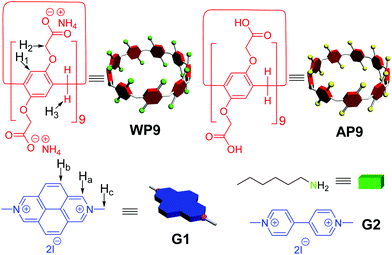
Dual-pH responsive host–guest complexation between a water-soluble pillar[9]arene and a 2,7-diazapyrenium salt†
Zhengtao
Li
,
Guocan
Yu
and
Jie
Yang
*
Department of Chemistry, Zhejiang University, Hangzhou 310027, P. R. China. E-mail: jieyang@zju.edu.cn; Fax: +86-571-8795-3189; Tel: +86-571-8795-3189
First published on 26th October 2016
Abstract
The host–guest complexation between a water-soluble pillar[9]arene and a 2,7-diazapyrenium salt not only can be controlled by the sequential addition of an acid and a base (HCl and NaOH, respectively) but also can be switched through the sequential addition of a base and an acid (hexylamine and TFA, respectively).
In supramolecular chemistry, stimuli-responsive molecular recognition motifs have played an important role due to their topological importance and extensive application in the construction of various interesting supramolecular systems, such as molecular switches and machines,1 supramolecular polymers,2 and other functional materials.3 Up to now, light, metal ions, pH, redox, enzyme and other external stimuli have been widely employed in the construction of responsive host–guest systems on the basis of macrocyclic compounds (such as crown ethers,4 cyclodextrins,5 calixarenes6 and cucurbiturils7). As a new type of macrocyclic hosts, pillar[n]arenes8 have gained increasing attention since their first report in 2008.8a Owing to their symmetrical pillar architecture and accessible functionalization, pillar[n]arenes have seen superior host–guest properties towards various guests and been further explored in the construction of numerous supramolecular systems, such as daisy chains,9 supramolecular polymers,10 drug-release systems,11 transmembrane channels12 and other advanced functional materials.13 By adopting different derivatization methods, anionic,14 cationic15 and neutral16 water-soluble pillararenes can be obtained according to previous reports. Among these water-soluble pillararenes, anionic ones decorated with carboxylate groups have an inherent advantage: they are responsive to acid/base reagent pairs (such as HCl and NaOH). Based on these pH-responsive pillararenes, plenty of molecular switches and corresponding supramolecular systems have been reported.11,17 However, these switches or systems can only be operated through a simple two-directional way: an acid turns them off/on and a base turns them on/off. Due to the increasing need for logic gates that can provide different inputs and various functions, we now have a great urge in preparing a dual-pH responsive host–guest recognition motif which can be switched on and off not only through the action of a specific acid/base pair but also through the addition of another base/acid pair.
With the pH-responsive hosts in hand, we speculate that if one guest which also responds to pH can be found or designed, a dual-pH controlled host–guest complex may be obtained. Luckily enough, such guests were already found! 2,7-Diazapyrenium (DAP) dications, which unite the merits of π-electron-deficient character and extended π-surface, have been proved to exhibit excellent supramolecular associations with a number of π-electron-rich host partners and been applied successfully to establish various host–guest complexes and corresponding advanced mechanically interlocked architectures. It is worth noting that DAP dications can form stable adducts with aliphatic amines and be recovered to original states upon the addition of equivalent trifluoroacetic acid (TFA).18 That is to say, DAP dications are pH-responsive and they can be treated as desired counterparts to construct dual-pH responsive host–guest complexes with anionic water-soluble pillar[n]arenes. Recently, Xue et al. reported some studies about DAP-based host–guest complexes with pillar[10]arenes in water or organic solution.19 However, dual-pH responsive complexation between pillararenes and DAP dications has never been reported, presumably because of the difficulty in preparing suitable and complementary host/guest pairs which can respond to specific acid/base pairs and base/acid pairs, respectively. Herein, we describe a unique dual-action host–guest complex whose uncomplexed and complexed states can be controlled through the employment of not only an acid/base pair but also a base/acid pair in water. In this work, dimethyldiazapyrenium (DMDAP) dicationic salt G1 served as the guest and anionic water-soluble pillar[9]arene WP9 acted as the macrocyclic host. The larger cavity17a of WP9 compared with those of pillar[5,6]arenes should be more suitable for this guest to thread into (Scheme 1).
WP9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17a and G1
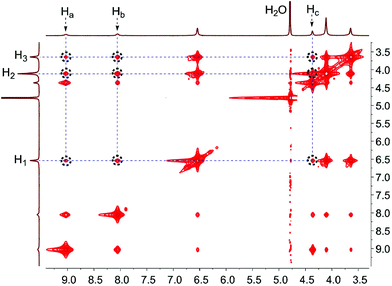
17a and G1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18d were synthesized according to previously reported procedures. Firstly, the host–guest complexation between WP9 and G1 was investigated by 1H NMR spectroscopy. Fig. 1 shows the 1H NMR spectra of G1 in D2O recorded in the absence and presence of approximately 1.0 equiv. of the host. Fast exchange on the NMR time scale was observed for this complex. After complexation with WP9, the peaks corresponding to the protons on complexed G1 became broad and shifted upfield significantly compared with those on free G1 (Fig. 1a). The reason was that the protons on G1 located within the cavity of WP9, thereby generating a shielding effect. On the other hand, the signals related to WP9 also exhibited slight chemical shift changes. Then, a 2D NOESY NMR experiment was conducted as it's a powerful tool for exploring the conformations of host–guest complexes. As shown in Fig. 2, nuclear overhauser effect (NOE) correlation signals were clearly observed between protons H1,2,3 on the pillar[9]arene unit and protons Ha,b,c on the guest unit, indicating that guest G1 penetrated into the cavity of WP9, in good agreement with the results from 1H NMR study.
18d were synthesized according to previously reported procedures. Firstly, the host–guest complexation between WP9 and G1 was investigated by 1H NMR spectroscopy. Fig. 1 shows the 1H NMR spectra of G1 in D2O recorded in the absence and presence of approximately 1.0 equiv. of the host. Fast exchange on the NMR time scale was observed for this complex. After complexation with WP9, the peaks corresponding to the protons on complexed G1 became broad and shifted upfield significantly compared with those on free G1 (Fig. 1a). The reason was that the protons on G1 located within the cavity of WP9, thereby generating a shielding effect. On the other hand, the signals related to WP9 also exhibited slight chemical shift changes. Then, a 2D NOESY NMR experiment was conducted as it's a powerful tool for exploring the conformations of host–guest complexes. As shown in Fig. 2, nuclear overhauser effect (NOE) correlation signals were clearly observed between protons H1,2,3 on the pillar[9]arene unit and protons Ha,b,c on the guest unit, indicating that guest G1 penetrated into the cavity of WP9, in good agreement with the results from 1H NMR study.
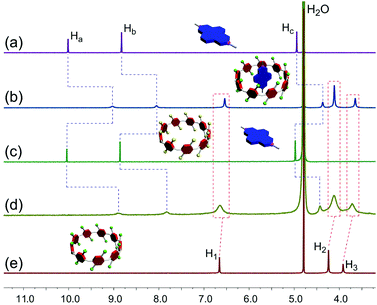 | ||
| Fig. 1 Partial 1H NMR spectra (500 MHz, D2O, 293 K): (a) G1 (2.00 mM); (b) WP9 (2.00 mM) and G1 (2.00 mM); (c) after addition of DCl to b; (d) after addition of NaOD to c; (e) WP9 (2.00 mM). | ||
UV-vis absorption spectroscopy gives further evidence for the existence of host–guest complexation between WP9 and G1. When WP9 was mixed with equimolar G1 in water, the colour of the resulting solution turned to yellow (Fig. S6a, ESI†) and the UV-vis spectrum of the solution exhibited a clear charge-transfer band above 450 nm (Fig. S5, ESI†), indicating the charge-transfer interaction existed between electron-rich WP9 and electron-deficient G1.
Fluorescence titration experiments (Fig. S2–S4, ESI†) were carried out to estimate the association constant (Ka) of the complexation between WP9 and G1 at room temperature in aqueous solution. Fluorescence intensity of G1 decreased significantly upon gradual addition of WP9 (Fig. S2, ESI†). A mole ratio plot (Fig. S3, ESI†) showed the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of the complex between WP9 and G1, which was in good agreement with the result obtained from ESI mass spectrometry (Fig. S7, ESI†). An ion peak at m/z 1188.9 for [WP9⊃G1 − 2I − 18NH3]2+ was observed to reveal the formation of the complex WP9⊃G1. By means of non-linear curve-fitting, the Ka value for WP9⊃G1 was estimated to be (9.42 ± 1.33) × 108 M−1 (Fig. S4, ESI†), which is much higher than the corresponding Ka value for the complex between WP9 and the paraquat guest G2 (2.27 × 106 M−1) reported previously by us.17a We assumed that WP9 had only one cavity when it was dissolved in water because of the repulsive electrostatic force resulting from the eighteen anionic carboxylate groups on both rims of the host molecule. So, we speculated that the architecture of DMDAP guest G1 was more suitable than that of G2 to locate in the cavity of WP9 due to the size selectivity. The reason for the remarkably high binding affinity of this host–guest complex may be ascribed to the cooperative interactions of electrostatic interaction, hydrophobic interaction and π–π interaction between WP9 and G1.
1 stoichiometry of the complex between WP9 and G1, which was in good agreement with the result obtained from ESI mass spectrometry (Fig. S7, ESI†). An ion peak at m/z 1188.9 for [WP9⊃G1 − 2I − 18NH3]2+ was observed to reveal the formation of the complex WP9⊃G1. By means of non-linear curve-fitting, the Ka value for WP9⊃G1 was estimated to be (9.42 ± 1.33) × 108 M−1 (Fig. S4, ESI†), which is much higher than the corresponding Ka value for the complex between WP9 and the paraquat guest G2 (2.27 × 106 M−1) reported previously by us.17a We assumed that WP9 had only one cavity when it was dissolved in water because of the repulsive electrostatic force resulting from the eighteen anionic carboxylate groups on both rims of the host molecule. So, we speculated that the architecture of DMDAP guest G1 was more suitable than that of G2 to locate in the cavity of WP9 due to the size selectivity. The reason for the remarkably high binding affinity of this host–guest complex may be ascribed to the cooperative interactions of electrostatic interaction, hydrophobic interaction and π–π interaction between WP9 and G1.
Additionally, the assembly and disassembly of the inclusion complex between WP9 and G1 can be reversibly controlled by sequential addition of HCl and NaOH aqueous solutions. Proton NMR spectroscopy was conducted to confirm this reversible process (Fig. 1). When DCl (20.0 equiv.) was added to the equimolar solution of WP9 and G1, the signals for the protons on WP9 disappeared (Fig. 1c) due to the precipitation of the resulted carboxylic acid pillar[9]arene (AP9) from the solution (Fig. S6b, ESI†). Meanwhile, the peaks of Ha–c on G1 migrated back to their free original values, suggesting that the guest dethreaded from the cavity of AP9 (Fig. 1c). On the other hand, after the addition of NaOD (20.0 equiv.) to the mixed solution, the system returned to the original complexed state and significant upfield shifts corresponding to protons on G1 were observed again (Fig. 1d), indicating the reformation of the inclusion complex between WP9 and G1. Thus, the sequential addition of HCl and NaOH to the solution of WP9 and G1 can be employed to switch the two components from unthreaded to rethreaded states, i.e., this host–guest complex can behave as an acid/base-controlled molecular switch.
Furthermore, we explored the reverse strategy: base/acid-controlled mode. The addition of hexylamine to an equimolar solution of WP9 and G1 caused a colour change from yellow to dark green because the more stable adduct between G1 and the aliphatic amines formed18a while the complex WP9⊃G1 dissociated. Subsequently, it can be recovered again when enough TFA was used to neutralize hexylamine accompanied with the reappearance of the yellow colour associated with the host–guest complex (Fig. S6c, ESI†). Proton NMR studies were performed to testify this reversible process (Fig. 3). When hexylamine (40.0 equiv.) was added to the mixture solution of WP9 and G1, the peaks corresponding to the protons on G1 disappeared substantially while the signals of WP9 returned to the original positions as the free host (Fig. 3c). On the other hand, when TFA (40.0 equiv.) was added to the mixed solution of WP9, G1, and hexylamine, an 1H NMR spectrum similar to that of the original solution of WP9 and G1 was obtained (Fig. 3d),20 indicating the regeneration of the complex between the host and the guest in this solution. Therefore, the host–guest complex WP9⊃G1 can be controlled reversibly from its complexed state to its uncomplexed state upon the sequential addition of a base and an acid, and this complex can also act as a base/acid-controllable molecular switch.
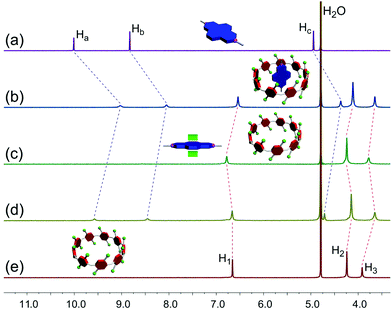 | ||
| Fig. 3 Partial 1H NMR spectra (500 MHz, D2O, 293 K): (a) G1 (2.00 mM); (b) WP9 (2.00 mM) and G1 (2.00 mM); (c) after addition of hexylamine to b; (d) after addition of TFA to c; (e) WP9 (2.00 mM). | ||
In summary, we reported a novel dual-pH responsive molecular recognition motif between water-soluble pillar[9]arene WP9 and DMDAP G1. It was demonstrated that WP9 and G1 formed a stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complex in water with an extraordinarily high association constant of 9.42 × 108 M−1. Furthermore, this host–guest complex can behave as a molecular switch that not only can be operated between its complexed and decomplexed states through the sequential addition of an acid and a base (HCl and NaOH, respectively) but also can be controlled upon the continual addition of a base and an acid (hexylamine and TFA, respectively). Future work will be focused on the fabrication of advanced controlled-release systems and other smart materials on the basis of this novel dual-pH responsive recognition motif.
1 inclusion complex in water with an extraordinarily high association constant of 9.42 × 108 M−1. Furthermore, this host–guest complex can behave as a molecular switch that not only can be operated between its complexed and decomplexed states through the sequential addition of an acid and a base (HCl and NaOH, respectively) but also can be controlled upon the continual addition of a base and an acid (hexylamine and TFA, respectively). Future work will be focused on the fabrication of advanced controlled-release systems and other smart materials on the basis of this novel dual-pH responsive recognition motif.
This work was supported by the Fundamental Research Funds for the Central Universities.
Notes and references
- (a) T. Han and C.-F. Chen, Org. Lett., 2007, 9, 4207–4210 CrossRef CAS PubMed; (b) M. Zhang, X. Yan, F. Huang, Z. Niu and H. W. Gbison, Acc. Chem. Res., 2014, 47, 1995–2005 CrossRef CAS PubMed.
- (a) L. Chen, Y.-K. Tian, Y. Ding, Y.-J. Tian and F. Wang, Macromolecules, 2012, 45, 8412–8419 CrossRef CAS; (b) J.-F. Xu, Y.-Z. Chen, D. Wu, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Angew. Chem., Int. Ed., 2013, 52, 9738–9742 CrossRef CAS PubMed; (c) Y.-J. Tian, E. W. Meijer and F. Wang, Chem. Commun., 2013, 49, 9197–9199 RSC; (d) H.-Q. Peng, J.-F. Xu, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Chem. Commun., 2013, 49, 9197–9199 RSC; (e) S. Cantekin, A. J. Markvoort, J. A. A. W. Elemans, A. E. Rowan and R. J. M. Nolte, J. Am. Chem. Soc., 2015, 137, 3915–3923 CrossRef CAS PubMed; (f) Y.-K. Tian, Y.-G. Shi, Z.-S. Yang and F. Wang, Angew. Chem., Int. Ed., 2014, 53, 6090–6094 CrossRef CAS PubMed; (g) Y.-K. Tian, Y.-F. Han, Z.-S. Yang and F. Wang, Macromolecules, 2016, 49, 6455–6461 CrossRef CAS.
- (a) A.-J. Avestro, M. E. Belowich and J. F. Stoddart, Chem. Soc. Rev., 2012, 41, 5881–5895 RSC; (b) V. N. Vukotic and S. J. Loeb, Chem. Soc. Rev., 2012, 41, 5896–5906 RSC; (c) D.-S. Guo and Y. Liu, Chem. Soc. Rev., 2012, 41, 5907–5921 RSC; (d) Y. Liu, Z. Wang and X. Zhang, Chem. Soc. Rev., 2012, 41, 5922–5932 RSC; (e) J. Hu, G. Zhang and S. Liu, Chem. Soc. Rev., 2012, 41, 5933–5949 RSC; (f) S.-L. Li, T. Xiao, C. Lin and L. Wang, Chem. Soc. Rev., 2012, 41, 5950–5968 RSC; (g) H. Jin, W. Huang, X. Zhu, Y. Zhou and D. Yan, Chem. Soc. Rev., 2012, 41, 5986–5997 RSC; (h) X. Yan, D. Xu, X. Chi, J. Chen, S. Dong, X. Ding, Y. Yu and F. Huang, Adv. Mater., 2012, 24, 362–369 CrossRef CAS PubMed.
- (a) M. J. Langton and P. D. Beer, Acc. Chem. Res., 2014, 47, 1935–1949 CrossRef CAS PubMed; (b) Y. Han, Z. Meng, Y.-X. Ma and C.-F. Chen, Acc. Chem. Res., 2014, 47, 2026–2040 CrossRef CAS PubMed.
- (a) X. Ma and H. Tian, Acc. Chem. Res., 2014, 47, 1971–1981 CrossRef CAS PubMed; (b) Q.-D. Hu, G.-P. Tang and P. K. Chu, Acc. Chem. Res., 2014, 47, 2017–2025 CrossRef CAS PubMed; (c) J. Hu and S. Liu, Acc. Chem. Res., 2014, 47, 2084–2095 CrossRef CAS PubMed.
- (a) D.-S. Guo and Y. Liu, Acc. Chem. Res., 2014, 47, 1925–1934 CrossRef CAS PubMed; (b) I. Pochorovski and F. Diederich, Acc. Chem. Res., 2014, 47, 2096–2105 CrossRef CAS PubMed.
- (a) L. Isaacs, Acc. Chem. Res., 2014, 47, 2052–2062 CrossRef CAS PubMed; (b) A. E. Kaifer, Acc. Chem. Res., 2014, 47, 2160–2167 CrossRef CAS PubMed.
- (a) T. Ogoshi, S. Kanai, S. Fujinami, T.-A. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS PubMed; (b) D. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang and H. Meier, Angew. Chem., Int. Ed., 2009, 48, 9721–9723 CrossRef CAS PubMed; (c) Y. Yao, M. Xue, J. Chen, M. Zhang and F. Huang, J. Am. Chem. Soc., 2012, 134, 15712–15715 CrossRef CAS PubMed; (d) M. Xue, Y. Yang, X. Chi, Z. Zhang and F. Huang, Acc. Chem. Res., 2012, 45, 1294–1308 CrossRef CAS PubMed; (e) X. Shu, S. Chen, J. Li, Z. Chen, L. Weng, X. Jia and C. Li, Chem. Commun., 2012, 48, 2967–2969 RSC; (f) H. Li, D.-X. Chen, Y.-L. Sun, Y. B. Zheng, L.-L. Tan, P. S. Weiss and Y.-W. Yang, J. Am. Chem. Soc., 2012, 135, 1570–1576 CrossRef PubMed; (g) G. Yu, Y. Ma, C. Han, Y. Yao, G. Tang, Z. Mao, C. Gao and F. Huang, J. Am. Chem. Soc., 2013, 135, 10310–10313 CrossRef CAS PubMed; (h) T. Ogoshi, T.-A. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937–8002 CrossRef CAS PubMed.
- Z. Zhang, G. Yu, C. Han, J. Liu, X. Ding, Y. Yu and F. Huang, Org. Lett., 2011, 13, 4818–4821 CrossRef CAS PubMed.
- (a) Z. Zhang, Y. Luo, J. Chen, S. Dong, Y. Yu, Z. Ma and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1397–1401 CrossRef CAS PubMed; (b) Y. Guan, M. Ni, X. Hu, T. Xiao, S. Xiong, C. Lin and L. Wang, Chem. Commun., 2012, 48, 8529–8531 RSC; (c) C. Li, K. Han, J. Li, Y. Zhang, W. Chen, Y. Yu and X. Jia, Chem. – Eur. J., 2013, 19, 11892–11897 CrossRef CAS PubMed; (d) C. Li, Chem. Commun., 2014, 50, 12420–12433 RSC; (e) B. Shi, D. Xia and Y. Yao, Chem. Commun., 2014, 50, 13932–13935 RSC; (f) B. Shi, K. Jie, Y. Zhou, D. Xia and Y. Yao, Chem. Commun., 2015, 51, 4503–4506 RSC.
- (a) G. Yu, X. Zhou, Z. Zhang, C. Han, Z. Mao, C. Gao and F. Huang, J. Am. Chem. Soc., 2012, 134, 19489–19497 CrossRef CAS PubMed; (b) Y. Cao, X.-Y. Hu, Y. Li, X. Zou, S. Xiong, C. Lin, Y.-Z. Shen and L. Wang, J. Am. Chem. Soc., 2014, 136, 10762–10769 CrossRef CAS PubMed.
- (a) W. Si, L. Chen, X.-B. Hu, G. Tang, Z. Chen, J.-L. Hou and Z.-T. Li, Angew. Chem., Int. Ed., 2011, 50, 12564–12568 CrossRef CAS PubMed; (b) X.-B. Hu, Z. Chen, G. Tang, J.-L. Hou and Z.-T. Li, J. Am. Chem. Soc., 2012, 134, 8384–8387 CrossRef CAS PubMed; (c) L. Chen, W. Si, L. Zhang, G. Tang, Z.-T. Li and J.-L. Hou, J. Am. Chem. Soc., 2013, 135, 2152–2155 CrossRef CAS PubMed.
- (a) G. Yu, M. Xue, Z. Zhang, J. Li, C. Han and F. Huang, J. Am. Chem. Soc., 2012, 134, 13248–13251 CrossRef CAS PubMed; (b) G. Yu, C. Han, Z. Zhang, J. Chen, X. Yan, B. Zheng, S. Liu and F. Huang, J. Am. Chem. Soc., 2012, 134, 8711–8717 CrossRef CAS PubMed; (c) N. L. Strutt, H. Zhang, S. T. Schneebeli and J. F. Stoddart, Acc. Chem. Res., 2014, 47, 2631–2642 CrossRef CAS PubMed; (d) Z. Zhu, M. Hong, D. Guo, J. Shi, Z. Tao and J. Chen, J. Am. Chem. Soc., 2014, 136, 16461–16464 CrossRef CAS PubMed; (e) Y. Chang, K. Yang, P. Wei, S. Huang, Y. Pei, W. Zhao and Z. Pei, Angew. Chem., Int. Ed., 2014, 53, 13126–13130 CrossRef CAS PubMed; (f) B. Cheng and A. E. Kaifer, J. Am. Chem. Soc., 2015, 137, 9788–9791 CrossRef CAS PubMed; (g) R. Joseph, A. Naugolny, M. Feldman, I. M. Herzog, M. Fridman and Y. Cohen, J. Am. Chem. Soc., 2016, 138, 754–757 CrossRef CAS PubMed; (h) M. Ni, N. Zhang, W. Xia, X. Wu, C. Yao, X. Liu, X.-Y. Hu, C. Liu and L. Wang, J. Am. Chem. Soc., 2016, 138, 6643–6649 CrossRef CAS PubMed; (i) J. Yang, L. Shao and G. Yu, Chem. Commun., 2016, 52, 3211–3214 RSC; (j) T. Ogoshi, R. Sueto, K. Yoshikoshi, K. Yasuhara and T.-A. Yamagishi, J. Am. Chem. Soc., 2016, 138, 8064–8067 CrossRef CAS PubMed.
- (a) T. Ogoshi, M. Hashizume, T.-A. Yamagishi and Y. Nakamoto, Chem. Commun., 2010, 46, 3708–3710 RSC; (b) X.-Y. Hu, X. Liu, W. Zhang, S. Qin, C. Yao, Y. Li, D. Cao, L. Peng and L. Wang, Chem. Mater., 2016, 28, 3778–3788 CrossRef.
- Y. Ma, X. Ji, F. Xiang, X. Chi, C. Han, J. He, Z. Abliz, W. Chen and F. Huang, Chem. Commun., 2011, 47, 12340–12342 RSC.
- T. Ogoshi, R. Shiga and T.-A. Yamagishi, J. Am. Chem. Soc., 2012, 134, 4577–4580 CrossRef CAS PubMed.
- (a) Z. Li, J. Yang, G. Yu, J. He, Z. Abliz and F. Huang, Chem. Commun., 2014, 50, 2841–2843 RSC; (b) Z. Li, J. Yang, G. Yu, J. He, Z. Abliz and F. Huang, Org. Lett., 2014, 16, 2066–2069 CrossRef CAS PubMed; (c) J. Yang, X. Chi, Z. Li, G. Yu, J. He, Z. Abliz, N. Li and F. Huang, Org. Chem. Front., 2014, 1, 630–633 RSC; (d) J. Yang, Z. Li, L. Shao and G. Yu, RSC Adv., 2016, 6, 40418–40421 RSC.
- (a) R. Ballardini, V. Balzani, A. Credi, M. T. Gandolfi, S. J. Langford, S. Menzer, L. Prodi, J. F. Stoddart, M. Venturi and D. J. Williams, Angew. Chem., Int. Ed., 1996, 35, 978–981 CrossRef CAS; (b) V. Balzani, A. Credi, S. J. Langford, F. M. Raymo, J. F. Stoddart and M. Venturi, J. Am. Chem. Soc., 2000, 122, 3542–3543 CrossRef CAS; (c) M. A. Cejas and F. M. Raymo, Langmuir, 2005, 21, 5795–5802 CrossRef CAS PubMed; (d) M.-L. Yen, W.-S. Li, C.-C. Lai, I. Chao and S.-H. Chiu, Org. Lett., 2006, 8, 3223–3226 CrossRef CAS PubMed.
- (a) X. Chi and M. Xue, RSC Adv., 2014, 4, 37786–37789 RSC; (b) X. Chi and M. Xue, Chem. Commun., 2014, 50, 13754–13756 RSC.
- Chemical shifts corresponding to Ha, Hb, and Hc in the original solution of WP9 and G1 were not fully recovered (spectra b and d in Fig. 3). Two possible reasons are: (1) the solution was diluted because of the additions of trifluoroacetic acid and hexylamine; (2) the ionic strength of the solution increased because of the production of C6H13NH3+TFA− salt. Both reasons could change the chemical shifts and/or decrease the complexation percentage.
Footnote |
| † Electronic supplementary information (ESI) available: Determination of the association constant, UV-vis data, mass spectrometric data and other materials. See DOI: 10.1039/c6qo00579a |
| This journal is © the Partner Organisations 2017 |