Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unique microstructure of an oil resistant nitrile butadiene rubber/polypropylene dynamically vulcanized thermoplastic elastomer
Nanying Ninga,
Xiangyan Lia,
Hongchi Tiana,
Yueqing Huaa,
Hongli Zuoa,
Pengjun Yao*ab,
Liqun Zhangab,
Youping Wuab,
Guo-Hua Huc and
Ming Tian *ab
*ab
aKey Laboratory of Beijing City on Preparation and Processing of Novel Polymer Materials, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: tianm@mail.buct.edu.cn; yaopj171512@126.com; Fax: +86 10 64433964; Tel: +86 10 64434860
bKey Laboratory of Carbon Fiber and Functional Polymers, Beijing University of Chemical Technology, Beijing 100029, China
cLaboratory of Reactions and Process Engineering, University of Lorraine-CNRS, Nancy, France
First published on 17th January 2017
Abstract
This paper reports on the microstructure, morphological evolution and the properties of oil resistant nitrile butadiene rubber (NBR)/polypropylene (PP) thermoplastic vulcanizates (TPVs) prepared by dynamical vulcanization (DV). The as prepared NBR/PP TPVs exhibited good mechanical properties, good elasticity, easy processability and good oil resistance. Interestingly, the dispersed NBR microparticles in the NBR/PP TPVs were actually the agglomerates of secondary NBR microparticles with a diameter of about 1.7 μm and the phase inversion of the NBR/PP TPVs during DV was dominated by the formation and agglomeration of these secondary NBR microparticles. More interestingly, many PP domains were embedded in the dispersed crosslinked NBR phase, attributed to the chemical reaction between the compatibilizers amine-terminated butadiene-acrylonitrile copolymer (ATBN) and maleic anhydride grafted polypropylene (MP) and the voids among the spherical secondary NBR microparticles. As the DV proceeded, the size of the dispersed NBR agglomerates and the thickness of the PP ligaments in the NBR/PP TPVs decreased, leading to the increase in the density of the NBR agglomerates and the strengthening in the rubber network of the NBR/PP TPVs. As a result, the mechanical properties, the elasticity and the oil resistance of the NBR/PP TPVs were obviously improved as the DV proceeded.
1. Introduction
Thermoplastic vulcanizates (TPVs) are a special class of thermoplastic elastomers (TPEs) prepared by a special polymer reactive blending technique, dynamic vulcanization (DV).1 After DV, a high-content of selectively crosslinked rubber phase of micro- or nano-size is dispersed in a low-content of continuous thermoplastic phase in TPVs.2–4 Thus, TPVs combine the good elasticity of traditional thermoset rubbers and the good melt processability and recyclability of thermoplastics, and have been widely used in automobile, construction, and electronics.5 Nowadays, due to the requirements of resource saving, environmental protection and sustainable development, TPVs are considered as typical “green” polymers to replace the unrecyclable petroleum-based thermoset rubbers and have attracted more and more attention in both academia and industry.6,7Usually, the most important properties of TPVs including the mechanical property, the elasticity and the rheological property all depend on the microstructure of TPVs. Specifically, the content and crosslinking degree of the rubber phase and the blend ratio,8 the size and size distribution of the rubber phase9 and the corresponding rubber network structure,10 the thickness of the plastic ligaments11 and the compatibility between the plastic and the rubber phases12,13 play key roles in these properties of TPVs. A high-content (60 to 80 wt%) of rubber phase is required to obtain good elasticity of TPVs, resulting in a continuous rubber phase in plastic/rubber premix before DV. However, a continuous plastic phase is required to obtain easy processability. Thus, the phase inversion of the rubber phase from continuous phase (in premix) to dispersed phase (in TPVs) is a key to prepare TPVs.14,15 Thus, it is significant to study the microstructure, the formation mechanism and the microstructure–property relationship in order to provide guidance for the morphological control and preparing high-performance TPVs.16–18 Because the ethylene-propylene-diene terpolymer (EPDM)/polypropylene (PP) TPVs is the most widely-used industrialized TPVs products nowadays, most previous studies in the literature were focused on the phase morphology and properties of EPDM/PP TPVs during DV.19–23
Nitrile butadiene rubber (NBR) is well known for its good oil resistance. NBR/polypropylene (PP) TPVs show good mechanical properties, good cold resistance and ozone resistance which are bad in crosslinked NBR.12 What's more, NBR/PP TPVs show good oil resistance and heat resistance. Up to know, some studies have been focused on the preparation, crosslinking, compatibilization and mechanical properties of NBR/PP TPVs.24–27 However, few studies were focused on the microstructure, the formation mechanism and the microstructure–property relationship of NBR/PP TPVs.
In our previous studies, it was found that the dispersed rubber microparticles in miscible rubber/plastic systems such as EPDM/PP TPVs and bromo-isobutylene-isoprene rubber (BIIR)/PP TPVs were actually agglomerates of rubber nanoparticles (40 to 100 nm).28,29 In this study, we aim to find out what is the microstructure and formation mechanism in the immiscible oil resistant NBR/PP TPVs. We deeply studied the microstructure and its formation mechanism, and the microstructure-properties (including mechanical property, elasticity, rheological property and oil resistance) relationship of NBR/PP TPVs during DV to provide guidance for the preparation of high-performance NBR/PP TPVs. We also deeply studied the variation of the crosslinking degree of the rubber phase, the variation of the size of rubber phase and the rubber network, and the phase inversion during DV. To improve the compatibility between NBR and PP maleic anhydride grafted polypropylene (MP) and amine-terminated butadiene-acrylonitrile copolymer (ATBN) were used as compatibilizer.
2. Experimental
2.1. Materials
PP (4220), with melt flow index (230 °C, 2.16 kg) of 0.24 g/10 min, was supplied by Yanshan Petrochemical (China). NBR (230 s), with acrylonitrile content of 35%, was supplied by Nippon (Japan). The compatibilizer maleic anhydride grafted polypropylene (MP) (353 D) was supplied by Dupont (United State) and the compatibilizer amine-terminated butadiene-acrylonitrile copolymer (ATBN) (HYCAR-ATBN 1300 × 16) was provided by Noveon (China). SnCl2·H2O, dimethylol phenolic resin (SP 1045) and pentaerythritol tetrakis 3-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionate (antioxidant 1010) were all commercially available.2.2. Sample preparation
NBR/PP TPVs was prepared by DV in a Haake Rheomix (600 OS internal mixer, Thermo Fisher Scientific, United State) equipped with two counter-rotating rotors. The mass ratio of NBR/ATBN/MP/PP is 60/10/20/10.26 NBR and ATBN were first mixed at room temperature and then mixed with MP and PP in the Haake Rheomix at 180 °C. The curing agents were added into the cooled-down NBR/PP premix at room temperature, resulting in morphology of PP dispersed in NBR matrix. The obtained NBR/PP blend was dynamically vulcanized in the Haake Rheomix at 180 °C with a rotor speed of 80 rpm. Five samples (designated as A, B, C, D and E) with different extents of vulcanization were selected during DV. The compositions of the NBR/PP TPVs were optimized according to our previous study, and are shown in Table 1.| Ingredients | Content of the ingredients |
|---|---|
| NBR | 60 |
| ATBN | 10 |
| PP | 20 |
| MP | 10 |
| 1010 | 0.5 |
| SnCl2·H2O | 1.1 |
| SP 1045 | 5.6 |
2.3. Characterization
3. Results and discussion
3.1. Variation of crosslinking degree in NBR phase during DV
The crosslinking degree of the rubber phase has a significant effect on the phase morphology of TPVs. According to the torque–time curve and the temperature–time curve (see Fig. 1), five samples A to E were selected to study the variation of crosslinking degree during DV. The reciprocal volume swell ratios (1/Q) of the samples were measured to represent the crosslinking degree of the NBR phase during DV.30 A higher 1/Q value represents a higher crosslinking degree. At the initial stage of DV, the NBR/PP blend melted quickly and the torque declines dramatically to a minimum at A, where the crosslinking degree of the NBR phase is low. With the increase in DV time, the rapid crosslinking of the NBR phase leads to the rapid increase in the viscosity of the NBR phase and thus the large increase in the torque until a peak occurs at B. With the further increase in DV time, the crosslinking degree of the NBR phase continues to increase from B to C where the torque declines sharply, indicating the occurrence of phase inversion.19 As the DV further proceeds, the crosslinking degree of the NBR phase and the torque of the NBR/PP blend levels off from C to E, where sample D was selected at 5 min and sample E was selected at 10 min. Thus, the crosslinking of the NBR phase mainly occurs at the early stage of the DV process, consistent well with that reported in previous studies.18,28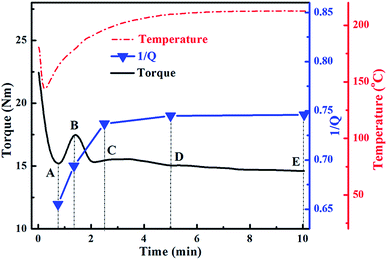 | ||
| Fig. 1 Variations of torque, temperature and reciprocal swell ratio 1/Q with mixing time during DV for NBR/PP TPVs. | ||
3.2. Phase morphology and morphological evolution during DV
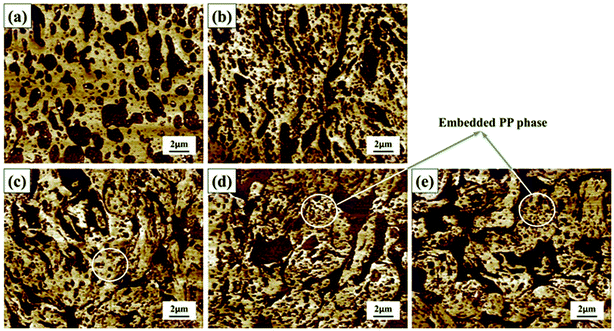
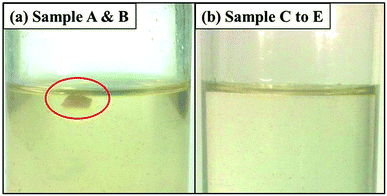
To study the morphological evolution of the NBR/PP TPVs during DV, the phase morphologies of sample A to E were observed by using AFM and the results are shown in Fig. 2. The darker regions represent the PP phase and the lighter regions represent the NBR phase. At the initial state, the PP phase is dispersed in the continuous NBR phase due to the high content and low crosslinking degree of the NBR phase, as shown in Fig. 2(a). The breakup and coalescence of the NBR phase occurs simultaneously under shear.28 As the DV proceeds, the crosslinking degree of the NBR phase increases from A to B (see Fig. 1) and the PP phase in sample B is still dispersed in the continuous NBR phase (see Fig. 2(b)), implying that the NBR phase can coalesce and the phase inversion has not occurred. As the crosslinking degree of the NBR phase further increases to C (see Fig. 1), the viscosity of the NBR phase largely increase15 and the NBR phase can not coalesce but transform into dispersed phase in the continuous PP matrix in sample C (see Fig. 2(c)), indicating the completion of the phase inversion. Here, the diameter of the dispersed NBR phase in sample C is bigger than 30 μm, and the shape of the dispersed NBR is irregular. With the further increase in DV time from C to E, the shape of the dispersed NBR in sample E is still irregular and the diameter of the dispersed NBR phase decreases to about 3–8 μm (see Fig. 2(c)–(e)), leading to the increase in the density of the dispersed NBR phase and the decrease in the thickness of the PP ligaments.18,33 Interestingly, it can be seen in Fig. 2(c)–(e) that many PP domains are embedded in the dispersed crosslinked NBR phase in samples C to E, ascribed to the chemical reaction between the compatibilizers ATBN and MP.12,26To further conform the phase morphologies and the phase inversion of the NBR/PP samples during DV, disintegrating tests were carried out and the photographs of samples A to E immersed in 1,2,4-trichlorobenzene (TCB) at 180 °C for 50 h or less are shown in Fig. 3. It should be noted here that PP can be dissolved in TCB at 180 °C within 10 h while crosslinked NBR does not dissolve in TCB. At the initial state, the PP phase is dispersed in the continuous NBR phase due to the low content of PP. Sample A and B do not disintegrate even after 50 h (see Fig. 3(a)), indicating that the NBR phase in samples A and B is the continuous phase or co-continuous phase and the phase inversion has not occurred. Fig. 3(b) shows that samples C to E are completely disintegrated after immersion in TCB for 20 h (sample C) to 27 h (sample E), respectively, indicating that the NBR phase is dispersed in the continuous PP phase and the phase inversion has completed in sample C. The increase in disintegration time of samples C to E implies that the rubber network in the samples is strengthened as the DV proceeds from C to E.
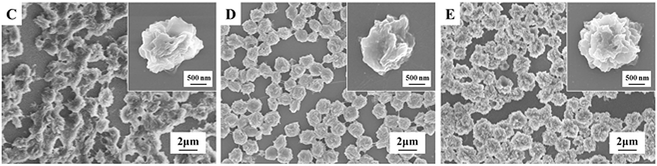
To investigate the dispersed NBR particles in samples C to E, the morphologies of the totally disintegrated samples were observed by using SEM and the micrographs are shown in Fig. 4. It can be seen that the dispersed NBR particles in samples C to E are all single spherical microparticles with a diameter of about 1.7 μm and the size of the microparticles is almost identical. The surfaces of these NBR microparticles are rough, ascribed to the chemical reaction between the compatibilizers ATBN and MP, as previously reported.12,26 Interestingly, the sizes of the NBR microparticles in the SEM micrographs of these samples are much smaller than that in AFM micrographs (Fig. 2) and the shape is more regular.
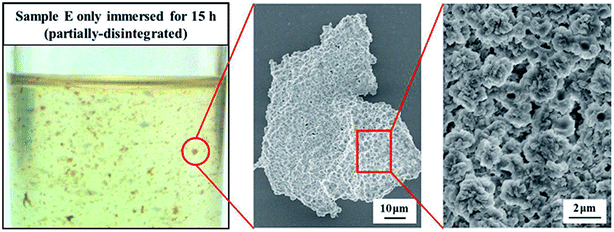
To further confirm the size of the dispersed NBR phase, sample E was partially disintegrated after immersed in TCB at 180 °C for 15 h. The photograph and SEM micrographs of partially disintegrated sample E were taken and the results are shown in Fig. 5. It can be seen that the dispersed NBR phase in the NBR/PP TPVs is actually the agglomerate of smaller NBR microparticles (called secondary NBR microparticles) with a diameter of about 1.7 μm, and the shape and size of these secondary NBR microparticles observed in the NBR agglomerate are similar with that in Fig. 4. The results are similar with that reported in our previous studies on EPDM/PP TPVs, in which EPDM microparticles are formed by agglomerates of EPDM nanoparticles.18,28 The difference is that the diameters of the EPDM particles range from 40–60 nm while the diameters of the secondary NBR microparticles are about 1.7 μm. In addition, the voids among the spherical secondary NBR microparticles results in the formation of embedded PP domains in NBR agglomerates.
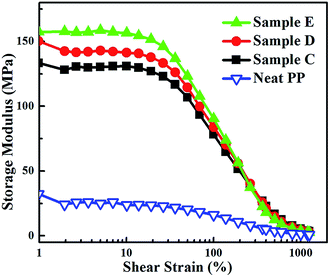
As previously reported, the rubber particles with high crosslinking degree and elastic modulus can be regarded as fillers.28 The phase inversion in the NBR/PP samples during DV indicates that samples C to E are TPVs where the NBR particles with high crosslinking degree are dispersed in the PP matrix. Thus, the rubber networks of samples C to E were characterized by the change in storage modulus with the increase in shear strain using rubber process analyzer (RPA), and the results are shown in Fig. 6. For comparison, neat PP was also characterized by using RPA. The change between the maximum and the minimum of the storage modulus G′ (ΔG′) represents the rubber network and the higher ΔG′ implies the stronger rubber network.34,35 The ΔG′ at the same shear strain increases as the DV proceeds from C to E, indicating the strengthening of the rubber network. The strengthening of the rubber network is consistent well with the AFM results and the disintegrating results, demonstrating the increase in the density of the dispersed NBR phase and the decrease in the thickness of PP ligaments. The rubber network has significant effect on the mechanical property, elasticity, and rheological property of the NBR/PP TPVs, which will be discussed later.
3.3. Mechanism for the formation of phase morphology
A schematic illustration of the morphological evolution in NBR/PP TPVs during DV was proposed in Fig. 7 to show the formation of the secondary NBR microparticles and the variation of the NBR agglomerates, which play an important role in the properties of TPVs. The yellow regions represent the NBR phase and the blue regions represent the PP phase. At the initial state, the PP phase is dispersed in the NBR phase due to the high content of the NBR phase, as is shown in Fig. 7(i). Many NBR domains are embedded in the dispersed PP phase, ascribed to the chemical reaction between the compatibilizers ATBN and MP, as previously reported.12,26 As the DV begins, the NBR phase can be broken up into a large number of NBR microdroplets under shear, as demonstrated in our previous study.18,28,29 Because of the inhomogeneity of the crosslinking degree, some of the NBR microdroplets with low crosslinking degrees can still coalesce while others are in situ vulcanized and transform into NBR microparticle agglomerates, as is shown in Fig. 7(ii). With the increase in the crosslinking degree, all the NBR microdroplets cannot coalesce but dispersed in the PP phase as NBR microparticles agglomerates, and the phase inverses, as is shown in Fig. 7(iii). Thus, the phase inversion in the NBR/PP TPVs depends on the formation and agglomeration of secondary NBR microparticles formed by the breakup and in situ vulcanization of NBR microdroplets at the early stage of the DV. And the voids among the spherical secondary NBR microparticles results in the formation of embedded PP domains in NBR agglomerates. With the further increase in DV time, the size of the NBR agglomerates decreases, resulting in the increase in the number of NBR agglomerates and the decrease in the thickness of the PP ligaments, as is shown in Fig. 7(iv). The mechanism of the morphological evolution of NBR/PP TPVs during DV is obviously different from that of EPDM/PP TPVs and BIIR/PP TPVs, and BIIR/Nylon-12 TPVs etc.18,28,29,31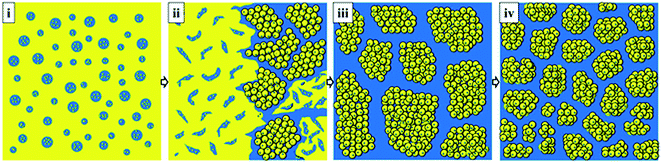 | ||
| Fig. 7 Schematic illustration of morphological evolution of NBR/PP TPVs during DV (the yellow regions represent PP phase and the blue regions represent the NBR phase). | ||
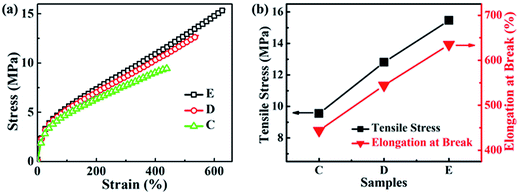
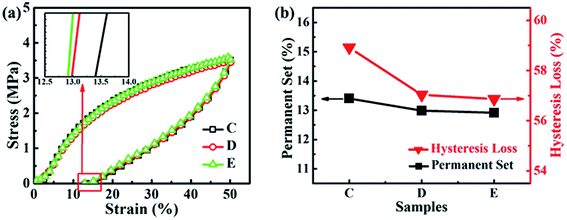
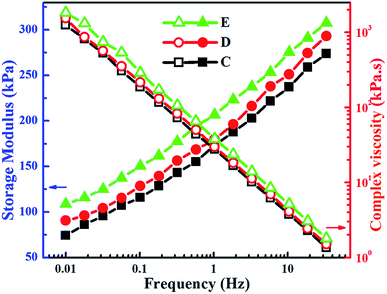
3.4. Properties of NBR/PP TPVs
The phase morphology and morphological evolution, especially the size of the dispersed rubber phase, have significant effects on the properties of TPVs.11 Thus, the properties including the mechanical property, the elasticity, the rheological property and the oil resistant, and the microstructure–property relationships of the samples C to E were studied in order to provide guidance for the preparation of high-performance NBR/PP TPVs for its application in oil-resistant products.| Changes | PP | NBR | C | D | E |
|---|---|---|---|---|---|
| ΔW, % | +10.6 | +2.2 | +5.6 | +4.9 | +4.5 |
| ΔV, % | +10.4 | +2.5 | +5.3 | +4.8 | +4.5 |
4. Conclusions
We successfully prepared NBR/PP TPVs by DV and studied the microstructure, the morphological evolution during DV and the properties of the NBR/PP TPVs. The corresponding mechanism for the formation and evolution of the microstructure was proposed based on the crosslinking degree of the rubber phase. The results indicate that the dispersed NBR microparticles in the NBR/PP TPVs are actually the agglomerates of secondary NBR microparticles with a diameter of about 1.7 μm formed by the breakup and in situ vulcanization of NBR microdroplets at the early stage of the DV. The phase inversion of the NBR/PP TPVs during DV is dominated by the formation and agglomeration of these secondary NBR microparticles, similar with those observed in EPDM/PP TPVs and BIIR/PP TPVs. Interestingly, many PP domains are embedded in the dispersed crosslinked NBR phase, ascribed to the chemical reaction between the compatibilizers ATBN and MP and the voids among the spherical secondary NBR microparticles. As the DV proceeds, the size of the dispersed NBR agglomerates and the thickness of the PP ligaments in NBR/PP TPVs decrease, leading to the increase in the density of the NBR agglomerates, the strengthening in the rubber network and the increase in the crystallinity of the NBR/PP TPVs. As a result, the rheological property is slightly deteriorated, and the mechanical property, the elasticity and the oil resistance are obviously improved as the DV proceeds.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (Grant No. 51525301, 51673014, 51521062) for financial supports.References
- S. S. Banerjee and A. K. Bhowmick, Polymer, 2015, 57, 105–116 CrossRef CAS.
- A. Thitithammawong, C. Nakason, K. Sahakaro and J. W. M. Noordermeer, Eur. Polym. J., 2007, 43, 4008–4018 CrossRef CAS.
- N. Ning, L. Hu, P. Yao, H. Wu, J. Han, L. Zhang, H. Tian and M. Tian, J. Appl. Polym. Sci., 2015, 133 DOI:10.1002/app.43043.
- J. Oderkerk and G. Groeninckx, Polymer, 2002, 43, 2219–2228 CrossRef CAS.
- C. Antunes, A. Machado and M. Van Duin, Rubber Chem. Technol., 2009, 82, 492–505 CrossRef CAS.
- K. Lu, M. van Duin, J. Loos and G. de With, Polymer, 2012, 53, 4171–4177 CrossRef CAS.
- A. Nicolini, T. L. Á. de Campos Rocha and M. A. Maldaner Jacobi, J. Appl. Polym. Sci., 2008, 109, 3093–3100 CrossRef CAS.
- M. D. Ellul, A. H. Tsou and W. Hu, Polymer, 2004, 45, 3351–3358 CrossRef CAS.
- K. Premphet and W. Paecharoenchai, J. Appl. Polym. Sci., 2002, 85, 2412–2418 CrossRef CAS.
- L. Wang, N. Ning, L. Zhang, Y. Lu, M. Tian and T. Chan, Composites, Part A, 2013, 47, 135–142 CrossRef CAS.
- M. Roy, M. van Duin, A. B. Spoelstra and J. G. Goossens, Soft Matter, 2010, 6, 1758–1768 RSC.
- M. Tian, J. Han, H. Zou, H. Tian, H. Wu, Q. She, W. Chen and L. Zhang, J. Polym. Res., 2012, 19, 1–13 CrossRef.
- J. D. Van Dyke, M. Gnatowski and A. Burczyk, J. Appl. Polym. Sci., 2008, 109, 1535–1546 CrossRef CAS.
- J.-H. Wu, C.-H. Li, H.-T. Chiu and Z.-J. Shong, J. Appl. Polym. Sci., 2008, 108, 4114–4121 CrossRef CAS.
- C. Antunes, M. Van Duin and A. Machado, Polym. Test., 2011, 30, 907–915 CrossRef CAS.
- S. Shahbikian, P. J. Carreau, M. C. Heuzey, M. D. Ellul, J. Cheng, P. Shirodkar and H. P. Nadella, Polym. Eng. Sci., 2012, 52, 309–322 CAS.
- G. Martin, C. Barrès, P. Sonntag, N. Garois and P. Cassagnau, Eur. Polym. J., 2009, 45, 3257–3268 CrossRef CAS.
- H. Wu, M. Tian, L. Zhang, H. Tian, Y. Wu, N. Ning and T. W. Chan, ACS Sustainable Chem. Eng., 2014, 3, 26–32 CrossRef.
- C. F. Antunes, A. V. Machado and M. van Duin, Eur. Polym. J., 2011, 47, 1447–1459 CrossRef CAS.
- C. F. Antunes, M. van Duin and A. V. Machado, Mater. Chem. Phys., 2012, 133, 410–418 CrossRef CAS.
- F. Goharpey, A. Katbab and H. Nazockdast, J. Appl. Polym. Sci., 2001, 81, 2531–2544 CrossRef CAS.
- F. Goharpey, A. Katbab and H. Nazockdast, Rubber Chem. Technol., 2003, 76, 239–252 CrossRef CAS.
- A. V. Machado, C. F. Antunes and M. van Duin, in Novel Trends in Rheology Iv, AIP Conference Proceedings, ed. M. Zatloukal, 2011, p. 1375, DOI:10.1063/1.3604484.
- B. G. Soares, M. de Oliveira, D. Meireles, A. S. Sirqueira and R. S. Mauler, J. Appl. Polym. Sci., 2008, 110, 3566–3573 CrossRef CAS.
- J. Pan, H. Hu, Z. Huang and Y. Duan, Polym.-Plast. Technol. Eng., 2001, 40, 593–604 CrossRef CAS.
- M. Tian, J. Han, H. Wu, H. Tian, Q. She, W. Chen and L. Zhang, J. Appl. Polym. Sci., 2012, 124, 1999–2006 CrossRef CAS.
- W.-c. Lee and A. T. DiBenedetto, Polymer, 1993, 34, 684–690 CrossRef CAS.
- H. Wu, M. Tian, L. Zhang, H. Tian, Y. Wu and N. Ning, Soft Matter, 2014, 10, 1816–1822 RSC.
- P. Yao, H. Wu, N. Ning, L. Zhang, H. Tian, Y. Wu, G.-H. Hu, T. W. Chan and M. Tian, RSC Adv., 2016, 6, 11151–11160 RSC.
- N. Vennemann, K. Bökamp and D. Bröker, Macromol. Symp., 2006, 245(1), 641–650 CrossRef.
- P. Yao, H. Wu, N. Ning, L. Zhang, H. Tian, Y. Wu, G.-H. Hu, T. W. Chan and M. Tian, RSC Adv., 2016, 6, 30004–30013 RSC.
- S. Chattopadhyay, T. Chaki and A. K. Bhowmick, J. Appl. Polym. Sci., 2001, 79, 1877–1889 CrossRef CAS.
- S. Wu, J. Appl. Polym. Sci., 1988, 35, 549–561 CrossRef CAS.
- A. R. Payne, J. Appl. Polym. Sci., 1962, 6, 368–372 CrossRef CAS.
- J. Fröhlich, W. Niedermeier and H.-D. Luginsland, Composites, Part A, 2005, 36, 449–460 CrossRef.
- C. Rajesh, G. Unnikrishnan, E. Purushothaman and S. Thomas, J. Appl. Polym. Sci., 2004, 92, 1023–1030 CrossRef CAS.
- J. Oderkerk, G. de Schaetzen, B. Goderis, L. Hellemans and G. Groeninckx, Macromolecules, 2002, 35, 6623–6629 CrossRef CAS.
- M. C. Boyce, O. Yeh, S. Socrate, K. Kear and K. Shaw, J. Mech. Phys. Solids, 2001, 49, 1343–1360 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |