Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A comparative study of manganese–cerium doped metal–organic frameworks prepared via impregnation and in situ methods in the selective catalytic reduction of NO
Xiao Zhangab,
Boxiong Shen *a,
Xiaoqi Zhanga,
Fumei Wanga,
Guilong Chib and
Meng Sia
*a,
Xiaoqi Zhanga,
Fumei Wanga,
Guilong Chib and
Meng Sia
aSchool of Energy and Environmental Engineering, Hebei University of Technology, Tianjin, China. E-mail: shenbx@nankai.edu.cn; Fax: +86-022-60435784; Tel: +86-022-60435784
bCollege of Environmental Science and Engineering, Nankai University, Tianjin, China
First published on 17th January 2017
Abstract
Mn and Ce were loaded on metal–organic frameworks (MOFs) via impregnation and in situ doping methods. The catalytic capacities of the obtained composite materials were evaluated in the selective catalytic reduction (SCR) of NO. The existing form of Mn–Ce in the MOF originates from different doping methods and its effect on the catalytic performance was investigated. Mn–Ce introduced by impregnation was deposited on the surface of the MOF and exhibited high catalytic efficiency of more than 98% from 200 °C to 300 °C. According to the results of BET, XRD, XPS, and ICP analyses, it was concluded that Mn–Ce introduced via the in situ doping method was inserted in the crystal lattice structure of the MOF, which resulted in an enlarged surface area, low Mn concentration, and poor redox property as compared to that introduced via the impregnated method. By exploring these factors, it was proven that the limited redox ability was the direct reason that resulted in the low activity of the MnCeMOF. Using thermal decomposition, the in situ doped Mn–Ce was liberated from the MnCeMOF crystal lattice and subsequently, exhibited recovered redox properties and catalytic activity. In this study, we proved that different doping methods lead to different forms of Mn–Ce in the MOF, which exhibit different redox properties and thus directly lead to different catalytic performance.
1. Introduction
Metal–organic frameworks (MOFs) are compounds consisting of metal ions or clusters coordinated to organic ligands.1 The advantages of high porosity, large specific surface areas, and versatility of their structures make MOFs very attractive in many fields such as separation, gas adsorption, and catalysis.2 Recently, the synthesis of metal oxides supported on MOFs for catalytic applications has gained significant attention.3 Impregnation and in situ doping are the two most common strategies used in catalyst preparation. Henschel et al.4 reported that the palladium particles incorporated into MOFs via incipient wetness impregnation exhibited significantly higher catalytic activity than that of the catalyst comprising palladium supported on activated carbon during the hydrogenation reaction. Han et al.5 proved that the impregnation of MOF with cobalt followed by a mild heat treatment exhibited a significant improvement in the catalytic activity when compared with that of cobalt oxide or cobalt oxide supported on silica nanospheres. In situ doping is another method to prepare metal–MOF composites. The incorporation of various metals ions, such as Mn2+, Li+, Na+, and K+ into an MOF structure via an in situ doping method has been reported.6,7 Ebrahim et al.8 reported a feasible way for the in situ doping of Ce in an MOF using Ce as a co-metal precursor in the synthetic process. Due to the redox properties of the in situ doped Ce, the material exhibited a high affinity for nitrogen oxides. The effectiveness of Ce in situ doped chromium-based MOFs in the catalytic H2 production was also reported by Wen et al.9Nitric oxide (NO) is known to cause environmental pollution, such as photochemical smog, acid rain, ozone depletion, and greenhouse effects.10 Coal-fired power plants are considered as important sources of NO emission and the selective catalytic reduction (SCR) of NO with NH3 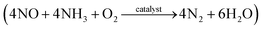 has been proven to be one of the most effective approaches for NO removal.11–13 Many transition metal oxides have been developed as NO-reduction catalysts. Among these, manganese oxides exhibit a significant enhancement in NO conversion at low temperatures.14–16 Cerium oxides have also been proven to be very effective during NO conversion due to their excellent redox behavior.16–18 Moreover, a synergistic improvement between Mn and Ce has been found in the mixed Mn–Ce catalysts.19–22 Various supporting materials, including carbon nanotubes,23 pillared-clay,20 glass-fiber,24 and zirconium oxide,25 have been employed for Mn–Ce loading and have exhibited high catalytic performance.
has been proven to be one of the most effective approaches for NO removal.11–13 Many transition metal oxides have been developed as NO-reduction catalysts. Among these, manganese oxides exhibit a significant enhancement in NO conversion at low temperatures.14–16 Cerium oxides have also been proven to be very effective during NO conversion due to their excellent redox behavior.16–18 Moreover, a synergistic improvement between Mn and Ce has been found in the mixed Mn–Ce catalysts.19–22 Various supporting materials, including carbon nanotubes,23 pillared-clay,20 glass-fiber,24 and zirconium oxide,25 have been employed for Mn–Ce loading and have exhibited high catalytic performance.
Although Mn–Ce-based catalysts used for the SCR of NO have been previously studied by researchers, the preparation of Mn–Ce and MOF composites via impregnation or in situ doping methods for the SCR of NO has not been reported. In this study, Mn–Ce species were loaded on the MOF via in situ doping or impregnation methods and their catalytic capabilities were evaluated in the SCR of NO reaction. It is very interesting to investigate the effect of the impregnation and in situ doping methods on the formation of the Mn–Ce catalyst and consequently, their influence on the catalytic performance.
In a pioneering work, Cavka et al. published the synthesis of the metal–organic framework UiO-67 (Zr6O4(OH)4(C12H10(CO2)2)6(DMF)x(H2O)y), which was composed of Zr clusters and linear 4,4′-biphenyldicarboxylic acid ligands.26 UiO-67 has proved to be a very promising candidate as a catalyst support due to its highly developed three-dimensional pore systems and high thermal, chemical, and mechanical stabilities.27,28 Therefore, in this study, UiO-67 was selected as the supporting MOF for Mn–Ce doping.
2. Experimental
2.1 Materials
Zirconium tetrachloride (ZrCl4), 4,4′-biphenyl-dicarboxylate (BDPC, 98.9%), cerium nitrate hexahydrate (Ce(NO3)3·6H2O, 99.5%), and manganese nitrate (Mn(NO3)2, 50 wt% solution in H2O) were obtained from Beijing J&K Co., Ltd. (Beijing, China). N,N-Dimethylformamide (DMF, 99%) and dichloromethane (DCM, 99%) were obtained from Tianjin Guangfu Reagent Co. Ltd. All chemicals were used without further purification.2.2 Preparation of the catalysts
2.3 Characterization
Powder X-ray diffraction (PXRD) patterns were obtained over the range of 5–80° (2θ) using a Bruker AXS GmbH X-ray diffractometer (Bruker D8 FOCUS, Germany) with a Cu Kα radiation. The textural characteristics of the samples were determined using N2 adsorption at −196 °C using an ASAP 2020/Tristar 3000 system (Micromeritics, America). The specific surface areas were calculated using the BET method and the specific pore volumes were calculated using the BJH method. The concentrations of the active species in the catalysts were determined by inductively coupled plasma optical emission spectrometry (ICP-OES; Varian Inc.). X-ray photoelectron spectroscopy (XPS) was performed using a Kratos Axis Ultra DLD spectrometer (Kratos Analytical, UK) operating at 10–9 Pa with an Al Kα radiation (1486.6 eV). H2-TPR was conducted using a PCA-1200 chemisorption analyzer (Builder, China). In the H2-TPR experiment, 50 mg of the catalyst was preheated at 150 °C for 1.5 h under N2 to remove the moisture in the sample. After cooling down to room temperature, the temperature programming was started with a 5 °C min−1 heating rate under 30 mL min−1 H2 (5 vol%)/N2.2.4 Catalytic activity test
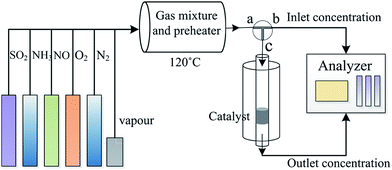
The catalytic activity of the catalysts was studied using a fixed-bed system (Fig. 1). The simulated flue gas included 500 ppm NO, 500 ppm NH3, 5% O2, and pure N2. In all the runs, the total gas flow rate was maintained at 450 mL min−1 over 0.5 g catalyst (with a gas hourly space velocity of 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1). The NO concentration in the inlet and outlet of the reactor was monitored using a Flue Gas Analyzer (KM940, Kane 100 international limited, UK). The NO conversion was calculated using the following equation:
000 h−1). The NO concentration in the inlet and outlet of the reactor was monitored using a Flue Gas Analyzer (KM940, Kane 100 international limited, UK). The NO conversion was calculated using the following equation:| NO conversion = (NOin − NOout)/NOin × 100% |
The influence of water vapor and SO2 on the NO conversion was studied by introducing 3% water vapor and 200 ppm SO2 into the flue gas.
3. Results and discussion
3.1 Catalytic performance of the catalysts
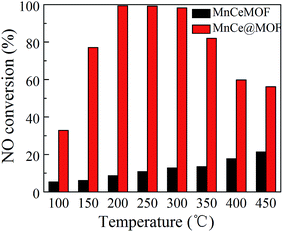
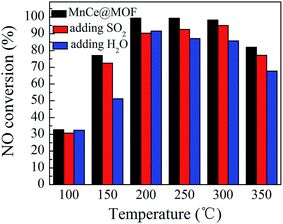
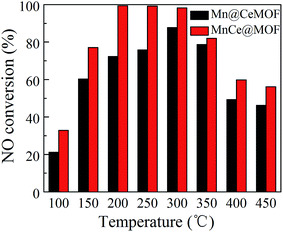
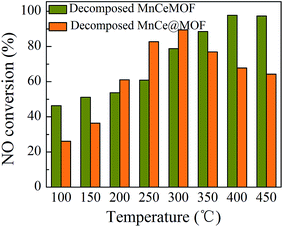
NO conversions in the temperature range of 100–450 °C for the SCR reaction over MnCeMOF and MnCe@MOF are shown in Fig. 2. It shows that the Mn–Ce impregnated catalyst MnCe@MOF exhibited a high activity with NO conversions of over 98% in the temperature range of 200–300 °C. The catalytic activity significantly decreased as the temperature was increased over 350 °C for MnCe@MOF, which could be attributed to the side reaction of ammonia where it reacts with oxygen to yield nitrogen oxides above 350 °C.29 Considering that traces of SO2 and moisture may exist in the flue gas, the effects of SO2 and H2O on the NO conversion over MnCe@MOF were investigated. As shown in Fig. 3, the addition of 200 ppm SO2 or 3% H2O to the flue gas resulted in a decrease in the NO removal performance. The prohibitive effect of H2O on NO conversion could be attributed to the competitive adsorption with the reactant and occupation of the active sites on the catalyst.30–32 The prohibitive effect of SO2 on NO conversion might be due to the reaction of SO2 with the active species on the surface of the catalyst, which inhibit the catalyst activity.33,34 Another reason for the prohibitive effect of SO2 may be the formation of ammonium sulfate or ammonium bisulfate on the surface of the catalyst. The prohibitive effect of SO2 or H2O was not significant, which indicated that the MnCe@MOF catalyst performed with an acceptable tolerance to SO2 and H2O in the SCR of NO. Therefore, via the impregnation method, the MOF was demonstrated to be a potential supporting material for the MnCe-based SCR catalysts.In the previous studies, it has been proven that in situ doped metals show catalytic properties.8,9 However, the in situ doped MnCeMOF showed a very low catalytic ability of below 20% in the SCR of NO (Fig. 2). It is very interesting to analyze the reasons for the low efficiency of MnCeMOF in the catalytic reaction. The mechanisms of different doping methods on the catalytic behavior will also be explored on the basis of the following characterization results.
3.2 Characterization of the catalysts
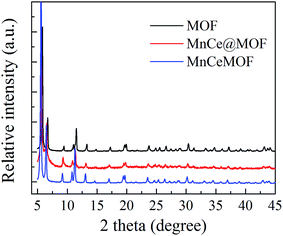
The XRD pattern of MnCeMOF was similar to that of the undoped MOF, indicating that doping the MOF with Mn–Ce did not alter the main octahedron structure of the material. However, XRD not only provides information on the crystalline structure but also some clue on the location and interactions between the doped Mn–Ce and the MOF matrix. Note that the strength of the peaks in the MnCeMOF pattern obviously increased and the maximum characteristic peaks in the MnCeMOF pattern (2θ = 5.6°, 6.5°, and 11.3°) shifted to a lower angle as compared to those of the undoped MOF pattern. This indicates that the in situ doped Mn–Ce affects the crystalline structure of the MOF.
| Undoped MOF | MnCeMOF | MnCe@MOF | Decomposed MnCe@MOF | Decomposed MnCeMOF | Mn@CeMOF | CeMOF | |
|---|---|---|---|---|---|---|---|
| BET surface area (m2 g−1) | 1376.95 | 2491.45 | 1048.41 | 113.63 | 30.95 | 1894.20 | 2252.95 |
| Pore volume (cm3 g−1) | 0.64 | 1.28 | 0.53 | 0.32 | 0.089 | 0.87 | 1.01 |
| Average pore width (nm) | 1.87 | 2.07 | 2.01 | 11.29 | 11.48 | 1.84 | 1.89 |
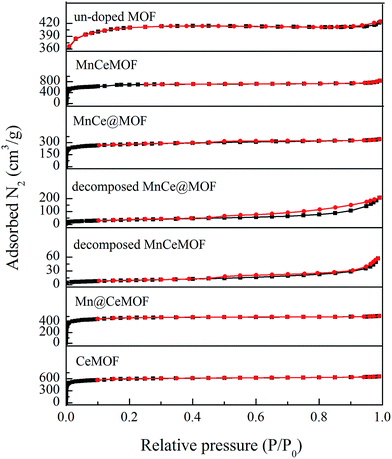
The BET surface area of a catalyst support is an important factor that affects the catalytic performance. A high surface area of the support can significantly enhance the catalytic activity of the metal oxides due to the advantages such as promoting the dispersion, avoiding metal oxide aggregation, and promoting charge transfer.5,23 The geometrical features and high BET surface area of the MOF are very likely to contribute to the desirable catalytic performance of MnCe@MOF. However, the MnCeMOF with a higher surface area exhibited low catalytic performance (Fig. 2). This indicates that the BET surface area was not the only factor that influenced the activity of the catalysts. The distinct difference of the impregnated and in situ doped materials in the catalytic behavior may be caused by other more important factors.
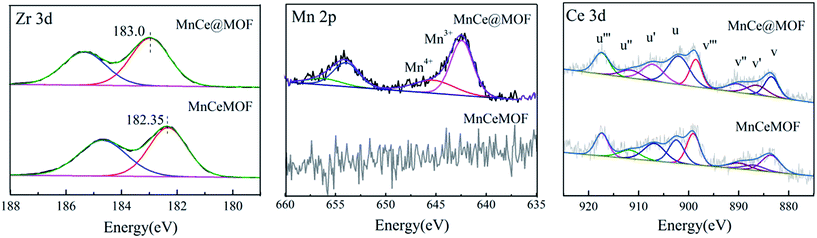
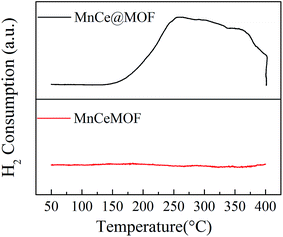
In the Mn spectrum for MnCe@MOF, two main peaks appeared representing Mn 2p1/2 and Mn 2p3/2. The spectra were divided into the Mn4+ peak (∼642.7 eV) and Mn3+ peak (∼641.2 eV) by peak-fitting deconvolution, which indicated that Mn2O3 and MnO2 were the major phases of Mn in MnCe@MOF. Moreover, no Mn was detected in the spectrum of the MnCeMOF sample, which may be because Mn was inserted in MnCeMOF or the concentration of Mn in MnCeMOF was below the detection limit of the instrument. The Ce spectra were divided and the sub-bands were denoted as “v” and “u”, which correspond to the Ce 3d5/2 and Ce 3d3/2 spin–orbit components. The v′ and u′ bands ascribed to Ce3+ and other six peaks represented the Ce4+ species on the surface.37 The ratio of Ce3+/Ce4+ was determined to be 22.6/77.4 for MnCe@MOF and 38.9/71.1 for MnCeMOF. The presence of Ce3+ on the surface is considered to create oxygen vacancies and facilitate the adsorption of oxygen species and, therefore, improves the redox activity in the SCR of NO.32 Considering this, the speciation of Ce in the MnCeMOF seemed more favorable for the catalytic reaction; however, MnCe@MOF displayed higher activity, indicating that there were other factors that affect the activity of the catalysts.
| MnCeMOF | MnCe@MOF | Mn@CeMOF | |
|---|---|---|---|
| Ce (%) | 4.98 | 4.92 | 4.79 |
| Mn (%) | 1.62 | 5.50 | 5.43 |
To verify this speculation, a Mn@CeMOF catalyst (impregnated Mn on the Ce in situ doped MOF) was prepared and compared with MnCe@MOF. The XRD pattern of Mn@CeMOF was similar to that of the undoped MOF, indicating that the synthesized Mn@CeMOF exhibited the characteristic octahedron structure. The physical properties of Mn@CeMOF and CeMOF were also analyzed by N2 adsorption (Table 1 and Fig. 6). The N2 adsorption measurement of Mn@CeMOF and CeMOF showed a type I property, suggesting that the microporous nature was dominant in the materials. Based on the discussion in our study, in situ doping led to an increase in the BET surface area and enlarged the pores, which might be due to the expansion of the MOF crystal. However, impregnation results in a decrease in the surface area due to the deposition of the impregnated metal and plugging of the pores in the MOF. Therefore, the surface area of Mn@CeMOF was higher than that of the undoped MOF but lower than that of the CeMOF. From the ICP results, the Mn and Ce contents between Mn@CeMOF and MnCe@MOF were basically the same, which was consistent with our designation. However, the results of the NO conversion tests showed that the catalytic activity of Mn@CeMOF was lower than that of MnCe@MOF (Fig. 8). This indicates that the content of Mn–Ce was not a key factor that leads to the different catalytic efficiencies between the in situ doped MnCeMOF and impregnated MnCe@MOF.
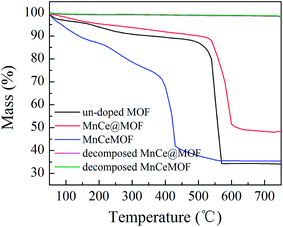
3.3 Comparative experiment results
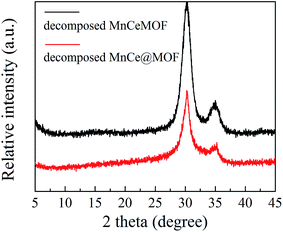
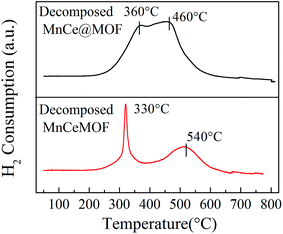
From the abovementioned experimental and characterization results, it was hypothesized that MnCeMOF exhibits low catalytic activity due to the Mn–Ce present in the MOF lattice possessing limited redox properties. To verify this assumption, Mn–Ce was liberated from the crystal lattice by heating the materials and a comparative experiment was performed. By the thermolysis of a MOF, metal oxides species could be obtained by the loss of organic ligands at high temperatures.39–41 TGA studies were conducted under N2 and the heating rate was set at 20 °C min−1. The TGA curves (Fig. 10) for the undoped MOF, MnCe@MOF, and MnCeMOF exhibited a similar trend. The curves revealed three steps that are mainly attributed to water and solvent removal, framework collapse with the decomposition of organic species, and the formation of inorganic oxides. It was observed that the structural collapse temperature for MnCeMOF was lower than that for the undoped MOF. This might be because Mn–Ce doping inhibited the complete growth of the MOF and led to crystal defects, which results in the decreased thermal stability of the framework. The TGA results indicate that the catalysts were stable when used below 400 °C. Moreover, based on the TGA curve obtained for the MOF, the thermal decomposition temperature was set at 600 °C for the preparation of the thermally decomposed samples. The catalytic performance and structural properties of the thermally decomposed MnCeMOF as well as the thermally decomposed MnCe@MOF were investigated.Moreover, the decomposed MnCe@MOF exhibited a broad H2 consumption peak in the temperature range from 250 to 550 °C, which could be divided into two peaks at 360 °C and 460 °C that are related to the reduction of surface Mn4+ to Mn3+ and Ce4+ to Ce3+, respectively.19,44 Note that that the Ce spectrum of the decomposed MnCeMOF shifted to a higher temperature when compared to that of the decomposed MnCe@MOF. Fallya et al.45 reported that the H2-peak of Ce oxides moved to a higher temperature when mixed with zirconium oxides. The shift in the CeO2 transition temperature in the decomposed MnCeMOF may indicate that there was interaction between Zr and Ce in the original MnCeMOF.
In addition, by comparing the decomposed and original impregnated MnCe@MOF, it was found that the decomposed sample showed a much lower catalytic efficiency. A support with a high BET surface area can effectively generate more surface active sites and promote charge transfer, which is beneficial for the catalytic performance.21,46 Based on the XRD and N2 adsorption results, it is most likely that the decreased catalytic efficiency of the decomposed sample can be attributed to the crushed special structure and the decreased surface area of the catalyst. From the comparative experimental results, the MOF was proven to be a potential supporting material for the impregnated Mn–Ce catalysts.
4. Conclusion
Mn–Ce doped MOF composites were synthesized via impregnation and in situ doping methods, and their catalytic capabilities were investigated in the SCR of NO. The results showed that the MnCe@MOF catalyst prepared via the impregnation method showed desirable NO conversion efficiency, whereas the MnCeMOF prepared via the in situ doping method exhibited low activity in the catalytic reaction. The characterization results indicated that Mn and Ce were spread on the surfaces of MnCe@MOF and displayed redox properties. The MOF was demonstrated to be a potential supporting material for the impregnated MnCe-based SCR catalyst. Using in situ doping, Mn–Ce was inserted into the crystal lattice structure of the MOF, which resulted in an enlarged surface area, low Mn concentration, and poor redox properties when compared with those of the impregnated material. Based on a comparative study, it was proved that the poor redox ability of the in situ doped Mn–Ce was the main factor that decided the activity of the catalyst. Moreover, this assumption was supported by the fact that upon the liberation of Mn and Ce from the crystal lattice, the decomposed MnCeMOF exhibited recovered redox properties and a high catalytic capability. In this study, we have proven that different doping methods can lead to different forms of Mn–Ce in the MOF, which exhibit different redox properties and directly lead to different catalytic performances in the SCR of NO.Acknowledgements
The project was supported by the National Natural Science Foundation of China (51541602) and Natural Science Foundation of Hebei Province (E2016202361).References
- J. L. C. Rowsell and O. M. Yaghi, Microporous Mesoporous Mater., 2004, 73, 3–14 CrossRef CAS.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- Y. Zhang, Y. Zhou, Y. Zhao and C.-J. Liu, Catal. Today, 2016, 263, 61–68 CrossRef CAS.
- A. Henschel, K. Gedrich, R. Kraehnert and S. Kaskel, Chem. Commun., 2008, 4192–4194 RSC.
- J. Han, D. Wang, Y. Du, S. Xi, J. Hong, S. Yin, Z. Chen, T. Zhou and R. Xu, J. Mater. Chem. A, 2015, 3, 20607–20613 CAS.
- M. Šimėnas, A. Ciupa, M. Mączka, A. Pöppl and J. R. Banys, J. Phys. Chem. A, 2015, 119, 24522–24528 Search PubMed.
- C.-L. Chu, J.-R. Chen and T.-Y. Lee, Int. J. Hydrogen Energy, 2012, 37, 6721–6726 CrossRef CAS.
- A. M. Ebrahim and T. J. Bandosz, ACS Appl. Mater. Interfaces, 2013, 5, 10565–10573 CAS.
- M. Wen, Y. Kuwahara, K. Mori, D. Zhang, H. Li and H. Yamashita, J. Mater. Chem. A, 2015, 3, 14134–14141 CAS.
- T. Castro, S. Madronich, S. Rivale, A. Muhlia and B. Mar, Atmos. Environ., 2001, 35, 1765–1772 CrossRef CAS.
- L. S. Wang, B. C. Huang, Y. X. Su, G. Y. Zhou, K. L. Wang, H. C. Luo and D. Q. Ye, Chem. Eng. J., 2012, 192, 232–241 CrossRef CAS.
- G. Liu and P.-X. Gao, Catal. Sci. Technol., 2011, 1, 552–568 CAS.
- S. Roy, M. S. Hegde and G. Madras, Appl. Energy, 2009, 86, 2283–2297 CrossRef CAS.
- D. K. Pappas, T. Boningari, P. Boolchand and P. G. Smirniotis, J. Catal., 2016, 334, 1–13 CrossRef CAS.
- Z. Chen, F. Wang, H. Li, Q. Yang, L. Wang and X. Li, Ind. Eng. Chem. Res., 2012, 51, 202–212 CrossRef CAS.
- W. Y. Yao, Y. Liu, X. Q. Wang, X. L. Weng, H. Q. Wang and Z. B. Wu, J. Phys. Chem. C, 2016, 120, 221–229 CAS.
- Z. Si, D. Weng, X. Wu, J. Yang and B. Wang, Catal. Commun., 2010, 11, 1045–1048 CrossRef CAS.
- F. Liu, H. He, Y. Ding and C. Zhang, Appl. Catal., B, 2009, 93, 194–204 CrossRef CAS.
- D. S. Krivoruchenko, N. S. Telegina, D. A. Bokarev and A. Y. Stakheev, Kinet. Catal., 2015, 56, 741–746 CrossRef CAS.
- Y. Y. Wang, B. X. Shen, C. He, S. J. Yue and F. M. Wang, Environ. Sci. Technol., 2015, 49, 9355–9363 CrossRef CAS PubMed.
- X. Fan, F. Qiu, H. Yang, W. Tian, T. Hou and X. Zhang, Catal. Commun., 2011, 12, 1298–1301 CrossRef CAS.
- Y. Wang, C. Ge, L. Zhan, C. Li, W. Qiao and L. Ling, Ind. Eng. Chem. Res., 2012, 51, 11667–11673 CrossRef CAS.
- X. Wang, Y. Zheng and J. Lin, Catal. Commun., 2013, 37, 96–99 CrossRef CAS.
- L. Li, Y. Diao and X. Liu, J. Rare Earths, 2014, 32, 409–415 CrossRef CAS.
- B. X. Shen, X. P. Zhang, H. Q. Ma, Y. Yao and T. Liu, J. Environ. Sci., 2013, 25, 791–800 CrossRef CAS.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- W. Salomon, C. Roch-Marchal, P. Mialane, P. Rouschmeyer, C. Serre, M. Haouas, F. Taulelle, S. Yang, L. Ruhlmann and A. Dolbecq, Chem. Commun., 2015, 51, 2972–2975 RSC.
- I. Luz, C. Rösler, K. Epp, F. X. Llabrés i Xamena and R. A. Fischer, Eur. J. Inorg. Chem., 2015, 3904–3912 CrossRef CAS.
- T. S. Park, S. K. Jeong, S. H. Hong and S. C. Hong, Ind. Eng. Chem. Res., 2001, 40, 4491–4495 CrossRef CAS.
- L. Qiu, J. Meng, D. Pang, C. Zhang and F. Ouyang, Catal. Lett., 2015, 145, 1500–1509 CrossRef CAS.
- Y. Zhang, W. Guo, L. Wang, M. Song, L. Yang, K. Shen, H. Xu and C. Zhou, Chin. J. Catal., 2015, 36, 1701–1710 CrossRef CAS.
- L. Chen, J. Li, M. Ge and R. Zhu, Catal. Today, 2010, 153, 77–83 CrossRef CAS.
- H. Xu, Z. Qu, C. Zong, F. Quan, J. Mei and N. Yan, Appl. Catal., B, 2016, 186, 30–40 CrossRef CAS.
- C. He, B. Shen and F. Li, J. Hazard. Mater., 2016, 304, 10–17 CrossRef CAS PubMed.
- X. Zhu, B. Li, J. Yang, Y. Li, W. Zhao, J. Shi and J. Gu, ACS Appl. Mater. Interfaces, 2015, 7, 223–231 CAS.
- F. Zhang, C. Chen, W.-M. Xiao, L. Xu and N. Zhang, Catal. Commun., 2012, 26, 25–29 CrossRef CAS.
- B. Shen, F. Wang and T. Liu, Powder Technol., 2014, 253, 152–157 CrossRef CAS.
- P. Wang, H. Zhao, H. Sun, H. Yu, S. Chen and X. Quan, RSC Adv., 2014, 4, 48912–48919 RSC.
- S. Zhang, H. Liu, C. Sun, P. Liu, L. Li, Z. Yang, X. Feng, F. Huo and X. Lu, J. Mater. Chem. A, 2015, 3, 5294–5298 CAS.
- H. Jiang, C. Wang, H. Wang and M. Zhang, Mater. Lett., 2016, 168, 17–19 CrossRef CAS.
- R. Das, P. Pachfule, R. Banerjee and P. Poddar, Nanoscale, 2012, 4, 591–599 RSC.
- J. M. Zamaro, N. C. Pérez, E. E. Miró, C. Casado, B. Seoane, C. Téllez and J. Coronas, Chem. Eng. J., 2012, 195–196, 180–187 CrossRef CAS.
- K. K. Yee, N. Reimer, J. Liu, S. Y. Cheng, S. M. Yiu, J. Weber, N. Stock and Z. T. Xu, J. Am. Chem. Soc., 2013, 135, 7795–7798 CrossRef CAS PubMed.
- S. Ding, F. Liu, X. Shi and H. He, Appl. Catal., B, 2016, 180, 766–774 CrossRef CAS.
- V. P. Fallya, H. Vidalb, J. Kasparb, G. Blancoc, J. M. Pintadoc, S. Bernalc, G. Colond, M. Daturie and J. C. Lavalleye, Catal. Today, 2000, 59, 373–386 CrossRef.
- H. Chang, X. Chen, J. Li, L. Ma, C. Wang, C. Liu, J. W. Schwank and J. Hao, Environ. Sci. Technol., 2013, 47, 5294–5301 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |