Open Access Article
Open Access ArticleA novel Eu3+/Eu2+ co-doped MgSrLa8(SiO4)6O2 single-phase white light phosphor for white LEDs
Xiaoli Gao,
Haitao Liu*,
Xinyu Yang*,
Yiguang Tian*,
Xue Lu and
Liyuan Han
College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou, Zhejiang 325035, China. E-mail: lht@wzu.edu.cn; gytian@wzu.edu.cn; yangxinyu13@126.com; Tel: +86 13777780689 Tel: +86 13858851825 Tel: +86 13706654740
First published on 5th January 2017
Abstract
A novel MgSrLa8−x(SiO4)6O2:xEu (MSLSO:xEu) phosphor was synthesized through a high-temperature solid-state reaction. The crystal structures, luminescent properties, fluorescence decay time, and oxygen vacancies were investigated systemically. XRD analysis shows a typical oxyapatite structure with the space group P63/m. Europium can enter crystal matrices simultaneously in the form of Eu3+ and Eu2+ and occupy nonequivalent crystallographic positions in a lattice, thus forming various optical centers. Under ultraviolet light excitation, the phosphors simultaneously show the blue-green emission of Eu2+ and the green-yellow-red emission of Eu3+. The optimal doping content of Eu is 7.5 mol% (x = 0.075). White light can be realized with a CIE coordinate of (0.3664, 0.3260) by adjusting the concentration of Eu. The lifetimes of Eu3+ and Eu2+ in this study are considerably longer than those in other references. The results suggest that the MSLSO:Eu2+/Eu3+ (0.075) phosphor is a promising candidate for white LEDs.
Introduction
White-light-emitting diodes (WLEDs) are regarded as eco-friendly light sources because of their small size, low energy consumption, high luminous efficiency, and long lifetime, and they are environmentally benign.1,2 The most commonly used method to obtain white light is by combining a blue LED chip and a YAG:Ce3+ yellow-emitting phosphor.3,4 However, this kind of WLED present numerous disadvantages, such as poor color-rendering index (CRI) and high color temperature because of the lack of a red component in the spectrum.5 Thus, this kind of WLED cannot satisfy illumination requirements. To solve this problem, we used an ultraviolet (UV)/n-UV LED chip coated with a single-phase white-light-emitting phosphor to fabricate WLEDs.6In recent years, silicate-based luminescent materials have been given considerable attention owing to their favorable luminescence properties and high chemical stability. Apatite-structure silicates are regarded as effective matrixes for the activation by rare-earth (RE) ions.7,8 Moreover, trivalent RE (RE3+) ions play an important role in producing highly efficient luminescent materials.9–12
Previously reported co-doped white phosphors present several shortcomings in the spectral distribution.13–30 For the Eu2+/Mn2+ or Ce3+/Mn2+ co-doped systems, the intensity of the peaks are relatively weak in the green region, whereas the Ce3+/Eu2+ or Ce3+/Tb3+ co-doped systems are lack the red component, which will affect the overall luminescent efficiency of materials. In general, the introduction of a variety of activated ions can compensate for the lack of spectrum, whereas this method may reduce the luminous intensity and the efficiency of energy transfer among the doped ions to a certain extent. Therefore, we focus on how to improve the loss of the doping system in the spectral distribution and how to obtain white phosphors to meet the requirements of different applications.
Among the various RE element species, europium has traditionally occupied a dominant role. Luminescence of the Eu3+ ion originates from its 4f6 → 4f6 transition consisting of sharp lines in the red region. The positions of its emission lines are independent of the host materials. Eu2+ presents broad emission from the parity-allowed transitions 4f65d1 → 4f7, which presents wide-emission range changing in the blue-green-yellow or red band. The emission band position can be tuned by selecting different host materials.31–35 Nevertheless, only a few studies have been considered this topic, thus inspiring us to explore the luminescence properties of Eu2+/Eu3+ co-doped single-phase white-light-emitting phosphors.
In this study, in Eu3+-doped material, part of Eu3+ is reduced to Eu2+. The crystal structures, luminescent properties, fluorescence decay time, and oxygen vacancies were investigated systemically. Finally, the phosphors simultaneously show blue emission of the Eu2+ ion and green-yellow-red emission of the Eu3+ ion under UV light excitation. Surprisingly, high energy level emission peaks of Eu3+ can be observed, i.e., {5D1–7FJ (J = 0, 1, 2) and 5D2–7FJ (J = 0, 1, 2, 3)}, which are our biggest innovation. White light can be realized by adjusting the concentration of Eu. Results show that MSLSO:Eu2+/Eu3+ is a promising white-emitting phosphor for WLEDs.
Experimental
Sample preparation
MSLSO doped with Eu was synthesized with a high-temperature solid phase method by calcining a mixture of La2O3 (99.9%), MgCO3·3H2O (99.99%), SrCO3, Eu2O3 (99.99%), SiO2 (99.9%), and H3BO3, where H3BO3 acted as flux. All calculations were performed assuming that Eu replaces La3+ exclusively and formally yielding MSLSO:xEu (x = 0, 0.05, 0.075, 0.09, 0.10, and 0.125). That is to say, n(Mg)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(Sr)
n(Sr)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(La)
n(La)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(Si)
n(Si)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(Eu) = 1
n(Eu) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (8 − x)
(8 − x)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x. Subsequently, the mixture was prefired at 1000 °C for 3 h in a weak reducing atmosphere with using carbon powder. Then, the products were cooled to room temperature naturally. After grinding for second time, and the samples were calcined at 1100 °C for 4 h under the same conditions.
x. Subsequently, the mixture was prefired at 1000 °C for 3 h in a weak reducing atmosphere with using carbon powder. Then, the products were cooled to room temperature naturally. After grinding for second time, and the samples were calcined at 1100 °C for 4 h under the same conditions.
Characterization
The X-ray diffraction (XRD) patterns of the samples were recorded on a Bruker D8 Advance diffractometer with Cu Kα1 radiation (λ = 1.5406 Å) in the range of 2θ = 10–90° with the step of Δ2θ = 0.02° operating at 40 mA and 40 kV. The fluorescent properties of the as-obtained samples were recorded by FluoroMax-4 (HORIBA Jobin Yvon) fluorescence spectrometer with a Xe-arc lamp of 150 W power, and the lifetime was measured with a phosphorimeter attached to the main system with a Xe-flash lamp (25 W power).Results and discussion
X-ray phase analysis
The solid solutions MSLSO:xEu (x = 0, 0.05, 0.075, 0.09, 0.10, and 0.125) belong to the oxyapatite structure type. According to ref. 36 the site-symmetry of atoms in Sr2La8(SiO4)6O2 crystals (space group P63/m, z = 1) is La1-4f, Sr-4f, La2-6h, Si-6h, O-6h, O-6h, O-12i, O-2a; z = 1. The structure contains isolated SiO4 tetrahedra. Oxygen O(4) in the 2a position does not enter into the composition of the tetrahedra and forms chains that are parallel to the hexagonal crystal axis.37 On this basis, we assumed that the Mg atoms also occupy the 4f position. Europium replacing the La atoms in Sr2La8(SiO4)6O2 can also be located in these two positions. In the unit cell, the six 6h sites are occupied exclusively by 6La3+, whereas the four 4f sites are randomly shared by 1Sr2+, 1Mg2+, and 2La3+.38Fig. 1(a) shows the characteristic diffraction patterns of the synthesized solid solutions MSLSO:xEu (x = 0, 0.05, 0.075, 0.09, 0.10, and 0.125). All diffraction peaks of the samples can be indexed in the hexagonal unit cell, and no second phase was detected at these doping levels, indicating that all as-prepared samples matched well with that of the standard Sr2La8Si6O26 phase (ICSD card no. 155625) and all samples have hexagonal apatite-like structure with the space group of P63/m. Therefore, the doped Eu3+ ions present no evident influence on the crystal line structure of the host MSLSO at various Eu3+ doping concentrations. Fig. 1(b) shows the energy dispersive X-ray spectroscopy (EDS) spectrum of the MSLSO, which confirmed the presence of Sr, La, Mg, Si, and O elements. In addition, n(Mg)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(Sr)
n(Sr)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(La)
n(La)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(Si)
n(Si)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(O) = 1
n(O) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26.
26.
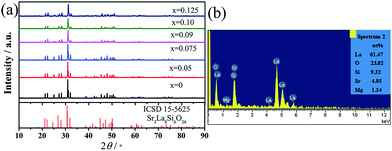 | ||
| Fig. 1 (a) Powder XRD patterns of Sr2La8Si6O26 (ICSD 15-5625) and MSLSO:xEu phosphors. (b) EDS spectrum. | ||
Photoluminescence
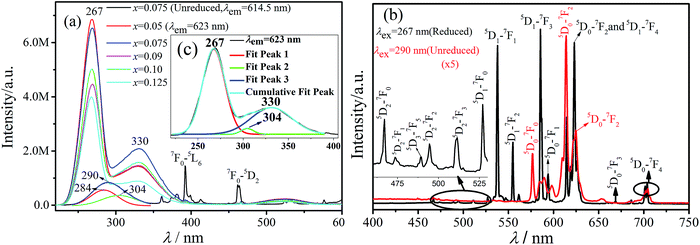
Fig. 2(a) illustrates that, in the PLE spectrum of 614.5 nm luminescence for the unreduced sample, two bands are centered at about 284 and 304 nm by using Gaussian fitting corresponding to the allowed O2− → Eu3+ charge transfer state (CTS1 and CTS2) of Eu3+ ion. The sharp excitation peaks between 350 and 600 nm are attributed to the intra-4f transitions from the Eu3+ ground state 7F0. Meanwhile, for the reduced samples, three bands are centered at about 267, 304, and 330 nm by using Gaussian fitting corresponding to the allowed O2− → Eu3+ charge transfer state (CTS1 and CTS2) of Eu3+ ion and the 4f7 → 4f65d1 from Eu2+, respectively, as shown in Fig. 2(c). CTS1 and CTS2 correspond to the 6h sites and the 4f sites, as is consistent with the reported literature.39 Weak sharp excitation peaks are found between 350 and 600 nm, meaning that for the emission of the Eu3+ ions when Eu2+ is excited and the CTS majorly contribute, and the contribution of f–f transition is smaller. An inflection around 290 nm of the band is centered at 330 nm, suggesting that Eu2+ in this site can exchange the excitation energy with Eu3+ probably located at the same site-type. For the reduced samples, by comparing the intensity of the peaks of the excitation spectrum, x = 0.075 is a more favorable condition. Next, in Fig. 2(b), we will research the emission spectra of the reduced and unreduced samples about MSLSO:0.075Eu excited at 267 and 290 nm, respectively. Compared with the unreduced sample, the reduced sample possess several sharp peaks, the intense emission peaks from transitions 5D1–7FJ (J = 0, 1, 2) and weak peaks from 5D2–7FJ (J = 0, 1, 2, 3) of Eu3+ appear to the emission spectra besides the peaks from 5D0–7FJ (J = 0, 1, 2, 3, 4) of Eu3+. According to our previous experimental results, given the energy transfer from Eu2+ ions to the Eu3+ ions, high-energy level emission of Eu3+. The emission spectra of the unreduced sample excited at 290 nm, where high energy level emission lines are not observed. The levels with J = 0 are normally not degenerated, so the 5D0–7F0 transition shows no more than one band at the spectrum. Two lines for the transition 5D0–7F0 indicate that the Eu3+ ions occupies the 4f and 6h positions.40 According to G. Blasse,41 for the unreduced sample, the Eu3+ 5D0–7F0 transition is observed as the strongest peak at 577 nm and the 5D0–7F0 emission of Eu3+ is rather intense, indicating a strong linear crystal-field component at the Eu3+ ion. This case is evident in the (6h) site. The site symmetry is Cs, with one free oxygen ion in the symmetry plane. For the reduced sample, we found two bands with peaks at 577 and 581 nm (see the Table 1) for the 5D0–7F0 transition, indicating that Eu3+ occupies two sites (6h and 4f, respectively) confirming the occurrence of two optical centers generated by Eu3+. The transitions of emission spectra were identified in Table 1.42–46| Wavelength (nm) | Transition | Wavelength (nm) | Transition |
|---|---|---|---|
| 489 | 5D3–7F5 | 585.5 | 5D1–7F3 |
| 460 | f6d–f7 (Eu2+) | 623 | 5D1–7F4 |
| 466 | 5D2–7F0 | 577, 581 | 5D0–7F0 |
| 473.5 | 5D2–7F1 | 587, 589.5, 594, 598 | 5D0–7F1 |
| 494.5 | 5D2–7F2 | 614.5, 619, 623 | 5D0–7F2 |
| 511 | 5D2–7F3 | 669 | 5D0–7F3 |
| 527 | 5D1–7F0 | 701.5, 704 | 5D0–7F4 |
| 538 | 5D1–7F1 | — | — |
| 555, 561 | 5D1–7F2 | — | — |
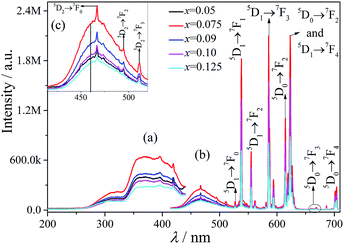
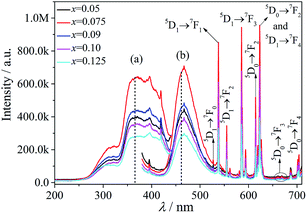
The excitation spectra of MSLSO:Eu2+/Eu3+ (x = 0.05, 0.075, 0.09, 0.10, and 0.125) are shown in Fig. 3(a) and 4(a) monitored at 460 nm, the excitation spectra consists of two distinct broad excitation bands peaking at 330 nm and 365 nm related to 4f7 (8S7/2) → 4f65d1 from Eu2+. The emission spectra of MSLSO:Eu2+/Eu3+ (x = 0.05, 0.075, 0.09, 0.10 and 0.125) are shown in Fig. 3(b) and (c) and 4(b), in addition, the insert Fig. 3(c) is from Fig. 3(b) between 415 and 520 nm, the whole emission spectra consists of a broad emission band peaking at 460 nm related to 4f65d1 → 4f7 (8S7/2) luminescence of Eu2+ and several sharp lines between the 530 nm and 710 nm region, ascribed to the 5DI → 7FJ (I = 0, 1, 2; J = 0, 1, 2, 3, 4) transitions in Eu3+. That is to say, part of the energy of Eu2+ transfer to Eu3+, another part for its own emission; in this case, it is possible to achieve white light emission. In the oxyapatite structure,34 Sr2+ occupy the 4f sites, the ionic radii of Eu2+ is very close to that of Sr2+, moreover, the Eu2+ ion match the Sr2+ electrovalence, so it is reasonable that Eu2+ will substitute for Sr2+ to occupy the 4f sites. Thus, the energy transfer between Eu2+ and Eu3+ occurs to the 4f sites.
The above results suggest that Eu2+ ions play a sensitizing agent role in the emission process and also acts as an activator.
To further understand the relationship plot between the intensity of 5D1, we constructed 5D0 emission and the Eu3+ ion concentration for the luminescence intensity of 5D0 and 5D1 under the excitation wavelength of 330 and 365 nm, as shown in Fig. 5. Evidently, the intensity of transition (5D0 and 5D1) increased with increasing Eu3+ ion concentration in the MSLSO host lattice from 5 mol% to 7.5 mol%; however, owing to concentration quenching, the emission intensity decreased when the Eu3+ ion concentration further increased over 7.5 mol%.
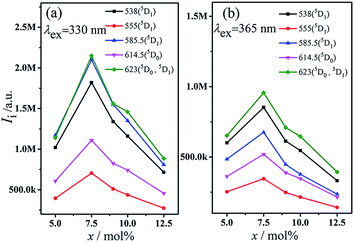 | ||
| Fig. 5 Intensity of 5D1, 5D0 emission excited at 330 (a) and 365 nm (b) for phosphors MSLSO:xEu2+/Eu3+ (x = 0.05, 0.075, 0.09, 0.10, and 0.125). | ||
In summary, whether excitation spectrum or emission spectrum, the intensity of the peaks gradually increase when x = 0.075, and the intensity of the peak reached the maximum and then gradually decreases with the increasing value of x. Overall, x = 0.075 is the optimal condition.
As seen from Fig. 3(b), 4(b), and 5, the ratio of the band intensity centered at 330 nm to that of centered at 365 nm in the excitation spectrum monitored at 460 nm is close to 1/2, whereas the ratio of the intensity of several emission peaks (538, 555, 585.5, 614.5, and 623 nm) of Eu3+ in the emission spectrum excited at 330 nm to the same emission peaks of Eu3+ in the emission spectrum excited at 365 nm is close to 2. This finding shows that the energy transfer from Eu2+ to Eu3+ mainly originated from the 330 nm excitation instead of the 365 nm excitation.
Moreover, according to the emission spectrum of the MSLSO:Eu2+/Eu3+ (0.075) excited at different wavelengths (λex = 267, 330, and 365 nm), the chromaticity coordinate were calculated to be (0.5008, 0.4518), (0.3664, 0.3260), and (0.2300, 0.2153). The coordinates are respectively displayed in the yellow, white, and blue regions. Both spectra are fitted in the 1931CIE chromaticity diagram as shown in Fig. 6(a), indicating that the emission hue is tunable from yellow to white and eventually to blue by adjusting the excitation wavelength. Moreover, Fig. 6(b) shows the color emission spectra of sample MSLSO:Eu2+/Eu3+ (0.075) excited at 330 nm, containing the composition of the three primary colors of white light so that white light emission is exhibited.
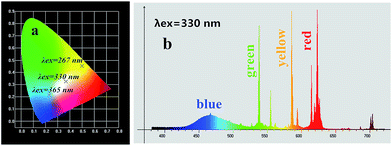 | ||
| Fig. 6 (a) CIE chromaticity diagram for MSLSO:Eu2+/Eu3+ (0.075) phosphor excited at 267, 330, and 365 nm; (b) the color emission spectra of sample MSLSO:Eu2+/Eu3+ (0.075) excited at 330 nm. | ||
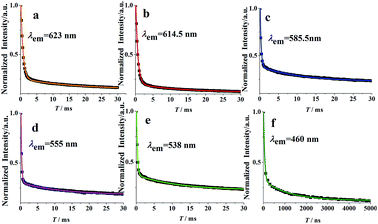
Fig. 7 illustrates the fluorescence decay curves of MSLSO:Eu2+/Eu3+ (0.075) phosphor at room temperature. The experimental decay curves of MSLSO:Eu2+/Eu3+ (0.075) can be best fitted with a double exponential eqn (1):
I(t) = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2 exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) + y0 exp(−t/τ2) + y0
| (1) |
Suggesting the significance of the energy transfer between Eu3+ ions and Eu2+ ions, where I is the luminescence intensity, A1 and A2 are constants, t is the time, τ1 and τ2 are the decay times for the exponential components. The average decay times τ can be estimated by the following eqn (2):47
| τ = (A1τ12 + A2τ22)/(A1τ1 + A2τ2) | (2) |
The fluorescence decay times of Eu2+ ions and Eu3+ ions are listed in Table 2. The lifetimes of 5D1 and 5D0 levels in the current study are considerably longer than those of the reports from ref. 48–51 and the lifetimes of 5D1 level are longer than those of the 5D0 level. We speculate that the energy transmission from 5D1 to 5D0 is interfered. As a result, the intense luminescence of 5D1 level is achieved due to the enhanced probability of radiation transition.
| Monitored wavelength (nm) | A1 | A2 | τ1 | τ2 | τ (ms) | Fitted function |
|---|---|---|---|---|---|---|
| 460 | 0.20 | 0.83 | 0.0008 | 0.00003 | 0.0007 | Double exponential |
| 538 | 0.92 | 0.15 | 0.18 | 7.96 | 7.01 | Double exponential |
| 555 | 1.09 | 0.12 | 0.15 | 8.10 | 6.95 | Double exponential |
| 586 | 0.89 | 0.16 | 0.19 | 7.51 | 6.60 | Double exponential |
| 614.5 | 0.97 | 0.08 | 0.41 | 8.34 | 5.50 | Double exponential |
| 623 | 0.90 | 0.11 | 0.45 | 11.02 | 8.34 | Double exponential |
Reduction of Eu3+ → Eu2+ was considered, which may be due to vacancy formation in the 4f crystal lattice position and the negative charge transfer by this vacancy to two types of Eu3+ ions.39
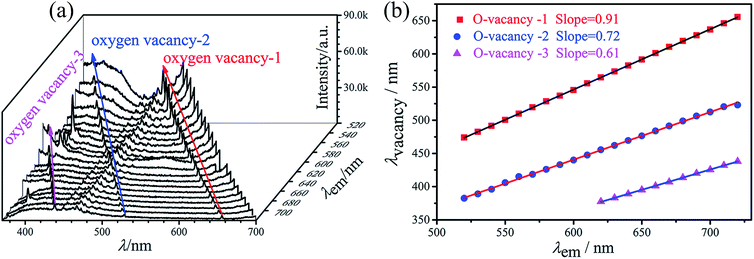
Fig. 8(a) displays three series of sharp peaks in the excitation spectra monitored at different wavelengths ranging from 474 nm to 655.5 nm, 382.5 nm to 523 nm, and 377.5 nm to 438 nm, respectively. These peaks all describe a number of sharp peaks with decreasing intensity, which are brought about by absorption of radiation by oxygen vacancies. As the wavelength of detected signals increases, the luminescence maxima of the peaks are displaced to the long-wave region of the spectrum. A similar displacement of lines in the excitation spectra is described in work.39,52,53 This displacement reflects an inhomogeneous character of oxygen vacancy distribution in the silicate matrix. Fig. 8(b) shows the peaks of oxygen vacancies vary from monitor wavelengths, the linear relationship formulas are as follows:
| λvacancy-1 = 0.91λmoni + 1.10 | (3) |
| λvacancy-2 = 0.72λmoni + 9.76 | (4) |
| λvacancy-3 = 0.61λmoni − 0.99 | (5) |
Under normal circumstances, transfer of energy from the 5D1 level to 5D0 level rapidly occurs in phosphors; therefore, the lifetime of the 5D1 level is relatively short and the lines of the intense 5D1 emission cannot be observed. In this host, the transfer of energy from 5D1 to 5D0 was hindered by oxygen vacancies; thus, the energy cannot be transmitted from 5D1 to 5D0. Therefore, the lines of the intense 5D1 emission can be observed and 5D1 level lifetimes are extremely long.
We infer two possible routes to transmit the excitation energy: one route is direct transmission to levels of Eu3+, and another route is through the oxygen vacancies trapping the energy and then releasing the energy again. Direct transmission is fast, and the release process is slow. As the slow release process leading to the decay curves displayed double exponential form, we summarize these two processes as a slow process that determines 5D1 level with extraordinary long lifetime and superior strength.
Conclusions
MSLSO doped with Eu phosphors were synthesized via high-temperature solid phase method. XRD analysis shows a typical oxyapatite structure with the space group of P63/m. The broad excitation spectra ranging from 220 nm to 430 nm, matched well with UV LED chips. Luminescence measurements indicate that the emission spectrum of as-obtained phosphors contain both the characteristic emissions of Eu2+ and Eu3+ ions, in which the broad band luminescence of Eu2+ and narrower 4f → 4f luminescent of Eu3+ can be observed upon 330 and 365 nm excitation. With increasing Eu contents, the relative intensity of the red component from Eu3+ become stronger gradually, whereas that of Eu2+ decreases, correspondingly. In addition, the optimal doping contents of Eu is confirmed to be 7.5 mol% (x = 0.075). Moreover, in the silicate lattice, inhomogeneously distributed oxygen vacancies are responsible for nonradiative transfer of excitation energy. The oxygen vacancies trap and release the energy, and the release process is slow. The slow release process leads to the decay curves displayed double exponential form. The CIE chromaticity diagram for MSLSO:Eu2+/Eu3+ (0.075) phosphor excited at 330 nm is displayed in the white region. In other words, by adjusting the concentration of Eu, the white light can be realized with the CIE coordinate (0.3664, 0.3260). Therefore, MSLSO:Eu2+/Eu3+ (0.075) is predicted to be a promising candidate for WLEDs.Acknowledgements
The work is financially supported by the National Natural Science Foundation of China (Grant no. 51202166).References
- M. Shang, X. Li and J. Lin, Chem. Soc. Rev., 2014, 43, 1372 RSC.
- X. Bai, G. Caputo, Z. D. Hao, V. T. Freitas, J. H. Zhang, R. L. Longo, O. L. Malta, R. A. S. Ferreira and N. Pinna, Nat. Commun., 2014, 5, 5702 CrossRef CAS PubMed.
- H. J. Yu, K. Park, W. Chung, J. Kim and S. H. Kim, Synth. Met., 2009, 159, 2474 CrossRef CAS.
- C. Yang, X. J. Liang, X. X. Di, P. Z. Li, G. C. Hu, R. Cao and W. D. Xiang, Ceram. Int., 2016, 42, 14526 CrossRef CAS.
- N. C. George, K. A. Denault and R. Seshadri, Annu. Rev. Mater. Res., 2013, 43, 481 CrossRef CAS.
- K. Li, J. Fan, M. M. Shang, H. Z. Lian and J. Lin, J. Mater. Chem. C, 2015, 3, 9989 RSC.
- Y. L. Ding, Y. X. Zhang, Z. Y. Wang, W. Li, D. L. Mao, H. B. Han and C. K. Chang, J. Lumin., 2009, 129, 294 CrossRef CAS.
- C. H. Lu and P. C. Wu, J. Alloys Compd., 2008, 466, 457 CrossRef CAS.
- Y. X. Cao, G. Zhu and Y. H. Wang, RSC Adv., 2015, 5, 65710 RSC.
- H. K. Liu, L. B. Liao, M. S. Molokeev, Q. F. Guo, Y. Y. Zhang and L. F. Mei, RSC Adv., 2016, 6, 24577 RSC.
- D. F. Peng, Q. Ju, X. Chen, R. H. Ma, B. Chen, G. X. Bai, J. H. Hao, X. S. Qiao, X. P. Fan and F. Wang, Chem. Mater., 2015, 27, 3115 CrossRef.
- D. Y. Wang, Y. J. Kang, X. C. Ye and C. B. Murray, Chem. Mater., 2014, 26, 6328 CrossRef CAS.
- C. H. Huang, T. S. Chan, W. R. Liu, D. Y. Wang, Y. C. Chiu, Y. T. Yeh and T. M. Chen, J. Mater. Chem., 2012, 22, 20210 RSC.
- Z. Chen, J. H. Zhang, S. Chen, M. Y. Lin, C. Q. He, G. D. Xu, M. M. Wang, X. F. Yu, J. Q. Zou and K. Guo, J. Alloys Compd., 2015, 632, 756 CrossRef CAS.
- Y. Y. Zhang, Z. G. Xia, H. K. Liu, Z. Y. Wang and M. L. Li, Chem. Phys. Lett., 2014, 593, 189 CrossRef CAS.
- W. Z. Lv, M. M. Jiao, Q. Zhao, B. Q. Shao, W. Lü and H. P. You, Inorg. Chem., 2014, 53, 11007 CrossRef CAS PubMed.
- S. H. Miao, Z. G. Xia, J. Zhang and Q. L. Liu, Inorg. Chem., 2014, 53, 10386 CrossRef CAS PubMed.
- M. M. Jiao, Y. C. Jia, W. Lü, W. Z. Lv, Q. Zhao, B. Q. Shao and H. P. You, J. Mater. Chem. C, 2014, 2, 90 RSC.
- P. L. Li, Z. J. Wang, Z. P. Yang and Q. L. Guo, J. Mater. Chem. C, 2014, 2, 7823 RSC.
- N. Guo, Y. H. Zheng, Y. C. Jia, H. Qiao and H. P. You, J. Phys. Chem. C, 2012, 116, 1329 CAS.
- Y. L. Jia, R. Pang, H. F. Li, W. Z. Sun, J. P. Fu, L. H. Jiang, S. Zhang, Q. Su, C. Y. Li and R. S. Liu, Dalton Trans., 2015, 44, 11399 RSC.
- Y. Liu, X. Zhang, Z. Hao, X. Wang and J. Zhang, Chem. Commun., 2011, 47, 10677 RSC.
- M. M. Jiao, Y. C. Jia, W. Lü, W. Z. Lv, Q. Zhao, B. Q. Shao and H. P. You, Dalton Trans., 2014, 43, 3202 RSC.
- W. Lü, N. Guo, Y. C. Jia, Q. Zhao, W. Z. Lv, M. M. Jiao, B. Q. Shao and H. P. You, Inorg. Chem., 2013, 52, 3007 CrossRef PubMed.
- X. Y. Mi, J. C. Sun, P. Zhou, H. Y. Zhou, D. Song, K. Li, M. M. Shang and J. Lin, J. Mater. Chem. C, 2015, 3, 4471 RSC.
- M. F. Zhang, Y. J. Liang, R. Tang, D. Y. Yu, M. H. Tong, Q. Wang, Y. L. Zhu, X. Y. Wu and G. G. Li, RSC Adv., 2014, 4, 40626 RSC.
- W. Lü, Y. C. Jia, W. Z. Lv, Q. Zhao and H. P. You, Opt. Mater., 2015, 42, 62 CrossRef.
- Z. J. Wang, P. L. Li, Z. P. Yang, Q. L. Guo and G. Y. Dong, Ceram. Int., 2014, 40, 15283 CrossRef CAS.
- H. K. Liu, Y. Luo, Z. Y. Mao, L. B. Liao and Z. G. Xia, J. Mater. Chem. C, 2014, 2, 1619 RSC.
- D. L. Geng, M. M. Shang, Y. Zhang, H. Z. Lian, Z. Y. Cheng and J. Lin, J. Mater. Chem. C, 2013, 1, 2345 RSC.
- W. P. Chen, Dalton Trans., 2015, 10, 1039 Search PubMed.
- R. J. Yu, J. Wang, Z. Zhao, M. X. Li, S. D. Huo, J. B. Li and J. Y. Wang, Mater. Lett., 2015, 160, 294 CrossRef CAS.
- A. Baran, S. Mahlik, M. Grinberg, P. Cai, S. I. Kim and H. J. Seo, J. Phys.: Condens. Matter, 2014, 26, 385401 CrossRef CAS PubMed.
- J. Sokolnicki and E. Zych, J. Lumin., 2015, 158, 65 CrossRef CAS.
- H. Bouchouicha, G. Panczer, D. deLigny, Y. Guyot, M. L. Baesso, L. H. C. Andrade, S. M. Lima and R. Ternane, J. Lumin., 2016, 169, 528 CrossRef CAS.
- Y. J. Masubuchi, M. Higuchi, T. Takeda and S. Kikkawa, Solid State Ionics, 2006, 177, 263 CrossRef CAS.
- L. C. Leu, S. Thomas, M. T. Sebastian, S. Zdzieszynski, S. Misture and R. Ubicz, J. Am. Ceram. Soc., 2011, 94, 2625 CrossRef CAS.
- C. Peng, X. J. Kang, G. G. Li, Z. Y. Hou, C. X. Li and J. Lin, J. Electrochem. Soc., 2011, 158, J208 CrossRef CAS.
- M. G. Zuev, A. M. Karpov and A. S. Shkvarin, J. Solid State Chem., 2011, 184, 52 CrossRef CAS.
- M. G. Zuev, S. Y. Sokovnin, V. G. Il'ves, I. V. Baklanova and A. A. Vasin, J. Solid State Chem., 2014, 218, 164 CrossRef CAS.
- G. Blasse, J. Solid State Chem., 1975, 14, 181 CrossRef CAS.
- C. A. Kodaira, H. F. Brito, O. L. Maltab and O. A. Serrac, J. Lumin., 2003, 101, 11 CrossRef CAS.
- H. Ronde, D. M. Krol and G. Blasse, J. Electrochem. Soc., 1977, 124, 1276 CrossRef CAS.
- Y. Y. Tsai, H. R. Shih, M. T. Tsai and Y. S. Chang, Macromol. Chem. Phys., 2014, 143, 611 CAS.
- F. Pelle, N. Gardant, M. Genotelle, P. Goldner and P. Porcher, J. Phys. Chem. Solids, 1995, 56, 1003 CrossRef CAS.
- H. Yu, D. G. Deng, L. F. Chen, D. Q. Chen, J. S. Zhong, H. T. Zhao and S. Q. Xu, Ceram. Int., 2015, 41, 3800 CrossRef CAS.
- N. Ruelle, M. P. Thi and C. Fouassier, Jpn. J. Appl. Phys., 1992, 31, 2786 CrossRef CAS.
- K. H. Jang, W. K. Sung, E. S. Kim, L. Shi, J. H. Jeong and H. J. Seo, J. Lumin., 2009, 129, 1853 CrossRef CAS.
- M. D. Chambers, P. A. Rousseve and D. R. Clarke, J. Lumin., 2009, 129, 263 CrossRef CAS.
- C. Bensalem, M. Mortier, D. Vivien and M. Diaf, Opt. Mater., 2011, 33, 791 CrossRef CAS.
- M. Gaft, R. Reisfeld, G. Panczer, P. Blank and G. Boulon, Spectrochim. Acta, Part A, 1998, 54, 2163 CrossRef.
- G. S. Huang, X. L. Wu, Y. F. Mei, X. F. Shao and G. G. Siu, J. Appl. Phys., 2003, 93, 582 CrossRef CAS.
- F. F. Komarov, A. V. Mudryi, L. A. Vlasukova, N. I. Mukhurov and A. V. Ivanyukovich, Opt. Spectrosc., 2008, 104, 235 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |