 4b could be explained by an intramolecular cyclization of the Mannich adducts between the amino group and one of the two cyano groups followed by tautomerization. Unfortunately, attempts to isolate the Mannich adducts, which are the precursors of cyclized products in these two cases, were unsuccessful because of the facile cyclization of the Mannich adducts during the operation of column chromatography. In the case of 2p with a tert-butyl group replacing the phenyl group in 2a, the reaction under standard conditions led to the formation of 3ap in 86% yield with 90% ee as well as a trace amount of the cyclized product 4c. While 4c could be obtained in 90% yield with a diminished enantioselectivity of 75% ee after a further reaction time of 6 h if the cooling bath was removed after the full consumption of 1a. The detriment to optical purity might be ascribed to racemization through a retro-Mannich reaction during cyclization. On the other hand, the products listed in Table 3 are quite stable, and no intramolecular cyclization occurred even under forcing conditions, e.g. heating in THF in the presence of NaH. The reason why α,α-dicyanoolefins 2n, 2o and 2p exhibited different reaction profiles is unclear currently. Possibly, for substrates 2n–2p, the Mannich adducts generated in the first step would adopt a conformation wherein the amino group and the cyano group were disposed near to each other to facilitate subsequential cyclization. While in the case of the products listed in Table 3, as exemplified by 3ah, the single crystal X-ray diffraction analysis revealed that amino and cyano groups were arranged in opposite directions (Table 3, entry 8).19
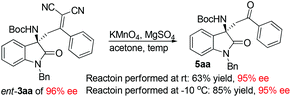
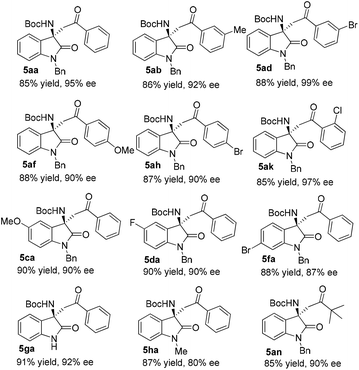
Next, transformation of the dicyanoolefin moiety of the Mannich adducts into a ketone moiety was next explored. Initially, the cleavage of the double bond was carried out via a known procedure reported by Jørgensen and co-workers.14g To our surprise, the oxidative cleavage of the double bond in 3aa completed immediately after the addition of solid KMnO4 in one portion, albeit in a rather modest yield of 63%. This result prompted us to evaluate the effect of temperature on the outcome of this reaction, and to our surprise the reaction performed at −10 °C with otherwise identical conditions proceeded smoothly to give ketone 5aa in 85% yield with excellent conservation of chiral enrichment (Scheme 2). Next, some representative Mannich adducts with different substitution patterns were subjected to the optimized oxidative cleavage procedure, and excellent yields were routinely attained in all cases without the erosion of the optical purity (Fig. 2). Thus, the present work shows that α,α-dicyanoolefins can act as suitable surrogates for aryl methyl ketones in the asymmetric catalytic Mannich reaction with N-alkoxycarbonyl isatin imines.
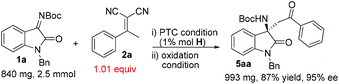
To prove the practicality of our catalytic system, we performed the reaction between 1a and 2a as well as the subsequent oxidative removal of the malonic nitrile moiety on a relatively large scale (Scheme 3). To our delight, the reaction proceeded smoothly with only a slight excess of 2a (1.01 equiv.) on a gram-scale, and it showed a clean spot on TLC after complete consumption of 1a. Simple filtration of the reaction mixture over a short pad of silica gel and evaporation of the volatiles afforded crude ent-3aa, which was pure enough for the subsequent oxidation step. Crude 3aa was treated with KMnO4 under the conditions mentioned above leading to ketone 5aa in an overall yield of 87% over 2 steps with 95% ee uneventfully.
Conclusions
In summary, we have developed an asymmetric vinylogous Mannich reaction between α,α-dicyanoolefins and N-Boc isatin imines catalyzed by 1 mol% bifunctional amide phosphonium salt in the presence of 1 equiv. solid K2CO3. For α,α-dicyanoolefins derived from aryl methyl ketones, the reactions proceeded smoothly to afford 3-substituted 3-aminooxindoles in good to excellent yields and high ees. When α,α-dicyanoolefins derived from tetralone, propiophenone and methyl t-butyl ketone were employed, cyclized products could be obtained in good yields with moderate ees through a cascade sequence of the Mannich reaction and an intramolecular cyclization. The malonic nitrile moiety in the Mannich adducts could be removed under mild conditions affording the corresponding ketones in excellent yields without the loss of ees. These results show that α,α-dicyanoolefins can act as suitable surrogates for aryl methyl ketones in the asymmetric catalytic Mannich reaction with N-Boc isatin imines. The practicality of the current method was demonstrated by a gram-scale synthesis of 5aa through a two-step sequence of the Mannich reaction and oxidation reaction requiring only one chromatography operation.Experimental section
General information
Thin-layer chromatography (TLC) was carried out on 0.25 mm silica gel plates visualized with UV light and/or by staining with ethanolic phosphomolybdic acid (PMA) or iodine. Flash column chromatography was performed on silica gel H (10–40 μ). 1H NMR spectra were recorded at 500 MHz and 13C NMR spectra were recorded at 125 MHz. Chemical shifts (δ) are given in ppm relative to TMS, and coupling constants (J) in Hz. High-resolution mass spectra were recorded by FTMS. Enantiomeric excesses were determined by HPLC analysis on a chiral stationary phase.General procedures for the preparation of phosphonium salts A–L and phosphines M–N
Amide phosphonium salts A–L were synthesized by a known literature procedure,17a among them A, C, H and J are new compounds. Phosphine catalysts M and N are known compounds, and were prepared according to a literature procedure.18j Phosphonium salts I and L were prepared from the corresponding phosphine-based compounds M and N according to a literature procedure.17b Phosphonium salt K was prepared from the corresponding N-methylated phosphine.17bGeneral procedures for reactions between isatin imines and α,α-dicyanoolefins
A mixture of N-Boc isatin imine4b1a (67 mg, 0.2 mmol), α,α-dicyanoolefin202a (37 mg, 0.22 mmol) and catalyst H (1.3 mg, 0.01 mmol) in toluene (0.5 mL) was cooled to –20 °C, and then K2CO3 (27 mg, 0.2 mmol) was added. The resulting mixture was stirred vigorously at the same temperature, and monitored by TLC. Upon the complete consumption of 1a, the reaction mixture was loaded directly onto a column packed with silica gel, and eluted with petroleum ether/ethyl acetate (4/1) to afford the addition products 3a (100 mg, 0.198 mmol) as a white solid in 99% yield with 96% ee.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 9.69 min (major enantiomer), tR = 27.60 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 9.69 min (major enantiomer), tR = 27.60 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 8.23 min (major enantiomer), tR = 13.55 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 8.23 min (major enantiomer), tR = 13.55 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.88 min (major enantiomer), tR = 14.26 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.88 min (major enantiomer), tR = 14.26 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 8.57 min (major enantiomer), tR = 15.42 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 8.57 min (major enantiomer), tR = 15.42 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 6.90 min (major enantiomer), tR = 10.62 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 6.90 min (major enantiomer), tR = 10.62 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 10.55 min (major enantiomer), tR = 32.19 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 10.55 min (major enantiomer), tR = 32.19 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 8.88 min (major enantiomer), tR = 28.52 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 8.88 min (major enantiomer), tR = 28.52 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 8.83 min (major enantiomer), tR = 28.66 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 8.83 min (major enantiomer), tR = 28.66 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 11.80 min (major enantiomer), tR = 46.87 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 11.80 min (major enantiomer), tR = 46.87 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 6.17 min (major enantiomer), tR = 21.43 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 6.17 min (major enantiomer), tR = 21.43 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 8.17 min (major enantiomer), tR = 19.38 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 8.17 min (major enantiomer), tR = 19.38 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.58 min (major enantiomer), tR = 23.55 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.58 min (major enantiomer), tR = 23.55 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 70
isopropanol = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 13.49 min (major enantiomer), tR = 14.40 min (minor enantiomer).
30, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 13.49 min (major enantiomer), tR = 14.40 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.76 min (major enantiomer), tR = 24.52 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.76 min (major enantiomer), tR = 24.52 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 95
isopropanol = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 14.57 min (minor enantiomer), tR = 15.78 min (major enantiomer).
5, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 14.57 min (minor enantiomer), tR = 15.78 min (major enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.61 min (major enantiomer), tR = 14.02 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.61 min (major enantiomer), tR = 14.02 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.45 min (major enantiomer), tR = 14.79 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.45 min (major enantiomer), tR = 14.79 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.29 min (major enantiomer), tR = 13.64 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.29 min (major enantiomer), tR = 13.64 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.28 min (major enantiomer), tR = 12.56 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.28 min (major enantiomer), tR = 12.56 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 10.56 min (major enantiomer), tR = 27.74 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 10.56 min (major enantiomer), tR = 27.74 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 6.38 min (major enantiomer), tR = 16.22 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 6.38 min (major enantiomer), tR = 16.22 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 6.75 min (major enantiomer), tR = 14.39 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 6.75 min (major enantiomer), tR = 14.39 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 5.94 min (major enantiomer), tR = 15.25 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 5.94 min (major enantiomer), tR = 15.25 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 11.53 min (major enantiomer), tR = 19.57 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 11.53 min (major enantiomer), tR = 19.57 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 13.89 min (major enantiomer), tR = 13.35 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 13.89 min (major enantiomer), tR = 13.35 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.91 min (major enantiomer), tR = 11.60 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.91 min (major enantiomer), tR = 11.60 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.31 min (major enantiomer), tR = 17.02 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.31 min (major enantiomer), tR = 17.02 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 10.34 min (major enantiomer), tR = 20.97 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 10.34 min (major enantiomer), tR = 20.97 min (minor enantiomer).
General procedure for oxidative cleavage of the malonic nitrile moiety of 3aa by KMnO4
Compound 3aa (50.4 mg, 0.1 mmol) was dissolved in acetone (1 mL). The resulting mixture was cooled with a cooling bath of −10 °C. Then MgSO4 (0.20 mmol) and KMnO4 (0.25 mmol) were added in one portion and the mixture was stirred for 10 min at the same temperature. Then the mixture was passed through a short pad of silica gel eluting with EtOAc/CH2Cl2 (1/1) to remove residual KMnO4 and MnO2. More water was added and the organic phase separated, and the aqueous phase was extracted twice with CH2Cl2. The resulting solution was concentrated, and the residue was purified via flash chromatography on silica gel (PE/AcOEt = 4/1) to give the product 5aa as a white solid (38.8 mg, 85% yield).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 8.92 min (major enantiomer), tR = 32.86 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 8.92 min (major enantiomer), tR = 32.86 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 75
isopropanol = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 6.90 min (major enantiomer), tR = 20.64 min (minor enantiomer).
25, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 6.90 min (major enantiomer), tR = 20.64 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 9.15 min (major enantiomer), tR = 37.58 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 9.15 min (major enantiomer), tR = 37.58 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 92
isopropanol = 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 16.91 min (minor enantiomer), tR = 20.21 min (major enantiomer).
8, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 16.91 min (minor enantiomer), tR = 20.21 min (major enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 8.83 min (major enantiomer), tR = 28.66 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 8.83 min (major enantiomer), tR = 28.66 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 9.90 min (major enantiomer), tR = 32.63 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 9.90 min (major enantiomer), tR = 32.63 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 92
isopropanol = 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 11.24 min (minor enantiomer), tR = 12.66 min (major enantiomer).
8, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 11.24 min (minor enantiomer), tR = 12.66 min (major enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 8.00 min (major enantiomer), tR = 25.26 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 8.00 min (major enantiomer), tR = 25.26 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 70
isopropanol = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 6.90 min (major enantiomer), tR = 20.64 min (minor enantiomer).
30, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 6.90 min (major enantiomer), tR = 20.64 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 12.43 min (major enantiomer), tR = 19.03 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 12.43 min (major enantiomer), tR = 19.03 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 70
isopropanol = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.09 min (major enantiomer), tR = 15.58 min (minor enantiomer).
30, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 7.09 min (major enantiomer), tR = 15.58 min (minor enantiomer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 80
isopropanol = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 5.83 min (major enantiomer), tR = 37.81 min (minor enantiomer).
20, flow rate = 1.0 mL min−1, λ = 210 nm): tR = 5.83 min (major enantiomer), tR = 37.81 min (minor enantiomer).
Acknowledgements
The generous financial support from the National Natural Science Foundation of China (no. 21272150 and No. 21672137) is acknowledged.Notes and references
- (a) L. Chevolot, A. M. Chevolot, M. Gajhede, C. Larsen, U. Anthoni and C. Christophersen, J. Am. Chem. Soc., 1985, 107, 4542 CrossRef CAS; (b) M. Ochi, K. Kawasaki, H. Kataoka and Y. Uchio, Biochem. Biophys. Res. Commun., 2001, 283, 1118 CrossRef CAS PubMed; (c) K. Bernard, S. Bogliolo and J. Ehrenfeld, Br. J. Pharmacol., 2005, 144, 1037 CrossRef CAS PubMed; (d) T. Shimazaki, M. Iijima and S. Chaki, Eur. J. Pharmacol., 2006, 543, 63 CrossRef CAS PubMed; (e) M. Rottmann, C. McNamara, B. S. K. Yeung, M. C. S. Lee, B. Zou, B. Russell, P. Seitz, D. M. Plouffe, N. V. Dharia, J. Tan, S. B. Cohen, K. R. Spencer, G. Gonzalez-Paez, L. Lakshiminarayana, A. Goh, R. Suwanarusk, T. Jegla, E. K. Schmitt, H.-P. Beck, R. Brun, F. Nosten, L. Renia, V. Dartois, T. H. Keller, D. A. Fidock, E. A. Winzeler and T. T. Diagana, Science, 2010, 329, 1175 CrossRef CAS PubMed; (f) T. Oost, G. Backfisch, S. Bhowmik, M. M. van Gaalen, H. Geneste, W. Hornberger, W. Lubisch, A. Netz, L. Unger and W. Wernet, Bioorg. Med. Chem. Lett., 2011, 21, 3828 CrossRef CAS PubMed.
- For selected recent reviews: (a) F. Zhou, Y.-L. Liu and J. Zhou, Adv. Synth. Catal., 2010, 352, 1381 CrossRef CAS; (b) K. Shen, X. Liu, L. Lin and X. Feng, Chem. Sci., 2012, 3, 327 RSC; (c) P. Chauhan and S. S. Chimni, Tetrahedron: Asymmetry, 2013, 24, 343 CrossRef CAS; (d) J. S. Yu, F. Zhou, Y. L. Liu and J. Zhou, Synlett, 2015, 2491 CAS; (e) J. Kaur, S. S. Chimni, S. Mahajan and A. Kumar, RSC Adv., 2015, 5, 52481 RSC; (f) Z. G. Mohammadi, R. Moradi and N. Lashgari, Tetrahedron: Asymmetry, 2015, 26, 517 CrossRef.
- For selected examples: (a) Y.-L. Liu, F. Zhou, J.-J. Cao, C.-B. Ji, M. Ding and J. Zhou, Org. Biomol. Chem., 2010, 8, 3847 RSC; (b) Q.-X. Guo, Y.-W. Liu, X.-C. Li, L.-Z. Zhong and Y.-G. Peng, J. Org. Chem., 2012, 77, 3589 CrossRef CAS PubMed; (c) J. J. Badillo, A. Silva-Garcia, B. H. Shupe, J. C. Fettinger and A. K. Franz, Tetrahedron Lett., 2011, 52, 5550 CrossRef CAS PubMed; (d) S. Duce, F. Pesciaioli, L. Gramigna, L. Bernardi, A. Mazzanti, A. Ricci, G. Bartoli and G. Bencivenni, Adv. Synth. Catal., 2011, 353, 860 CrossRef CAS.
- (a) N. Hara, S. Nakamura, M. Sano, R. Tamura, Y. Funahashi and N. Shibata, Chem. – Eur. J., 2012, 18, 9276 CrossRef CAS PubMed; (b) W. J. Yan, D. Wang, J. C. Feng, P. Li, D. Zhao and R. Wang, Org. Lett., 2012, 14, 2512 CrossRef CAS PubMed.
- aza-Friedel–Crafts reaction: (a) J. Feng, W. Yan, D. Wang, P. Li, Q. Sun and R. Wang, Chem. Commun., 2012, 48, 8003 RSC; (b) M. M. Magraner, C. Vila, R. Cantón, G. Blay, I. Fernández, M. C. Muñoz and J. R. Pedro, Angew. Chem., Int. Ed., 2015, 54, 6320 CrossRef PubMed; (c) P. Kumari, S. Barik, N. H. Khan, B. Ganguly, R. I. Kureshy, S. H. R. Abdi and H. C. Bajaj, RSC Adv., 2015, 5, 69493 RSC; (d) M. M. Magraner, C. Vila, A. R. Patiño, G. Blay, I. Fernández, M. C. Muñoz and J. R. Pedro, ACS Catal., 2016, 6, 2689 CrossRef; (e) X. Y. Zhang, J. L. Zhang, L. L. Lin, H. F. Zheng, W. B. Wu, X. H. Liu and X. M. Feng, Adv. Synth. Catal., 2016, 358, 3021 CrossRef CAS.
- (a) F. L. Hu, Y. Wei, M. Shi, S. Pindi and G. Li, Org. Biomol. Chem., 2013, 11, 1921 RSC; (b) X. Zhao, T. Z. Li, J. Y. Qian, F. Sha and X. Y. Wu, Org. Biomol. Chem., 2014, 12, 8072 RSC; (c) A. Kumar, V. Sharma, J. Kaur, N. Kumar and S. S. Chimni, Org. Biomol. Chem., 2015, 13, 5629 CAS; (d) Y. Yoshida, M. Sako, K. Kishi, H. Sasai, S. Hatakeyama and S. Takizawa, Org. Biomol. Chem., 2015, 13, 9022 RSC.
- (a) T. Z. Li, X. B. Wang, F. Sha and X. Y. Wu, J. Org. Chem., 2014, 79, 4332 CrossRef CAS PubMed; (b) Y. Guo, Y. Zhang, L. Qi, F. Tian and L. Wang, RSC Adv., 2014, 4, 27286 RSC; (c) X. B. Wang, T. Z. Li, F. Sha and X. Y. Wu, Eur. J. Org. Chem., 2014, 739 CrossRef; (d) Z. K. Tang, Y. Shi, H. B. Mao, X. B. Zhu, W. P. Li, Y. X. Cheng, W. H. Zheng and C. Zhu, J. Org. Biomol. Chem., 2014, 12, 6085 RSC; (e) J. Zhao, B. Fang, W. Luo, X. Hao, X. Liu, L. Lin and X. Feng, Angew. Chem., Int. Ed., 2015, 54, 241 CrossRef CAS PubMed; (f) M. X. Zhao, L. Jing, H. Zhou and M. Shi, RSC Adv., 2015, 5, 75648 RSC; (g) K. Zhao, T. Shu, J. Jia, G. Raabe and D. Enders, Chem. – Eur. J., 2015, 21, 3933 CrossRef CAS PubMed; (h) Y. Zhu, E. G. Zhang, C. Luo, X. Li and J. P. Cheng, Tetrahedron, 2015, 71, 4090 CrossRef CAS; (i) T. Liu, W. W. Liu, X. N. Li, F. Z. Peng and Z. H. Shao, J. Org. Chem., 2015, 80, 4950 CrossRef CAS PubMed; (j) O. D. Engl, S. P. Fritz and H. Wennemers, Angew. Chem., Int. Ed., 2015, 54, 8193 CrossRef CAS PubMed; (k) X. Z. Bao, B. M. Wang, L. C. Cui, G. D. Zhu, Y. L. He, J. P. Qu and Y. M. Song, Org. Lett., 2015, 17, 5168 CrossRef CAS PubMed; (l) F. I. Amr, C. Vila, G. Blay, M. C. Muñoz and J. R. Pedro, Adv. Synth. Catal., 2016, 358, 1583 CrossRef CAS; (m) C. Vila, F. I. Amr, G. Blay, M. C. Muñoz and J. R. Pedro, Chem. – Asian J., 2016, 11, 1532 CrossRef CAS PubMed.
- (a) A. Kumar, J. Kaur and S. S. Chimni, RSC Adv., 2014, 4, 24816 RSC; (b) Y. H. Wang, Y. L. Liu, Z. Y. Cao and J. Zhou, Asian J. Org. Chem., 2014, 3, 429 CrossRef CAS; (c) T. Arai, E. Matsumura and H. Masu, Org. Lett., 2014, 16, 2768 CrossRef CAS PubMed; (d) M. Holmquist, G. Blay and J. R. Pedro, Chem. Commun., 2014, 50, 9309 RSC; (e) B. Fang, X. Liu, J. Zhao, Y. Tang, L. Lin and X. Feng, J. Org. Chem., 2015, 80, 3332 CrossRef CAS PubMed.
- (a) D. Wang, J. Liang, J. Feng, K. Wang, Q. Sun, L. Zhao, D. Li, W. Yan and R. Wang, Adv. Synth. Catal., 2013, 355, 548 CAS; (b) Y. L. Liu and J. Zhou, Chem. Commun., 2013, 49, 4421 RSC.
- (a) H. F. Zheng, X. H. Liu, C. R. Xu, Y. Xia, L. L. Lin and X. M. Feng, Angew. Chem., Int. Ed., 2015, 54, 10958 CrossRef CAS PubMed; (b) K. Zhao, Y. Zhi, X. Y. Li, R. Puttreddy, K. Rissanen and D. Enders, Chem. Commun., 2016, 52, 2249 RSC; (c) X. Y. Han, W. L. Chan, W. J. Yao, Y. J. Wang and Y. X. Lu, Angew. Chem., Int. Ed., 2016, 55, 6492 CrossRef CAS PubMed; (d) M. G. Sankar, M. Garcia-Castro, C. Golz, C. Strohmann and K. Kumar, Angew. Chem., Int. Ed., 2016, 55, 9709 CrossRef CAS PubMed; (e) M. G. Sankar, M. Garcia-Castro, C. Golz, C. Strohmannb and K. Kumar, RSC Adv., 2016, 6, 56537 RSC.
- For other examples of asymmetric catalytic reactions involving N-Boc isatin imines as reaction components, see: (a) Q. He, L. Wu, X. Z. Kou, N. Butt, G. Q. Yang and W. B. Zhang, Org. Lett., 2016, 18, 288 CrossRef CAS PubMed; (b) C. S. Marques and A. J. Burke, Eur. J. Org. Chem., 2016, 806 CrossRef CAS; (c) H. Lv, B. Tiwari, J. M. Mo, C. Xing and Y. G. Chi, Org. Lett., 2012, 14, 5412 CrossRef CAS PubMed; (d) T. Z. Li, X. B. Wang, F. Sha and X. Y. Wu, Tetrahedron, 2013, 69, 7314 CrossRef CAS; (e) J. George, B. Sridhar and B. V. S. Reddy, Org. Biomol. Chem., 2014, 12, 1595 RSC; (f) A. Kumar, V. Sharma, J. Kaur, V. Kumar, S. Mahajan, N. Kumar and S. S. Chimni, Tetrahedron, 2014, 70, 7044 CrossRef CAS; (g) T. Arai, K. Tsuchiya and E. Matsumura, Org. Lett., 2015, 17, 2416 CrossRef CAS PubMed; (h) C. Beceño, P. Chauhan, A. Rembiak, A. Wang and D. Enders, Adv. Synth. Catal., 2015, 357, 672 CrossRef; (i) B. X. Feng, J. D. Yang, J. Y. Li and X. Li, Tetrahedron Lett., 2016, 57, 3457 CrossRef CAS.
- Asymmetric catalytic detrifluoroacetylative Mannich reaction of N-Boc isatin imine: (a) X. H. Liu, J. L. Zhang, L. Zhao, S. X. Ma, D. X. Yang, W. J. Yan and R. Wang, J. Org. Chem., 2015, 80, 12651 CrossRef CAS PubMed. Ph3PAuOTf catalyzed Mukaiyama–Mannich reaction of N-Boc isatin imine: (b) J. S. Yu and J. Zhou, Org. Biomol. Chem., 2015, 13, 10968 RSC.
- (a) D. Xue, Y. C. Chen, Q. W. Wang, L. F. Cun, J. Zhu and J. G. Deng, Org. Lett., 2005, 7, 5293 CrossRef CAS PubMed; (b) T. B. Poulsen, C. Alemparte and K. A. Jørgensen, J. Am. Chem. Soc., 2005, 127, 11614 CrossRef CAS PubMed.
- For reviews on asymmetric catalyzed reactions involving α,α-dicyanoolefins as reaction components, see: (a) H. L. Cui and Y. C. Chen, Chem. Commun., 2009, 4479 RSC; (b) J. Kaur, P. Chauhana and S. S. Chimni, Org. Biomol. Chem., 2016, 14, 7832 RSC. For selected recent examples of this topic, see: (c) J. W. Xie, L. Yue, D. Xue, X. L. Ma, Y. C. Chen, Y. Wu, J. Zhu and J. G. Deng, Chem. Commun., 2006, 1563 RSC; (d) J. W. Xie, W. Chen, R. Li, M. Zeng, W. Du, L. Yue, Y. C. Chen, Y. Wu, J. Zhu and J. G. Deng, Angew. Chem., Int. Ed., 2007, 46, 389 CrossRef CAS PubMed; (e) J. Lu, F. Liu and T.-P. Loh, Adv. Synth. Catal., 2008, 350, 1781 CrossRef CAS; (f) J. Lu, W.-J. Zhou, F. Liu and T.-P. Loh, Adv. Synth. Catal., 2008, 350, 1796 CrossRef CAS; (g) J. Alemán, C. B. Jacobsen, K. Frisch, J. Overgaard and K. A. Jørgensen, Chem. Commun., 2008, 632 RSC; (h) S. Rout, S. K. Ray, R. A. Unhale and V. K. Singh, Org. Lett., 2014, 16, 5568 CrossRef CAS PubMed; (i) T. B. Poulsen, M. Bell and K. A. Jørgensen, Org. Biomol. Chem., 2006, 4, 63 RSC; (j) L. Jiang, H.-T. Zheng, T.-Y. Liu, L. Yue and Y.-C. Chen, Tetrahedron, 2007, 63, 5123 CrossRef CAS; (k) H. L. Cui, J. Peng, X. Feng, W. Du, K. Jiang and Y. C. Chen, Chem. – Eur. J., 2009, 15, 1574 CrossRef CAS PubMed; (l) J. Peng, X. Huang, H.-L. Cui and Y.-C. Chen, Org. Lett., 2010, 12, 4260 CrossRef CAS PubMed; (m) T. Y. Liu, H. L. Cui, J. Long, B. J. Li, Y. Wu, L. S. Ding and Y. C. Chen, J. Am. Chem. Soc., 2007, 129, 1878 CrossRef CAS PubMed; (n) B. Niess and K. A. Jørgensen, Chem. Commun., 2007, 1620 RSC; (o) Q. A. Chen, W. Zeng, D. W. Wang and Y. G. Zhou, Synlett, 2009, 2236 CAS; (p) X.-F. Xiong, Z.-J. Jia, W. Du, K. Jiang, T.-Y. Liu and Y.-C. Chen, Chem. Commun., 2009, 6994 RSC; (q) J. Lu and W. Y. Chen, Bull. Korean Chem. Soc., 2012, 33, 3175 CrossRef CAS; (r) X.-L. Zhu, W.-J. He, L.-L. Yu, C.-W. Cai, Z.-L. Zuo, D.-B. Qin, Q.-Z. Liu and L.-H. Jing, Adv. Synth. Catal., 2012, 354, 2965 CrossRef CAS; (s) M.-R. Zhang and J.-L. Zhang, Chem. – Eur. J., 2014, 20, 399 CrossRef CAS PubMed; (t) X. Y. Li, X. Y. Xu, W. W. Wei, A. J. Lin and H. Q. Yao, Org. Lett., 2016, 18, 428 CrossRef CAS PubMed.
- Y. Zhu, Y. Li, Q. B. Meng and X. Li, Org. Chem. Front., 2016, 3, 709 RSC.
- For recent reviews on phase transfer catalysis, see: (a) K. Maruoka and T. Ooi, Chem. Rev., 2003, 103, 3013 CrossRef CAS PubMed; (b) M. J. O'Donnell, Acc. Chem. Res., 2004, 37, 506 CrossRef PubMed; (c) S. Jew and H. Park, Chem. Commun., 2009, 7090 RSC; (d) K. Maruoka, Chem. Rec., 2010, 10, 254 CrossRef CAS PubMed.
- For selected examples of bifunctional phase transfer catalysts, see: (a) X. Y. Wu, Q. Liu, Y. Liu, Q. Wang, Y. Zhang, J. Chen, W. G. Cao and G. Zhao, Adv. Synth. Catal., 2013, 355, 2701 CrossRef CAS; (b) D. D. Cao, Z. Chai, J. X. Zhang, Z. Q. Ye, H. Xiao, H. Y. Wang, J. H. Chen, X. Y. Wu and G. Zhao, Chem. Commun., 2013, 49, 5972 RSC; (c) S. Shirakawa, L. J. Wang, R. J. He, S. Arimitsu and K. Maruoka, Chem. – Asian J., 2014, 9, 1586 CrossRef CAS PubMed; (d) H. Y. Wang, J. X. Zhang, D. D. Cao and G. Zhao, ACS Catal., 2013, 3, 2218 CrossRef CAS; (e) Y. Liu, S. Shirakawa and K. Maruoka, Org. Lett., 2013, 15, 1230 CrossRef CAS PubMed; (f) S. Shirakawa, T. Tokuda, A. Kasai and K. Maruoka, Org. Lett., 2013, 15, 3350 CrossRef CAS PubMed; (g) D. D. Cao, J. X. Zhang, H. Y. Wang and G. Zhao, Chem. – Eur. J., 2015, 21, 9998 CrossRef CAS PubMed; (h) S. Wen, X. Li, W. J. Yao, A. Waheed, N. Ullah and Y. X. Lu, Eur. J. Org. Chem., 2016, 4298 CrossRef.
- (a) F. R. Zhong, X. W. Dou, X. Y. Han, W. J. Yao, Q. Zhu, Y. Z. Meng and Y. X. Lu, Angew. Chem., Int. Ed., 2013, 52, 943 CrossRef CAS PubMed; (b) H. Y. Wang, K. Zhang, C. W. Zheng, Z. Chai, D. D. Cao, J. X. Zhang and G. Zhao, Angew. Chem., Int. Ed., 2015, 54, 1775 CrossRef CAS PubMed. For selected examples of amino acid-derived phosphines as catalysts in asymmetric catalytic reactions, see: (c) X. Y. Han, W. L. Chan, W. J. Yao, Y. J. Wang and Y. X. Lu, Angew. Chem., Int. Ed., 2016, 55, 6492 CrossRef CAS PubMed; (d) T. L. Wang, Z. Y. Yu, D. L. Hoon, C. Y. Phee, Y. Lan and Y. X. Lu, J. Am. Chem. Soc., 2016, 138, 265 CrossRef CAS PubMed; (e) Y. N. Gao, Q. Xu and M. Shi, ACS Catal., 2015, 5, 6608 CrossRef CAS; (f) D. Wang, G. P. Wang, Y. L. Sun, S. F. Zhu, Y. Wei, Q. L. Zhou and M. Shi, Chem. Sci., 2015, 6, 7319 RSC; (g) Y. P. Lou, C. W. Zheng, R. M. Pan, Q. W. Jin, G. Zhao and Z. Li, Org. Lett., 2015, 17, 688 CrossRef CAS PubMed; (h) W. J. Yao, X. Y. Dou and Y. X. Lu, J. Am. Chem. Soc., 2015, 137, 54 CrossRef CAS PubMed; (i) X. Y. Han, Y. Q. Wang, F. R. Zhong and Y. X. Lu, J. Am. Chem. Soc., 2011, 133, 1726 CrossRef CAS PubMed; (j) H. Xiao, Z. Chai, C.-W. Zheng, Y.-Q. Yang, W. Liu, J.-K. Zhang and G. Zhao, Angew. Chem., Int. Ed., 2010, 49, 4467 CrossRef CAS PubMed.
- CCDC 1496438 and 1496437 contain the supplementary crystallographic data of 3ah and 4b for this paper.
- J. Lu, F. Liu, W.-J. Zhou and T.-P. Loh, Tetrahedron Lett., 2008, 49, 5389 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental details, and copies of 1H, 13C NMR and HPLC spectra. CCDC 1496437 and 1496438. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00546b |
| This journal is © the Partner Organisations 2017 |