Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metastable intermolecular composites of Al and CuO nanoparticles assembled with graphene quantum dots†
Yue Taoa,
Jiali Zhangb,
Yaoyao Yangb,
Haixia Wub,
Lan Huc,
Xiaohu Dongc,
Jian Luc and
Shouwu Guo *b
*b
aDepartment of Polymer Science and Engineering, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai 200240, P. R. China
bDepartment of Electronic Engineering, School of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University, Shanghai 200240, P. R. China. E-mail: swguo@sjtu.edu.cn
cXi'an Modern Chemistry Institute, Xi'an, Shaanxi 710065, P. R. China
First published on 5th January 2017
Abstract
Metastable intermolecular composites (MICs) have attracted great attention during the last two decades owing to their potential applications for both civilian and military purposes. In this work, a novel category of MICs are assembled using Al and CuO nanoparticles (NPs), and graphene quantum dots (GQDs) as building blocks. It has been demonstrated that the as-assembled Al/GQDs/CuO MICs show unique energetic performance in contrast to the physical mixture of Al and CuO NPs. More specifically, the MICs assembled at relatively higher temperature and with proper Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GQDs
GQDs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuO ratio of 20
CuO ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 (in weight) exhibit overall better energetic performances, including a higher heat releasing rate and larger specific heat. It was also illustrated that the specific heat amount released during the reaction can be lifted by whittling the oxide layer of the Al NPs surface, revealing that the inactive oxide layer on Al NPs should be one of the main reasons influencing the energetic performances of the MICs. Nevertheless, this work shows that the GQDs should be a useful media for MICs assembling.
90 (in weight) exhibit overall better energetic performances, including a higher heat releasing rate and larger specific heat. It was also illustrated that the specific heat amount released during the reaction can be lifted by whittling the oxide layer of the Al NPs surface, revealing that the inactive oxide layer on Al NPs should be one of the main reasons influencing the energetic performances of the MICs. Nevertheless, this work shows that the GQDs should be a useful media for MICs assembling.
Introduction
Metastable intermolecular composites (MICs), called also nanoenergetic materials (EMs) or thermite nanocomposites, can release rapidly abundant heat energy upon a thermal actuation.1–3 Hence, the MICs have been considered as one of the most promising energetic materials for propulsion fuels, pyrotechnics, and explosives for military purposes,1,4–7 as well as lead-free electric matches, thermal batteries and automotive air-bag propellants in civilian applications.8,9 Generally, the MICs are composed of a fuel and oxidizer both with nanometer sizes.10–12 In most cases, Al NPs are chosen as the fuel owing to their high energy densities, and several different metal oxide NPs, such as CuO, Fe2O3, Bi2O3, Mn2O3, are employed as the oxidizer in MICs.13–19 In comparison with the bulk thermites consisting of micrometer sized or even larger Al and metal oxides particles, the MICs show superb energetic performances because their nanosized components assume usually larger surface areas, which reinforce the mass transportation and interfacial contact between the fuel and oxidizer particles. Additionally, the thickness of the passivation oxide layer on the fuel surface, and the arrangement of the nanosized fuel and oxidizer within the MICs are also the key factors influencing their energetic performances, especially, the specific heat and the heat release rate.11,15,19–23During last two decades, several protocols such as physical mixing,24–26 sol–gel processing,20,27 high-energy ball milling,15 and molecular self-assembling2,9,28,29 have been developed for the preparation of MICs. Among these, the self-assembly techniques are used commonly. For instance, Gangopadhyay et al. assembled MICs of CuO nanorods and Al NPs with NH4NO3 as media.30 Bancaud et al. reported a DNA-directed assembly procedure for Al/CuO MICs and found that the as-prepared MICs showing better energetic performance in comparison with the physically mixed counterparts.9 More recently, Wang et al. developed an electrostatic based assembly method for preparing Al/MnO2 MICs. Briefly, the surfaces of MnO2 nanowires were positively charged by chemical modification with polydiallyldime-thylammonium chloride, and then were assembled with negatively charged Al NPs in aqueous solution.31 Similarly, using poly(4-vinylpyridine) as binder, Gangopadhyay et al. also prepared MICs of Al NPs and CuO nanorods through a self-assembly procedure, and the as-prepared MICs showed improved energetic properties than the ones prepared with other methods.32 However, it is worth pointing out that the media suitable for the MICs assembly procedure seems still limited.
Graphene sheets, owing to its unique single atomic layered structure, exhibit amazing physical/chemical properties, including large specific surface area, high thermal conductivity, and extraordinary carrier mobility.33,34 Graphene quantum dots (GQDs) assume the similar atomic layered structural character and properties with graphene sheets, but are less than one hundred nanometer in lateral dimensions and have more periphery carboxylic groups.35–38 The smaller sized GQDs could also be dispersed in water and some organic solvents moderately. Putting together, the GQDs are most probably the best media candidates for MIC assembly.
In the work, we report for the first time the MICs of Al and CuO NPs assembled using GQDs as media. The composition, structure, and morphologies of as-obtained MICs were characterized complementally using high resolution microscopic and variety of spectroscopic techniques. Their energetic performances including the heat release rate and the specific heat of reaction were evaluated quantitatively. As will be shown, the heat release rate and the specific heat of reaction of the MICs assembled with GQDs are significantly higher than those of the MICs prepared simply through physical mixing of the Al and CuO NPs. In addition, the ratio of Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GQDs
GQDs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuO, and the thickness of native oxide layer on Al NPs which may severely affect the energetic performances of MICs were explored.
CuO, and the thickness of native oxide layer on Al NPs which may severely affect the energetic performances of MICs were explored.
Experimental
Materials
Copper(II) sulfate pentahydrate (CuSO4·5H2O), sodium carbonate (Na2CO3), polyethylene glycol 400 (PEG400), ethanol, hydrogen peroxide (30% in water) (H2O2) were purchased from Sinopharm Chemical Reagent Co., Ltd, Shanghai, China. Sodium hydroxide (NaOH) was bought from Shanghai Lingfeng Chemical Reagent Co., Ltd, Shanghai, China. Al NPs were acquired from Xi'an Modern Chemistry Research Institute, Xi'an, China. All chemicals were used as received. Graphene oxide and GQDs were prepared following the procedures described in our previous works.39,40Preparation of CuO NPs
In a typical experiment, 12 ml (0.1 M) of CuSO4·5H2O were added in the mixture of 150 ml of deionized water and 150 ml of ethanol that contains 60 ml of PEG400 under vigorous magnetic stirring. Then, 100 ml of saturated Na2CO3 aqueous solution were added dropwisely. The as-obtained solution was stirred continuously for 2 h at room temperature (∼25 °C). The precipitate was separated by centrifuge and washed 3 times with deionized water and alcohol alternatively, dried at 60 °C for 12 h in vacuum oven. Finally, the solid product was grinded and calcined at 300 °C for 5 h, then 500 °C for 1 h in air to obtain the CuO NPs.Assembling of Al/CuO MICs with GQDs
The procedure used for Al/GQDs/CuO assembling is schematically shown in Fig. 1. Specifically, 8.1 mg of Al nanoparticles was first dispersed in 50 ml deionized water under ultrasonication for 5 min at 30 °C. Then, aqueous dispersion of GQDs was added with Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GQDs ratios of 20
GQDs ratios of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 20
0.5, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20
1, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 20
2, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 20
5, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (in weight) at pH ≈ 8, that was adjusted using diluted NaOH solution and stirred continuously for 1 h. Meanwhile, 36 mg of CuO NPs was dispersed in 100 ml deionized water under ultrasonication for 5 min at 30 °C. Finally, the CuO NPs dispersion was added into aforementioned Al/GQDs suspension and stirred vigorously for 1 h. The final ratios of Al
10 (in weight) at pH ≈ 8, that was adjusted using diluted NaOH solution and stirred continuously for 1 h. Meanwhile, 36 mg of CuO NPs was dispersed in 100 ml deionized water under ultrasonication for 5 min at 30 °C. Finally, the CuO NPs dispersion was added into aforementioned Al/GQDs suspension and stirred vigorously for 1 h. The final ratios of Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GQDs
GQDs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuO are of 20
CuO are of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, 20
90, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, 20
90, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, 20
90, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, and 20
90, and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 (in weight). At the end, the precipitate was separated by centrifuging and washed 3 times with deionized water, the solid product was dried at 50 °C for 1 h in vacuum. Notably, to compare the temperature effects, the MICs was also assembled at 10 °C through the same procedure.
90 (in weight). At the end, the precipitate was separated by centrifuging and washed 3 times with deionized water, the solid product was dried at 50 °C for 1 h in vacuum. Notably, to compare the temperature effects, the MICs was also assembled at 10 °C through the same procedure.
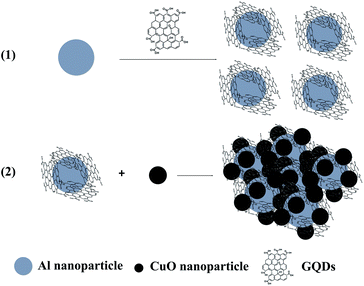 | ||
| Fig. 1 Schematic representation of the assembling procedure of Al/GQDs/CuO MICs with Al and CuO NPs, and GQDs as building blocks. | ||
Instrumental
The atomic force microscopy (AFM) images were acquired in tapping mode using a Multimode Nanoscope V scanning probe microscopy system (Bruker, USA), and AN-NSC 10AFM cantilever tips (SHNIT Co., Russia) with a force constant of ∼37 N m−1 and resonance vibration frequency of ∼300 kHz. Samples for AFM were prepared by solution casting the aqueous suspension of GQDs on a freshly cleaved mica surface and dried in air. The SEM and TEM images were acquired using Ultra 55 field emission scanning electron microscope (Zeiss, Germany) with the working voltage is 10.0 kV, and JEOL JEM-2100F transmission electron microscope (JEOL, Japan) with the operation voltage is 200 kV, and the high-resolution TEM (HRTEM) images were measured on the same instrument. The TEM specimens were prepared by placing the aqueous suspensions on the copper grids and drying under ambient condition. X-ray diffraction (XRD) patterns were recorded on a Bruker D8 Advance PC diffractometer (Bruker, Germany) using Cu/Kα radiation (λ = 1.55406 Å), with the 2θ range of 10 to 80° and the scan rate of 0.2° s−1. Differential scanning calorimetry (DSC) measurements were conducted on a STA 449 F3 Jupiter (NETZSCH, Germany) at a heating rate of 10 °C min−1 under argon gas flow (99.999%, in purity).Results and discussion
Characterizations of Al and CuO NPs, and GQDs
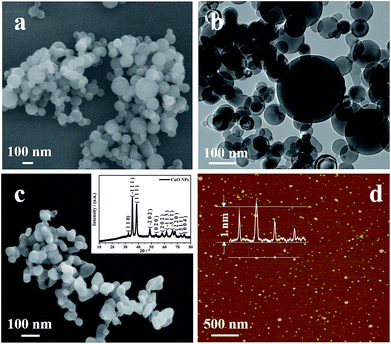
The morphology and size of Al NPs used in the work were characterized first through SEM and TEM imaging, and the results were shown in Fig. 2a and b. Clearly, the Al NPs assume well defined spherical morphology, and their average size estimated from the TEM image is of ∼94 nm in diameter, but with a wide size distribution. As depicted in Fig. 2c, the sizes of as-prepared CuO NPs are ranged from 50 to 100 nm in diameter, and are aggregated somehow. The structure and composition of the CuO NPs were also verified with X-ray powder diffraction pattern (inset in Fig. 2c) which indicated that the CuO NPs assume a monoclinic crystalline phase (JCPDS standard no. 48-1548). Considering that PEG400 was used as capping reagent during the CuO NPs preparation, one may doubt the residual of the PEG400 in the CuO NPs aggregate. To clarify this, TGA data of CuO NPs was collected and depicted in Fig. S1† illustrating that the PEG400 was extirpated at 500 °C. The morphology of GQDs used in the work was studied with AFM imaging and the result is represented in Fig. 2d. The average size of GQDs is ∼10 nm in diameter and the height is ∼1 nm (inset in Fig. 2d), revealing the single atomic layer motif.40,41Assembling of Al/GQDs/CuO MICs
It is well known that there is a native oxide layer on the surface of Al NPs,11,21 and the GQDs used in the work containing abundant periphery carboxylic groups.39 Thus, when Al NPs were introduced into the aqueous suspensions of GQDs, the peripheral carboxylic groups on GQDs should react with the native oxide layer on Al NPs, and the GQDs can be anchored chemically onto the Al NPs. Similarly, the as-prepared CuO NPs can also react with carboxylic groups on GQDs. Therefore, to obtain Al/GQDs/CuO MICs, after the formation of Al/GQDs, the CuO NPs were added in the reaction system under vigorous stirring. The detailed assembling procedure is schematically shown in Fig. 1. Considering that the CuO NPs and the native oxidation layer of Al NPs can be decomposed in both basic and acidic reaction systems that may affect further the formation of MICs,42 the pH value of the reaction system was kept at ∼8 during the MICs assembling. On the other hand, the assembling of Al NPs, GQDs, and CuO NPs may also be originated from the electrostatic interaction among them. To clarify the assumption, the zeta potentials of Al NPs, GQDs and CuO NPs at different pH values were measured (Fig. S2†). It shows that Al NPs and GQDs are negatively charged, CuO NPs are positively charged at pH 8, implying the electrostatic interaction is not dominating factor for the MICs assembling. To evaluate the effect of temperature on the Al/GQDs/CuO assembling, two different kinds of MICs were assembled at 10 °C and 30 °C with other conditions be the same. For comparisons, the MICs with Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GQDs
GQDs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuO ratios of 20
CuO ratios of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, 20
90, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, 20
90, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, 20
90, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 (in weight) were also prepared.
90 (in weight) were also prepared.
Fig. 3a and b show selectively the SEM images of the Al/GQDs/CuO MICs, with Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GQDs
GQDs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuO ratio of 20
CuO ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, prepared at 10 and 30 °C, respectively. The morphologies of the two MICs are similar. In both cases, the Al NPs were surrounded with the relatively smaller CuO NPs which is different from the physical mixture of Al and CuO NPs without GQDs (Fig. S3a†). This implies that the GQDs do play a key role for the assembling of the Al and CuO NPs. Notably, the all as-prepared MICs with different Al
90, prepared at 10 and 30 °C, respectively. The morphologies of the two MICs are similar. In both cases, the Al NPs were surrounded with the relatively smaller CuO NPs which is different from the physical mixture of Al and CuO NPs without GQDs (Fig. S3a†). This implies that the GQDs do play a key role for the assembling of the Al and CuO NPs. Notably, the all as-prepared MICs with different Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GQDs
GQDs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuO ratio assume similar morphologies, Fig. 3b and S3.† The hyperfine structure of the MICs was further elaborated using the high resolution TEM (Fig. 3d). The inter plane lattice constants fit well with Al and CuO crystals, respectively. More significantly, GQDs aggregates can be observed clearly between Al and CuO NPs, and the d-spacing between the GQDs sheets is around 0.36 nm, very close to that of the graphite (0.34 nm).43 This result provides a direct evidence of the formation of Al/GQDs/CuO MICs.
CuO ratio assume similar morphologies, Fig. 3b and S3.† The hyperfine structure of the MICs was further elaborated using the high resolution TEM (Fig. 3d). The inter plane lattice constants fit well with Al and CuO crystals, respectively. More significantly, GQDs aggregates can be observed clearly between Al and CuO NPs, and the d-spacing between the GQDs sheets is around 0.36 nm, very close to that of the graphite (0.34 nm).43 This result provides a direct evidence of the formation of Al/GQDs/CuO MICs.
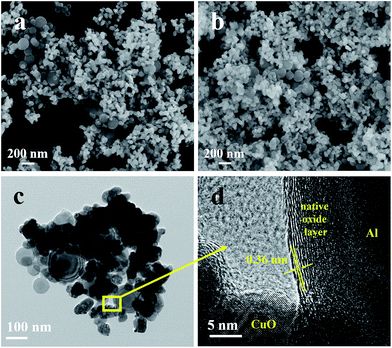 | ||
Fig. 3 (a) SEM image of Al/GQDs/CuO MICs with Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GQDs GQDs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuO ratio of 20 CuO ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 and assembled at 10 °C. (b–d) SEM, TEM, HRTEM images of the Al/GQDs/CuO MICs assembled at 30 °C. 90 and assembled at 10 °C. (b–d) SEM, TEM, HRTEM images of the Al/GQDs/CuO MICs assembled at 30 °C. | ||
Energetic performances of as-assembled Al/GQDs/CuO MICs
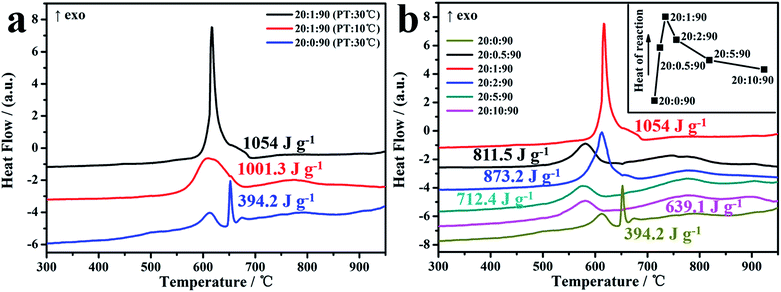
To evaluate the energetic performances of the as-assembled Al/GQDs/CuO MICs, including mainly the specific heat of the reaction and the heat release rate, the DSC curves of the MICs were acquired and shown in Fig. 4. The specific heat estimated from the DSC data for the Al/GQDs/CuO MICs with Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GQDs
GQDs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuO ratio of 20
CuO ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 assembled at 10 °C and 30 °C can reach to 1001.3 and 1054.0 J g−1, respectively, which are much higher than those of the physically mixed Al and CuO NPs, 394.2 J g−1, and also the physically mixed Al, GQDs and CuO NPs, 475.9 J g−1 (Fig. S4†). Comparably, the specific heat of the MICs assembled at 30 °C seems larger than that of the one prepared at 10 °C, revealing that the MICs assembled at relatively high temperature assume better energetic performance. The reason might be that at the relatively high temperature, the building blocks, GQDs, and Al, CuO NPs can be mixed more sufficiently, and thus, the fuel and oxidizer can approach to each other more easily during the reaction. Additionally, for the Al/GQDs/CuO MICs, their initial exothermal temperatures are about 50 °C lower than that of the physically mixed one. Further, the heat release rates of MICs were also enhanced dramatically. There is no exothermal peak appeared around 660 °C for Al NPs melting on the DSC curves indicating the solid–solid reaction between the Al and CuO NPs occurred, which is highly demanded for the MICs (thermite). This might be raised from the single atomic layered character and also the pronounced thermal conductivity of GQDs.44,45
90 assembled at 10 °C and 30 °C can reach to 1001.3 and 1054.0 J g−1, respectively, which are much higher than those of the physically mixed Al and CuO NPs, 394.2 J g−1, and also the physically mixed Al, GQDs and CuO NPs, 475.9 J g−1 (Fig. S4†). Comparably, the specific heat of the MICs assembled at 30 °C seems larger than that of the one prepared at 10 °C, revealing that the MICs assembled at relatively high temperature assume better energetic performance. The reason might be that at the relatively high temperature, the building blocks, GQDs, and Al, CuO NPs can be mixed more sufficiently, and thus, the fuel and oxidizer can approach to each other more easily during the reaction. Additionally, for the Al/GQDs/CuO MICs, their initial exothermal temperatures are about 50 °C lower than that of the physically mixed one. Further, the heat release rates of MICs were also enhanced dramatically. There is no exothermal peak appeared around 660 °C for Al NPs melting on the DSC curves indicating the solid–solid reaction between the Al and CuO NPs occurred, which is highly demanded for the MICs (thermite). This might be raised from the single atomic layered character and also the pronounced thermal conductivity of GQDs.44,45
To get insight into the effect of the GQDs on the energetic properties of the Al/GQDs/CuO MICs, the DSC data of a series of Al/GQDs/CuO MICs containing different amount of GQDs prepared at 30 °C were collected and compared. As illustrated in Fig. 4b, in general, the specific heats of the Al/GQDs/CuO MICs are always higher than that of physically mixed Al and CuO NPs. However, the fact is that the specific heat seems not continuously increased with increasing of the GQDs content in the MICs. Specifically, the specific heat released by the MICs with the Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GQDs
GQDs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuO ratio of 20
CuO ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 (in weight) is about 811.5 J g−1. When the Al
90 (in weight) is about 811.5 J g−1. When the Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GQDs
GQDs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuO ratio is increased to 20
CuO ratio is increased to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, the specific heat reached to the maximum. With the further increasing of the GQDs content, the specific heat starts to decrease. The reasons might be that when the GQDs content is too high, the interaction between the fuel Al NPs and the oxidizer CuO NPs may be blocked somehow, thus the solid–solid reaction between Al and CuO NPs may transfer to solid–liquid reaction. On the other hand, the increase of the GQDs content declines the relative content of the active components (Al and CuO NPs) in the MICs which will be discussed in details later on. Additionally, the initial temperatures for the reactions of MICs are also lower than that of the physically mixed one. The detailed relationship between the GQDs content in MICs and the specific heat is summarized in the inset in Fig. 4b. Further, the narrowed FWHMs (full width at half maximum) of the exothermic peaks on DSC illustrate that the heat release rates of MICs can be accelerated with increasing of the GQDs content. The reason should be raised from the high thermal conductivity of graphene derivatives including GQDs.44,45 Consequently, it may be concluded that using GQDs as the media for Al and CuO NPs assembling, the as-obtained MICs have more exquisite energetic performances in contrast to the physically mixed Al/CuO counterparts.
90, the specific heat reached to the maximum. With the further increasing of the GQDs content, the specific heat starts to decrease. The reasons might be that when the GQDs content is too high, the interaction between the fuel Al NPs and the oxidizer CuO NPs may be blocked somehow, thus the solid–solid reaction between Al and CuO NPs may transfer to solid–liquid reaction. On the other hand, the increase of the GQDs content declines the relative content of the active components (Al and CuO NPs) in the MICs which will be discussed in details later on. Additionally, the initial temperatures for the reactions of MICs are also lower than that of the physically mixed one. The detailed relationship between the GQDs content in MICs and the specific heat is summarized in the inset in Fig. 4b. Further, the narrowed FWHMs (full width at half maximum) of the exothermic peaks on DSC illustrate that the heat release rates of MICs can be accelerated with increasing of the GQDs content. The reason should be raised from the high thermal conductivity of graphene derivatives including GQDs.44,45 Consequently, it may be concluded that using GQDs as the media for Al and CuO NPs assembling, the as-obtained MICs have more exquisite energetic performances in contrast to the physically mixed Al/CuO counterparts.
Honestly, it should be point out that the specific heats of the as-assembled MICs are far below to the theoretical specific heat of the Al/CuO thermite (3900 J g−1).9 In principle, there are several factors may contribute to the specific heat of Al/GQDs/CuO MICs. First, as mentioned above, the introduction of the inactive GQDs may be one of the reasons. However, the maximum GQDs content in the MICs with the Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GQDs
GQDs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuO ratio of 20
CuO ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
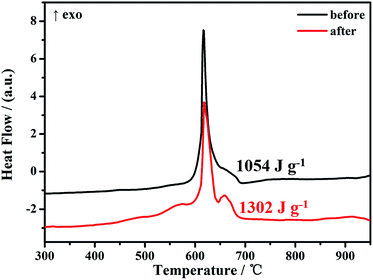
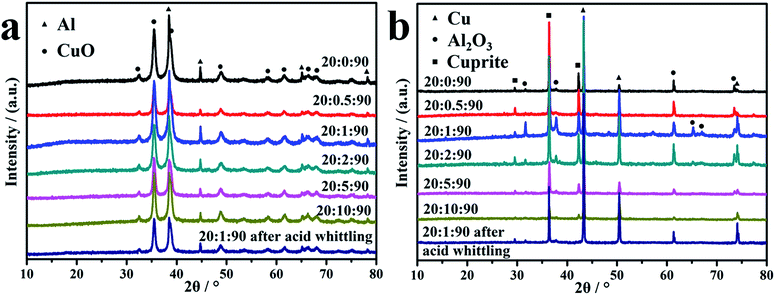
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 is 8.3% (the content of active components is of 91.7%) (in weight), but the corresponding specific heat is only 639.1 J g−1, equaling 16% of the theoretical data. This implies that the GQDs can reduce the specific heat somehow, but not the dominating factor. Second, the native oxide layer on the Al NPs can be another drawback for the effective heat release of reaction of thermite. To elaborate this, in the work, we attempt to whittle the native oxide layer on Al NPs through a quick etching procedure with diluted HCl (∼0.1 M) before the MIC assembling. As depicted in Fig. S5,† the oxide layer can be whittled, but not completely because the etching reaction is conducted in aqueous solution. Nevertheless, the Al/GQDs/CuO MICs were assembled with as-whittled Al NPs and characterized. As shown in Fig. S6,† the newly assembled MICs assume similar morphology with that using original Al NPs. Remarkably, the DSC curve, Fig. 5, shows that the energetic performance of the MICs with the partially whittled Al NPs was improved dramatically. Its specific heat reaches to 1302 J g−1, that is much higher than that of the MICs with original Al NPs (1054 J g−1). This implies that the inactive oxide layer on Al NPs should be a major reason reducing the specific heat of the as-prepared MICs. Additionally, once the oxide layer getting thinner, the reactivity of Al NPs with CuO should be improved which is also beneficial to the increase of the heat release rate. Third, the reaction products of MICs can also affect their energetic performances. To monitor this, the XRD patterns of MICs before and after the reaction were acquired and shown in Fig. 6. Besides Al2O3 and Cu, cuprite was detected in the final products (Fig. 6b). This should be another reason why the specific heat of the as-assembled MICs is lower than the theoretical value of Al/CuO thermite. Because, in principle, the products of MIC after the reaction should be only Cu and Al2O3 reflecting the stoichiometric ratio of Al/GQDs/CuO should be optimized further.
90 is 8.3% (the content of active components is of 91.7%) (in weight), but the corresponding specific heat is only 639.1 J g−1, equaling 16% of the theoretical data. This implies that the GQDs can reduce the specific heat somehow, but not the dominating factor. Second, the native oxide layer on the Al NPs can be another drawback for the effective heat release of reaction of thermite. To elaborate this, in the work, we attempt to whittle the native oxide layer on Al NPs through a quick etching procedure with diluted HCl (∼0.1 M) before the MIC assembling. As depicted in Fig. S5,† the oxide layer can be whittled, but not completely because the etching reaction is conducted in aqueous solution. Nevertheless, the Al/GQDs/CuO MICs were assembled with as-whittled Al NPs and characterized. As shown in Fig. S6,† the newly assembled MICs assume similar morphology with that using original Al NPs. Remarkably, the DSC curve, Fig. 5, shows that the energetic performance of the MICs with the partially whittled Al NPs was improved dramatically. Its specific heat reaches to 1302 J g−1, that is much higher than that of the MICs with original Al NPs (1054 J g−1). This implies that the inactive oxide layer on Al NPs should be a major reason reducing the specific heat of the as-prepared MICs. Additionally, once the oxide layer getting thinner, the reactivity of Al NPs with CuO should be improved which is also beneficial to the increase of the heat release rate. Third, the reaction products of MICs can also affect their energetic performances. To monitor this, the XRD patterns of MICs before and after the reaction were acquired and shown in Fig. 6. Besides Al2O3 and Cu, cuprite was detected in the final products (Fig. 6b). This should be another reason why the specific heat of the as-assembled MICs is lower than the theoretical value of Al/CuO thermite. Because, in principle, the products of MIC after the reaction should be only Cu and Al2O3 reflecting the stoichiometric ratio of Al/GQDs/CuO should be optimized further.
Conclusions
In conclusion, a novel category of MICs are designed and assembled with Al and CuO NPs, and GQDs as building blocks. It was demonstrated that with GQDs as media, Al and CuO NPs can be assembled through the chemical interactions among the carboxylic groups of GQDs and the CuO and the native oxide layer on Al NPs, forming homogeneous Al/GQDs/CuO MICs. The as-assembled MICs show improved energetic performances, including the higher specific heat and faster heat release rate over the physically mixed counterparts. The specific heat of the as-assembled MICs can be tuned by varying the GQDs content, and it was illustrated that the MIC with Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GQDs
GQDs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuO ratio of 20
CuO ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 has overall better energetic performances, but is still below the theoretical specific heat of Al/CuO thermite. The reason was assigned to the native oxide layer on Al NPs and also the formation of cuprite in the reaction product. The works how to whittle completely the native oxide layer on Al and to optimize the stoichiometric ratio of Al, CuO, and GQDs is undergoing in our laboratory, and we hope to show them in our coming work.
90 has overall better energetic performances, but is still below the theoretical specific heat of Al/CuO thermite. The reason was assigned to the native oxide layer on Al NPs and also the formation of cuprite in the reaction product. The works how to whittle completely the native oxide layer on Al and to optimize the stoichiometric ratio of Al, CuO, and GQDs is undergoing in our laboratory, and we hope to show them in our coming work.
Acknowledgements
The work was financially supported by the National “973 Program” of China (No. 2014CB260411 and 2015CB931801), the National Natural Science foundation of China (No. 11374205).References
- B. W. Asay, S. F. Son, J. R. Busse and D. M. Oschwald, Propellants, Explos., Pyrotech., 2004, 29, 216 CrossRef CAS.
- S. H. Kim and M. R. Zachariah, Adv. Mater., 2004, 16, 1821 CrossRef CAS.
- V. K. Patel and S. Bhattacharya, ACS Appl. Mater. Interfaces, 2013, 5, 13364 CAS.
- J. J. Granier and M. L. Pantoya, Combust. Flame, 2004, 138, 373 CrossRef CAS.
- R. A. Guidotti and P. Masset, J. Power Sources, 2006, 161, 1443 CrossRef CAS.
- C. R. Kaili Zhang, M. Petrantoni and N. Mauran, J. Microelectromech. Syst., 2008, 17, 832 CrossRef.
- M. Petrantoni, C. Rossi, L. Salvagnac, V. Conédéra, A. Estève, C. Tenailleau, P. Alphonse and Y. J. Chabal, J. Appl. Phys., 2010, 108, 084323 CrossRef.
- S. Valliappan, J. Swiatkiewicz and J. A. Puszynski, Powder Technol., 2005, 156, 164 CrossRef CAS.
- F. Séverac, P. Alphonse, A. Estève, A. Bancaud and C. Rossi, Adv. Funct. Mater., 2012, 22, 323 CrossRef.
- S. F. Son, R. Yetter and V. Yang, J. Propul. Power, 2007, 23, 643 CrossRef.
- V. E. Sanders, B. W. Asay, T. J. Foley, B. C. Tappan, A. N. Pacheco and S. F. Son, J. Propul. Power, 2007, 23, 707 CrossRef CAS.
- C. Rossi, A. Estève and P. Vashishta, J. Phys. Chem. Solids, 2010, 71, 57 CrossRef CAS.
- B. S. Bockmon, M. L. Pantoya, S. F. Son, B. W. Asay and J. T. Mang, J. Appl. Phys., 2005, 98, 064903 CrossRef.
- M. L. Pantoya and J. J. Granier, J. Therm. Anal. Calorim., 2006, 85, 37 CrossRef CAS.
- S. M. Umbrajkar, M. Schoenitz and E. L. Dreizin, Thermochim. Acta, 2006, 451, 34 CrossRef CAS.
- T. Foley, A. Pacheco, J. Malchi, R. Yetter and K. Higa, Propellants, Explos., Pyrotech., 2007, 32, 431 CrossRef CAS.
- M. Schoenitz, S. M. Umbrajkar and E. L. Dreizin, J. Propul. Power, 2007, 23, 683 CrossRef CAS.
- S. F. Son, B. W. Asay, T. J. Foley, R. A. Yetter, M. H. Wu and G. A. Risha, J. Propul. Power, 2007, 23, 715 CrossRef CAS.
- J. Y. Ahn, J. H. Kim, J. M. Kim, D. W. Lee, J. K. Park, D. Lee and S. H. Kim, Powder Technol., 2013, 241, 67 CrossRef CAS.
- T. M. Tillotson, A. E. Gash, R. L. Simpson, L. W. Hrubesh, J. H. Satcher Jr and J. F. Poco, J. Non-Cryst. Solids, 2001, 285, 338 CrossRef CAS.
- J. Wang, A. Hu, J. Persic, J. Z. Wen and Y. Norman Zhou, J. Phys. Chem. Solids, 2011, 72, 620 CrossRef CAS.
- R. J. Jacob, G. Jian, P. M. Guerieri and M. R. Zachariah, Combust. Flame, 2015, 162, 258 CrossRef CAS.
- A. Bach, P. Gibot, L. Vidal, R. Gadiou and D. Spitzer, J. Energ. Mater., 2015, 33, 260 CrossRef CAS.
- L. Wang, D. Luss and K. S. Martirosyan, J. Appl. Phys., 2011, 110, 074311 CrossRef.
- J. Z. Wen, S. Ringuette, G. Bohlouli-Zanjani, A. Hu, N. H. Nguyen, J. Persic, C. F. Petre and Y. N. Zhou, Nanoscale Res. Lett., 2013, 8, 1 CrossRef PubMed.
- L. Glavier, G. Taton, J.-M. Ducéré, V. Baijot, S. Pinon, T. Calais, A. Estève, M. Djafari Rouhani and C. Rossi, Combust. Flame, 2015, 162, 1813 CrossRef CAS.
- A. E. Gash, T. M. Tillotson, J. Joe, H. Satcher, J. F. Poco, L. W. Hrubesh and R. L. Simpson, Chem. Mater., 2001, 13, 999 CrossRef CAS.
- J. Y. Malchi, T. J. Foley and R. A. Yetter, ACS Appl. Mater. Interfaces, 2009, 1, 2420 CAS.
- H. Wang, G. Jian, G. C. Egan and M. R. Zachariah, Combust. Flame, 2014, 161, 2203 CrossRef CAS.
- R. Thiruvengadathan, A. Bezmelnitsyn, S. Apperson, C. Staley, P. Redner, W. Balas, S. Nicolich, D. Kapoor, K. Gangopadhyay and S. Gangopadhyay, Combust. Flame, 2011, 158, 964 CrossRef CAS.
- Y. Yang, P. P. Wang, Z. C. Zhang, H. L. Liu, J. Zhang, J. Zhuang and X. Wang, Sci. Rep., 2013, 3, 1694 Search PubMed.
- R. Shende, S. Subramanian, S. Hasan, S. Apperson, R. Thiruvengadathan, K. Gangopadhyay, S. Gangopadhyay, P. Redner, D. Kapoor, S. Nicolich and W. Balas, Propellants, Explos., Pyrotech., 2008, 33, 122 CrossRef CAS.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906 CrossRef CAS PubMed.
- X. Huang, Z. Yin, S. Wu, X. Qi, Q. He, Q. Zhang, Q. Yan, F. Boey and H. Zhang, Small, 2011, 7, 1876 CrossRef CAS PubMed.
- P. He, J. Sun, S. Tian, S. Yang, S. Ding, G. Ding, X. Xie and M. Jiang, Chem. Mater., 2014, 27, 218 CrossRef.
- S. H. Song, M.-H. Jang, J. Chung, S. H. Jin, B. H. Kim, S.-H. Hur, S. Yoo, Y.-H. Cho and S. Jeon, Adv. Opt. Mater., 2014, 2, 1016 CrossRef CAS.
- D. Chao, C. Zhu, X. Xia, J. Liu, X. Zhang, J. Wang, P. Liang, J. Lin, H. Zhang, Z. X. Shen and H. J. Fan, Nano Lett., 2015, 15, 565 CrossRef CAS PubMed.
- Y. Lin, R. Chapman and M. M. Stevens, Adv. Funct. Mater., 2015, 25, 3183 CrossRef CAS.
- X. Zhou, Y. Zhang, C. Wang, X. Wu, Y. Yang, B. Zheng, H. Wu, S. Guo and J. Zhang, ACS Nano, 2012, 6, 6592 CrossRef CAS PubMed.
- J. Zhang, H. Yang, G. Shen, P. Cheng, J. Zhang and S. Guo, Chem. Commun., 2010, 46, 1112 RSC.
- S. Park and R. S. Ruoff, Nat. Nanotechnol., 2009, 4, 217 CrossRef CAS PubMed.
- R. D. Letterman and S. G. Vanderbrook, Water Res., 1983, 17, 195 CrossRef CAS.
- F. Zhang, F. Liu, C. Wang, X. Xin, J. Liu, S. Guo and J. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 2104 CAS.
- A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Nano Lett., 2008, 8, 902 CrossRef CAS PubMed.
- S. Ghosh, I. Calizo, D. Teweldebrhan, E. P. Pokatilov, D. L. Nika, A. A. Balandin, W. Bao, F. Miao and C. N. Lau, Appl. Phys. Lett., 2008, 92, 151911 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra25972c |
| This journal is © The Royal Society of Chemistry 2017 |