Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Laccase catalyzed grafting of –N–OH type mediators to lignin via radical–radical coupling†
L. Munka,
A. M. Puntb,
M. A. Kabelb and
A. S. Meyer *a
*a
aCenter for BioProcess Engineering, Department of Chemical and Biochemical Engineering, Technical University of Denmark, Building 229, DK-2800 Kgs. Lyngby, Denmark. E-mail: am@kt.dtu.dk
bLaboratory of Food Chemistry, Wageningen University, Bornse Weilanden 9, 6708 WG, Wageningen, The Netherlands
First published on 13th January 2017
Abstract
Lignin is an underexploited resource in biomass refining. Laccases (EC 1.10.3.2) catalyze oxidation of phenolic hydroxyls using O2 as electron acceptor and may facilitate lignin modification in the presence of mediators. This study assessed the reactivity of four different synthetic mediators by laccases from Trametes versicolor and Pleurotus ostreatus by quantitative analysis of the reaction outcome by pyrolysis gas chromatography mass spectroscopy. The two laccases were equally efficient in catalyzing grafting, but only –N–OH type mediators grafted. HPI (N-hydroxyacetanilide) grafted 7–10 times better than HBT (1-hydroxybenzotriazole). Three different mechanisms are suggested to explain the grafting of HPI and HBT, all involving radical–radical coupling to produce covalent bonding to lignin. Lignin from exhaustive cellulase treatment of wheat straw was more susceptible to grafting than beech organosolv lignin with the relative abundance of grafting being 35% vs. 11% for HPI and 5% vs. 1% for HBT on these lignin substrates. The data imply that lignin can be functionalized via laccase catalysis with –N–OH type mediators.
Introduction
Comprising 15–30% of the available carbon source in plant biomass, the utilization of lignin is important for development towards a future bioeconomy.1–3 Studies show that up to 60% more lignin is present in today's biorefineries than that needed for process energy.2 Hence, lignin may be considered as an abundantly available raw material for developing new value added biorefinery products without compromising internal process requirements. As an amorphous alkyl–aromatic biopolymer, the lignin structure is distinctly different from other biomass polymers. Lignin is synthesized by radical polymerization of primarily three different phenylpropanoid subunits p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) and minor amounts of related derivatives.4 These subunits are cross-linked through a network of several types of ether and C–C bonds, which closely surrounds and crosslinks to the carbohydrate polymers in the plant cell wall.3,5One option for valorisation of lignin is surface modification by heterogeneous catalysis, chemoenzymatic catalysis or biocatalysis.6 Biocatalytic or enzymatic lignin functionalization involves grafting of molecules of interest onto radical-reactive lignin moieties in turn changing the functionality of lignin.7,8 The biocatalytic approach has the advantage that it can be done under mild reaction conditions, notably with respect to temperature.6
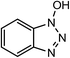
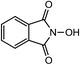
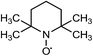
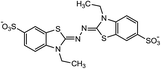
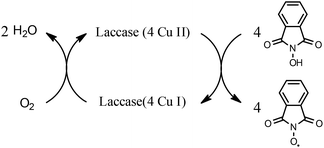
Laccases (EC 1.10.3.2) catalyze oxidation of hydroxyl groups of mono- and diphenols via the sequential removal of an electron and a proton from the phenolic hydroxyl groups; the catalysis takes place via transfer of 4 electrons per round of catalysis with simultaneous reduction of one molecule of O2 to two molecules of H2O.9,10 The oxidizing capacity of laccases is defined by the redox potential of the T1 copper site in the enzyme and the redox potential is known to be related to the ability of the enzyme to catalyze the abstraction of electrons from phenolic substrates.10 Among laccases, the fungally derived enzymes have particularly high redox potentials E0 ranging from 0.5 to 0.8 V vs. the Normal Hydrogen Electrode (NHE).11 Laccases have been shown to catalyze oxidation of the phenolic subunits representing 10–30% of the units in native lignin.12 The aliphatic alcohols and ether groups in native lignin are more resistant to oxidation (redox potential (E0) > 1.2 V vs. NHE),11 and cannot directly be oxidized by laccase catalysis.13,14 As indicated in lignin model systems, laccases may also catalyze oxidation of non-phenolic lignin units (C4-etherified) to radicals, but only when acting via a mediator.15–17 Upon oxidation by laccase, the oxidized mediator (often in form of a radical) oxidizes non-phenolic substrates according to mechanisms not available to laccases and return to its original reduced form.18 In this way the mediator works as a shuttle between laccase and lignin, which requires high stability of the compound acting as mediator both in its reduced and oxidized radical form.19 Artificial mediators commonly used in laccase catalysis studies include HBT (1-hydroxybenzotriazole), HPI (N-hydroxy-acetanilide), TEMPO (2,2,6,6-tetra-methyl-piperidin-1-yloxy) and ABTS (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonate)) (Table 1). Mediators such as HBT and HPI containing an –N–OH structure, react oxidatively with lignin model compounds via the radical Hydrogen Atom Transfer (HAT) route,10,20,21 whereas TEMPO and ABTS react via an ionic route and the socalled Electron Transfer (ET) route, respectively.20–22
Laccase treatments and laccase-mediator treatments (LMTs) of lignocellulosic biomass can thus facilitate different changes in lignin including direct oxidative modifications, where Cα oxidation from alcohol to carbonyl is a commonly reported change,23–25 and radical coupling where the outcome will vary depending on the extent of reaction and on the type of molecule being coupled to the lignin. When lignin precursors are continuously coupled to lignin to increase the molecular weight the process results in polymerization, whereas the case where single molecules are attached to the surface of lignin is referred to as grafting.13,26 Treatment of lignin with fungal laccases has thus been shown to graft molecules of phenolic nature including phenolic acids, phenolic amines, and fluorophenols onto lignocellulosic material.8 On this basis we hypothesized that LMT reaction systems would facilitate oxidative modification of lignin and that the effect might differ depending on the type of mediator used, the type of enzyme, and maybe even vary depending on the type of lignin-substrate employed.
This study was therefore undertaken to investigate modifications of lignin facilitated by laccase treatments and LMTs, and to explore the options for upgrading of lignin via such modifications. For this purpose high redox potential laccases from Trametes versicolor (TvL), (E0 = 0.79 V vs. NHE),27 and Pleurotus ostreatus (PoL), (E0 = 0.65 V vs. NHE),28,29 were each applied in combination with each of four selected mediators HBT, HPI, TEMPO, and ABTS (Table 1) to treat two types of lignin-enriched biomass, referred to as wheat straw lignin (WSL) and beech organosolv lignin (BOL). After reaction, modifications in the WSL and BOL were assessed by Pyrolysis-Gas Chromatography-Mass Spectrometry (Py-GC/MS) analysis.
Results and discussion
Lignin substrate changes after enzyme-mediator treatment
Py-GC/MS analysis of the two lignin substrates (having undergone 24 h control incubation in buffer at 50 C, pH 4.8, but without any laccase or mediator or laccase-mediator added) showed differences in their pyrogram profiles (Fig. 1). The WSL showed a characteristic high abundance of 4-vinylguaiacol (peak 4G, Fig. 1a and Table S2†) in accordance with what has been found by others.30 The pyrogram also contained a very high peak at approximately 25 min, which was identified as being levoglucosan (by using Amdis software (version 2.71, NIST, USA)). The pyrogram profile of the BOL showed relatively high abundancies of several peaks, including notably syringol (peak 7S), 4-methylsyringol (peak 10S), syringaldehyde (peak 20S), and trans-sinapyl alcohol (27S) (Fig. 1b, ESI Table S5†).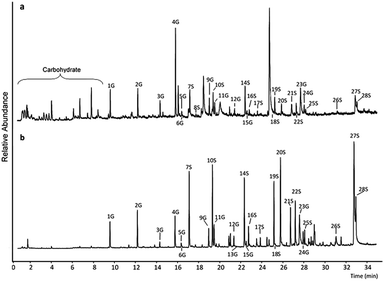 | ||
| Fig. 1 Pyrograms of WSL and BOL after pyrolysis. (a) Blank WSL sample. (b) Blank BOL sample. Annotated peaks were identified as G and S lignin residues originating from lignin. The annotations refer to Table S1 in the ESI.† | ||
The more frequent occurrence of peaks at low retention time (RT) in the pyrogram of WSL (Fig. 1a) compared to the pyrogram of BOL (Fig. 1b), is interpreted as being due to the higher abundance of carbohydrates in the WSL. The relative abundance of all identified and annotated syringyl (S) and guaiacyl (G) residues (ESI Table S1†) provided the basis for calculating the ratio of syringyl-like to guaiacyl-like lignin units (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratio) before and after the different laccase-mediator treatments (Table 2). The S
G ratio) before and after the different laccase-mediator treatments (Table 2). The S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratio for all the differently treated WSL samples (and the controls) varied from 0.58 to 0.65 and no particular pattern or effect of the treatments could be discerned. The data did however signify a dominance of G residues in wheat straw lignin, which is concurrent with data published by others.30 The S
G ratio for all the differently treated WSL samples (and the controls) varied from 0.58 to 0.65 and no particular pattern or effect of the treatments could be discerned. The data did however signify a dominance of G residues in wheat straw lignin, which is concurrent with data published by others.30 The S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratios for the similarly treated BOL samples did not show any differences among treatments, and varied from 2.48 to 2.74 (Table 2). The S
G ratios for the similarly treated BOL samples did not show any differences among treatments, and varied from 2.48 to 2.74 (Table 2). The S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G data for the BOL signified a dominance of S residues in beech wood lignin, which is in complete agreement with published data for organosolv hardwoods.31 Laccase treatments and LMTs thus did not seem to affect the overall S
G data for the BOL signified a dominance of S residues in beech wood lignin, which is in complete agreement with published data for organosolv hardwoods.31 Laccase treatments and LMTs thus did not seem to affect the overall S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratio in any of the lignin substrates.
G ratio in any of the lignin substrates.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratio for each treatment of the two substrates WSL and BOL. S
G ratio for each treatment of the two substrates WSL and BOL. S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratio was calculated based on total molar area of syringyl residues (S) and guaiacyl residues (G) pr. μg loaded sample; no lac is control without enzyme added
G ratio was calculated based on total molar area of syringyl residues (S) and guaiacyl residues (G) pr. μg loaded sample; no lac is control without enzyme added
| No lac | TvL | PoL | HBT | HPI | TEMPO | ABTS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No lac | TvL | PoL | No lac | TvL | PoL | No lac | TvL | PoL | No lac | TvL | PoL | |||||
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G G |
WSL | 0.62 | 0.64 | 0.63 | 0.61 | 0.61 | 0.61 | 0.63 | 0.65 | 0.63 | 0.58 | 0.61 | 0.6 | 0.62 | 0.6 | 0.62 |
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G G |
BOL | 2.69 | 2.71 | 2.74 | 2.51 | 2.53 | 2.63 | 2.54 | 2.68 | 2.63 | 2.52 | 2.53 | 2.48 | 2.48 | 2.55 | 2.5 |
Changes in the relative occurrence of lignin residues
Even though the overall S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratios did not change in response to any of the treatments it was assessed whether there were differences in the relative occurrence of individual residues within the S and G distribution after the different treatments. The relative occurrence for each lignin related residue was presented in percent, i.e. each G-compound compared to the total G molar area and per S-compound compared to the total S molar area (ESI Tables S2–S5†). Generally, only minor differences within the distribution of S and G residues were observed in the lignin after laccase treatments and laccase-mediator treatments (LMTs), and differences were moreover almost exclusively seen in the WSL samples. The most dominant change was a decrease in the relative occurrence of 4-vinyl guaiacol (G), which were seen for all laccase and laccase-mediator treated samples, but not when the mediators were added alone (ESI: Table S2†).
G ratios did not change in response to any of the treatments it was assessed whether there were differences in the relative occurrence of individual residues within the S and G distribution after the different treatments. The relative occurrence for each lignin related residue was presented in percent, i.e. each G-compound compared to the total G molar area and per S-compound compared to the total S molar area (ESI Tables S2–S5†). Generally, only minor differences within the distribution of S and G residues were observed in the lignin after laccase treatments and laccase-mediator treatments (LMTs), and differences were moreover almost exclusively seen in the WSL samples. The most dominant change was a decrease in the relative occurrence of 4-vinyl guaiacol (G), which were seen for all laccase and laccase-mediator treated samples, but not when the mediators were added alone (ESI: Table S2†).
It was also observed that the occurrence of other residues in the S and G distribution decreased or increased, and the residues affected correlated with the use of mediator (ESI Tables S2 and S3†). Changes in BOL were only observed after treatments involving ABTS.
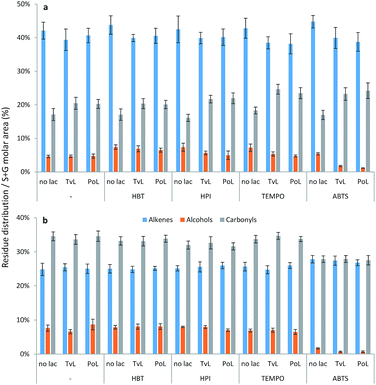
By grouping the relative abundances of S and G residues according to occurrence of aliphatic alkenes, alcohols, and carbonyls (alkanes not included), the mild effects of laccase treatments and LMTs on the substrates became more distinct (Fig. 2).
In the WSL samples, both sole laccase treatments and LMTs decreased the occurrence of aliphatic alkene residues by 2–6% and simultaneously produced an increase of aliphatic carbonyl residues by 3–7% compared to the controls (Fig. 2a) thus indicating oxidation. Laccase–TEMPO and laccase–ABTS treatments were most effective in catalyzing these oxidative conversions as these treatments induced decreases in the relative occurrence of aliphatic alkene residues of 5–6% and a corresponding increase in the aliphatic carbonyl residues of 6–7% (Fig. 2a); the slightly higher level of carbonyl residues appearing than alkene residues disappearing may be due to oxidative conversion of aliphatic alcohols, too. Similar grouping of S and G residues in BOL did not reveal any modification by LMTs (Fig. 2b).
Only the presence of ABTS resulted in a substantial decrease of alkenes and alcohols with a resulting increase of aliphatic alkanes (from 30% to 40% – data not shown). There were no differences in outcome between samples treated with TvL and PoL.
Grafting of mediators
Upon comparing pyrograms of samples having undergone LMTs with HBT and HPI, respectively (Fig. 3b, c, e and f and 4b, c, e and f) with corresponding pyrograms of samples having undergone sole mediator treatments (Fig. 4a and d and 5a and d), it became evident that new peaks occurred in pyrograms of samples treated with laccase-HBT at RT = 24.35 min, and at RT = 21.80 min for samples treated with laccase-HPI. Analysis of the pure mediator compounds with Py-GC/MS confirmed that the peaks originated from each of the mediators, HBT (m/z 64, 91, 119) and HPI (m/z 76, 104, 147). This proved, that for both WSL and BOL, the mediators were present after LMTs and subsequent washing of the biomass, but absent in the samples treated with mediators alone. These observations suggest that the laccases (TvL and PoL) catalyzed incorporation of HBT and HPI into the insoluble part of lignin by grafting.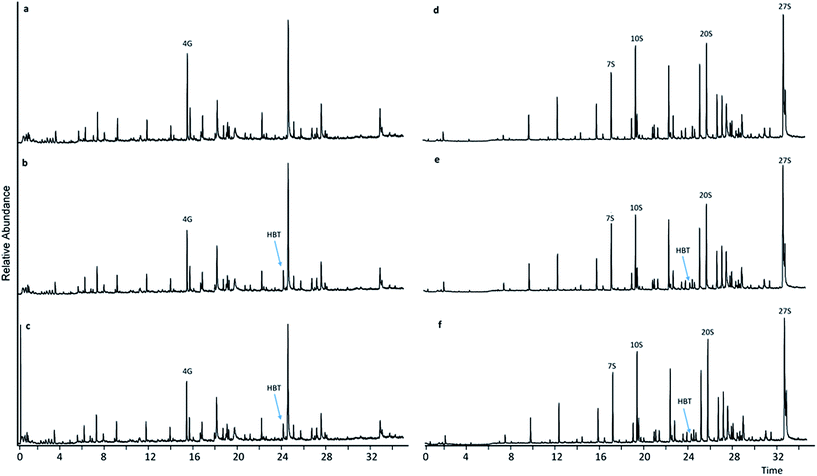 | ||
| Fig. 3 Pyrograms of WSL (a–c) and BOL (d–f) pyrograms. (a) HBT treatment, (b) HBT + TvL treatment, (c) HBT + PoL treatment, (d) HBT treatment, (e) HBT + TvL treatment, (f) HBT + PoL treatment. | ||
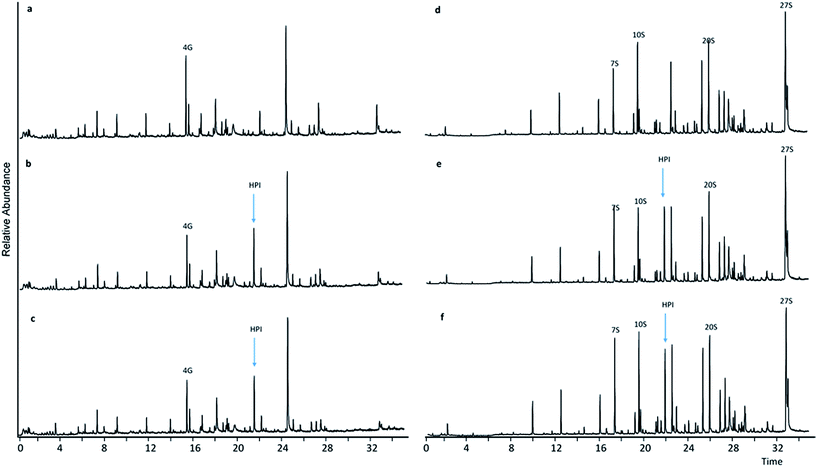 | ||
| Fig. 4 Pyrograms of WSL (a–c) and BOL (d–f) pyrograms. (a) HPI treatment, (b) HPI + TvL treatment, (c) HPI + PoL treatment, (d) HPI treatment, (e) HPI + TvL treatment, (f) HPI + PoL treatment. | ||
In contrast, in pyrograms of WSL and BOL treated with laccase-TEMPO or laccase-ABTS there was no trace of either TEMPO or ABTS in the lignin residues. It was therefore assumed that in the case any new compounds had formed via the latter treatments, they would have been removed in the washing steps and not grafted onto lignin.
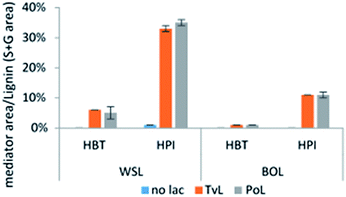
The relative abundance of the grafted mediator structures was assessed from the molar area of the mediator/total S + G molar area (Fig. 5). In WSL samples treated with laccase-HPI, the occurrence of HPI in the biomass comprised what corresponded to 32% (TvL) and 35% (PoL) of the molar amount of total S and G residues, whereas treatment with laccase-HBT only resulted in 6% (TvL) and 5% (PoL) occurrence relative to the molar amount of total S and G residues.
On BOL a similar result, i.e. greater tendency of incorporating the HPI compared to HBT was seen. Even though the grafting of mediators was also apparent, the data showed that BOL was much less susceptible towards having mediators incorporated. LMTs with both TvL and PoL resulted in 11% and 1% occurrence relative to the molar amount of the total S and G residues for HPI and HBT, respectively. The almost equal relative occurrence of mediators by use of either TvL or PoL indicated an equal efficiency of grafting mediators onto lignin.
Activity of enzymes during treatment
The activity retainment of TvL and PoL under the enzymatic treatment conditions showed that TvL and PoL responded uniformly to the different conditions, although the T1/2 values suggest a slightly more stable nature of the PoL compared to the TvL (Table 3).| Lignin | Laccase | — | HBT | HPI |
|---|---|---|---|---|
| T1/2 [min] | T1/2 [min] | T1/2 [min] | ||
| — | TvL | 372 ± 12b,x | 28 ± 1b,y | 26 ± 1c,y |
| PoL | 452 ± 18a,x | 27 ± 1b,y | 30 ± 2c,y | |
| WSL | TvL | 264 ± 23c,x | 223 ± 6a,y | 243 ± 15a,b,x,y |
| PoL | 355 ± 21b,x | 231 ± 9a,y | 263 ± 23a,y | |
| BOL | TvL | 140 ± 7d,y | 229 ± 32a,x | 210 ± 11b,x |
| PoL | 175 ± 12c,y | 226 ± 31a,x,y | 252 ± 11a,b,x |
When TvL and PoL were incubated with mediators, without any biomass present, rapid activity decay occurred equivalent to the T1/2 dropping from 372–452 min (no mediators) to 26–30 min (with mediators) (Table 3, ESI Fig. S1a and b† (only data for the –N–OH type mediators, HBT and HPI are shown)). In the presence of biomass the activity retainment of the enzymes with mediators present improved roughly 10 times (Table 3).
In the presence of WSL without any mediators, the T1/2 indicated a slightly faster decay of activity compared to the control in water (TvL: 264 vs. 372 min, PoL: 355 vs. 452 min, Table 3). T1/2 was reduced by more than half in presence of BOL compared to in water (TvL: 140 vs. 372 min, PoL: 175 vs. 452 min, Table 3). These data suggest that the BOL had a more negative effect on the enzyme activity than the WSL – however, in the presence of mediators, the two substrates improved activity retainment equally well. As a last point, HBT and HPI did not show any profoundly different effects on enzyme activity (Table 3).
Action and robustness of the laccases
The two laccases, TvL and PoL, displayed similar effects and appeared to act equally well on the substrates confirming that the equal activity dosing of the two laccases measured on syringaldazine could be transferred to LMTs on the two lignin biomasses. The uniform effect also points to the fact that in spite of a lower redox potential of PoL (E0 = 0.65 V vs. NHE) the observed changes in lignin were just as extensive as those observed for TvL (E0 = 0.79 V vs. NHE), thus indicating that the impact of redox potential might be less significant in LMTs of biomass.Measurements of activity during treatment and calculation of T1/2 provided information about how long the LMTs retained the capacity to oxidize. PoL was found to be slightly more stable than TvL, but this was not reflected in the Py-GC/MS results from LMTs. Instability of the laccase-mediator system in absence of biomass might be explained by the generation of radicals that, instead of acting on the biomass, presumably act on the enzymes themselves, which causes degradation. The acidic nature of BOL is also assumed to be the cause of a faster decline in the relative activity of the laccase in presence of BOL.
The reactivity of the two different lignin substrates
Results from Py-GC/MS analyses consistently showed that the lignin in the biomass from wheat straw was more prone to modification than the lignin from the beech wood (Fig. 2–5). The differences in the propensity to modification of the two lignin substrates might be due to a range of different factors. First, the higher content of guaiacyl-like units as compared to syringyl-like units in the wheat straw lignin, WSL, compared to the beech wood lignin, BOL (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratios of 0.6 vs. 2.7, Table 2), may have impacted the accessibility of the laccases to the phenolic –OH; the phenolic –OH group in the guaiacyl-like units may be more accessible than the equivalent –OH group in the syringyl, because the additional methoxy group in the syringyl-like units may contribute to steric hindrance, and thereby less reactivity.32 Another possible influencing factor related to the lignin biomass origin, is that beech wood (hardwood) lignin contains relatively more core-lignin units compared to wheat straw lignin, which contain considerable amounts of ester linked ferulates from crosslinking and p-coumarates linked to terminal units of the core-lignin.30,33
G ratios of 0.6 vs. 2.7, Table 2), may have impacted the accessibility of the laccases to the phenolic –OH; the phenolic –OH group in the guaiacyl-like units may be more accessible than the equivalent –OH group in the syringyl, because the additional methoxy group in the syringyl-like units may contribute to steric hindrance, and thereby less reactivity.32 Another possible influencing factor related to the lignin biomass origin, is that beech wood (hardwood) lignin contains relatively more core-lignin units compared to wheat straw lignin, which contain considerable amounts of ester linked ferulates from crosslinking and p-coumarates linked to terminal units of the core-lignin.30,33
However, the different types of pretreatment employed for the two biomasses may be of even more importance. WSL had been hydrothermically pretreated and enzymatically hydrolyzed, a treatment known to be mild, maintaining many of the native characteristics of lignin, whereas the BOL was a direct result of organosolv (ethanol) pretreatment. Even though organosolv processing is also known as a gentle way of extracting lignin, studies on hardwood organosolv lignin have shown that this process can change the lignin by breaking the β-O-4 bonds and create more condensed C–C bonds.34,35 Such change would decrease the reactivity of lignin, since β-O-4 and other ether bonds are known to be the most reactive bond in lignins, and the expected outcome of LMTs on such lignin must be a less modified lignin compared to a lignin with a more native structure.36
Changes in the composition of lignin in response to laccase-mediator treatments
The relatively limited modifications observed in the lignin substrates by Py-GC/MS after LMTs was expected since treatments of lignin with laccase-mediator systems must be regarded as mild treatments. The fact that no changes in lignin were observed after sole mediator treatments (except with ABTS) (Fig. 2), confirmed that laccase is needed to activate the mediators.Of the individual lignin residues, modification of 4-vinylguaiacol (G), as analyzed by Py-GC/MS, was responsible for the most pronounced relative change in WSL, being reduced by all laccase and laccase-mediator treatments (ESI, Table S2†). Presence of ferulates in wheat straw contributes to the relative amount of the 4-vinylguaiacol residues, when analyzed by Py-GC/MS. Thus, it is not known if the decrease is caused by modification on the core-lignin or the ferulate cross-linkages in wheat straw. A relative decrease of 4-vinylguaiacol (G) has been reported before as result of growth on wheat straw compost of the fungus Agaricus bisporus, that expresses laccase.37 A decrease in guaiacyl-like lignin units, related with increased S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratios and associated with lignin degradation after laccase-mediator treatment has also been reported by others,24 supporting the hypothesis that syringyl-like units are more recalcitrant towards modification by LMTs compared to guaiacyl-like units. In the present work, minor relative changes were observed within the S and G distribution after LMTs, but a clear effect on the overall S
G ratios and associated with lignin degradation after laccase-mediator treatment has also been reported by others,24 supporting the hypothesis that syringyl-like units are more recalcitrant towards modification by LMTs compared to guaiacyl-like units. In the present work, minor relative changes were observed within the S and G distribution after LMTs, but a clear effect on the overall S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratio was not observed. A plausible explanation for the lacking shift in S
G ratio was not observed. A plausible explanation for the lacking shift in S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratio could be that LMTs under the given conditions tended to modify (e.g. oxidize) the surface of lignin rather than degrading it; such oxidation would not change the S
G ratio could be that LMTs under the given conditions tended to modify (e.g. oxidize) the surface of lignin rather than degrading it; such oxidation would not change the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratio analyzed by Py-GC/MS.
G ratio analyzed by Py-GC/MS.
Dependent on the type of mediator used, different individual G and S residues were affected after treatment (ESI, Tables S2–S5†). This selectivity may be related to the different mechanisms by which the mediators act.18,38 HBT and HPI are both N–OH type mediators. In LMTs, they promote a Hydrogen Atom Transfer (HAT) route through the formation of a corresponding N-oxyl radical (Table 1).20,29 In contrast, TEMPO operates through an ionic route in a laccase-mediator system by formation of an oxammonium ion, whereas ABTS follows an Electron Transfer (ET) route (Table 1).39 These different routes have been shown to favor formation of different radical intermediates, which in turn appear to influence which subunits in lignin are activated and how these are modified. F. d'Acunzo et al.40 reported that a laccase–TEMPO system was more competent for oxidation of benzyl alcohols (model compound for aliphatic alcohols in lignin), whereas a corresponding laccase–HBT treatment was more competent for oxidation of ether model compounds. In the present study, laccase–ABTS treatments were most efficient in diminishing (presumably oxidizing) aliphatic alcohols in the lignin substrates, and most profoundly so in the WSL (Fig. 2). It cannot be unequivocally concluded, however, if the observed changes in distribution of S and G residues of treated samples can be directly related to the different route of oxidation, but the present results support that mediators introduce selectivity towards lignin subunits in biomass.
Correlations between residues that decreased in relative amounts and plausible oxidation products that ideally would be expected to increase simultaneously, were not evident e.g. oxidation of 4-vinylguaiacol (G) would be expected to result in an increase of acetovanillone. Instead, the identified syringyl-like and guaiacyl-like residues from Py-GC/MS were grouped according to their aliphatic characteristics e.g. alkene, alcohol, and carbonyl, which turned out to provide a relative measurement of the oxidizing effect of the treatments (Fig. 2). As expected, the relative amounts of aliphatic alcohols (i.e. non-phenolic alcohols) did not decrease after laccase treatment, because the sole action of laccase is oxidation of phenolic –OH groups. By including mediators, laccase-mediator systems were created which enabled oxidation of non-phenolic alcohols and thus extended the oxidation capacity of laccase, with laccase–TEMPO and laccase–ABTS appearing to be most efficient in catalyzing the decrease in the amount of the aliphatic alcohols – and the effect being most pronounced on the WSL (Fig. 2).
The potency of ABTS towards oxidation of lignin was surprising since the cation of ABTS, known to be readily created by laccase, has the lowest redox potential of the included mediators (Table 1). It has however, been suggested that oxidation of the ABTS+ cation may induce further oxidation of ABTS to form the di-cation ABTS++ which has a redox potential of 1.1 V vs. NHE compared to 0.76 V vs. NHE for the cation radical.41 If such further oxidation of ABTS occurs, ABTS may provide a redox potential similar to those of HBT and HPI at 1.08 and 1.09 V vs. NHE, respectively. However, despite a much lower redox potential of 0.76 V vs. NHE for TEMPO, the laccase–TEMPO appeared to be more effective in oxidation of the WSL compared to the HBT–laccase system (Fig. 2). Comparison of the lignin oxidation efficiency and the redox potential of the mediators thus suggest that lignin modification is not only governed by the redox potential of the mediators, but is also most likely influenced by other parameters including radical stability and kinetic characteristics of the mediators.20
Laccase induced lignin grafting
Both laccases were shown to be able to graft HBT and HPI onto both of the lignin biomass samples (Fig. 3 and 4). To our knowledge, this is the first demonstration of LMT facilitated grafting of –N–OH type mediators, HBT and HPI, onto lignin. Both of these –N–OH type mediators become N-oxyl radicals upon one-electron oxidation by laccase (reaction for HPI shown in Scheme 1).Comparison of the relative occurrence of grafted molecules revealed that the extent of grafting with HPI was more pronounced than with HBT (Fig. 5). The better grafting ability of HPI is most likely due to differences in radical stability among the oxidized form of the mediators. Hence, formation of the N-oxyl radical from HBT has been reported to be followed by a spontaneous decay with a half-life (first cycle) of 100 s42 This is much faster than the decay reported for the N-oxyl radical generated from HPI, which has a longer half-life (first cycle) of 7900 s, most probably due to its symmetric structure.43 Such a notable difference in half-life has been reported to affect the selectivity of mediators,44 and may explain why the N-oxyl radical of HPI appeared more available to graft onto the activated lignin surface.
Possible mechanisms of grafting
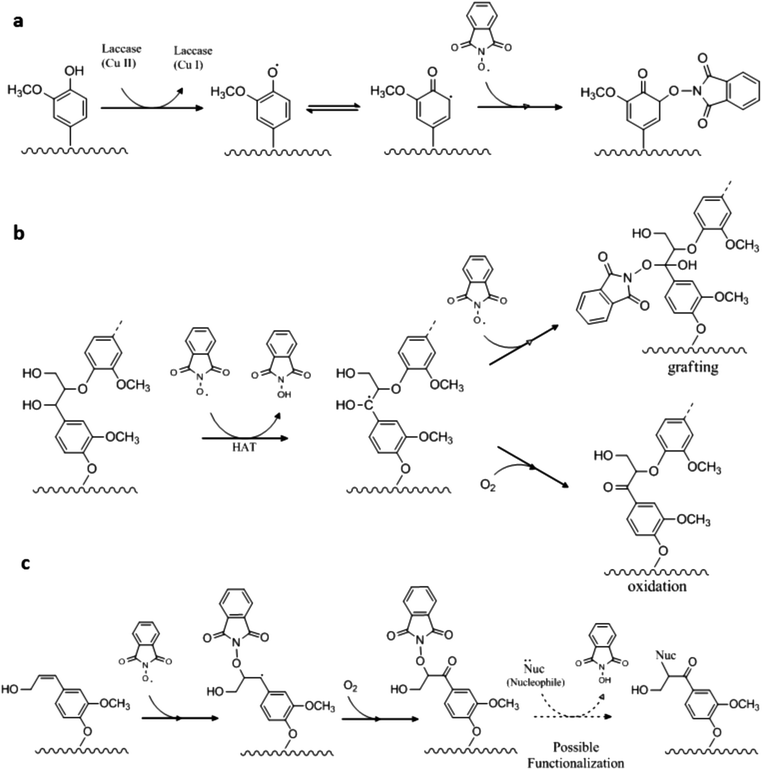
There is no generic theory related to the laccase catalyzed grafting of mediators onto lignin. However, it is tempting to consider a generic grafting mechanism that is a result of radical coupling between two radicals with a resulting formation of a covalent bond between the lignin and the mediator.26 When a mediator is present with the laccase, the grafting may occur either via the phenolic moiety or via the non-phenolic moiety in lignin. Phenolic subunits are available in lignin, and thus presumably also in both of the pretreated lignin substrates employed here.45 Phenolic subunits are known to be activated by laccase oxidation and we therefore suggest that a plausible route to grafting could be through radical coupling between the N-oxyl radical and a phenoxy radical on an activated lignin subunit (Scheme 2a). Since the mediator enables oxidation of non-phenolic moieties, another plausible route of the LMT induced grafting might be via activation of a non-phenolic subunit in lignin, where a benzylic radical is generated by an N-oxyl radical, which in turn either would result in a Cα oxidation or radical coupling between the benzylic cation and a second N-oxyl radical as these are present in high quantities (Scheme 2b). A third option, supported by results obtained by R. Bag et al.,46 suggests that N-oxyl radicals under mild conditions and in the presence of a Cu catalyst (in the present work this would be laccase) may react with double bonds in lignin (aliphatic alkenes) thereby attaching the radical mediator with a resulting oxidation of the Cα (neighbor C-atom) (Scheme 2c). This latter mechanism is a novel mechanism for LMT action on lignin, but more work is required, such as e.g. understanding the lifetime of the different radicals, before the most likely among the three suggested mechanisms can be ascertained.It is likely that the extent of grafting may be optimized, e.g. by using conditions (pH and temperature) that favors the stability of the radicals involved. Grafting on lignin by laccase action has mainly been carried out with phenolic molecules7,8 and only few have succeeded in grafting aliphatic compounds such as amino acids, acrylamide and alkylamines.1,47–49 Different types of lignin have been employed in each study (kraft pulp, flax fibers, and even various wood pulps or chips). A grafting mechanism similar to the one outlined in Fig. 2a has been suggested for radical coupling of a phenolic compound to lignin.7 It has, however, to our knowledge not been reported that the mediator itself graft onto lignin to produce “hetero-functionalization”, i.e. going beyond phenolic polymerization. Moreover, in the present study the laccase catalyzed reaction is suggested to be the same on different types of lignin substrates.
The grafting of the mediator itself presumably induces changes in the properties of lignin, but it may also serve as potential pathway for controlling modification of lignin. HBT or HPI are used in organic chemistry as coupling facilitators because they both have the properties of being good leaving groups.50,51 Andia et al.52 have demonstrated how HBT and HPI can be added to alkenes that have similar structures to the subunits found in lignin. The mediators subsequently serve as leaving groups for substitution of compounds possessing nucleophilic properties. In a two-step design, where conditions are optimized to improve grafting of –N–OH mediators to native lignin, where after conditions are optimized to facilitate substitution, these mediators may open a new pathway to functionalize lignin. In addition, it is not unlikely that the use of mediators as intermediates may expand the possibilities for grafting non-aromatic and more diverse compounds including phosphorus, nitrogen, and sulphur nucleophiles to give high-value products.52
Conclusions
High redox potential laccases were found to be capable of grafting HBT and HPI mediators, both being –N–OH type mediators, to lignin. We suggest that the grafting occurred by radical–radical coupling mechanisms involving laccase oxidation of the mediator, and then different types of either direct (Scheme 2a) or indirect catalytic oxidation in lignin moieties (Scheme 2b and c). The coupling efficiency appeared highly dependent on the type of lignin substrate, an effect likely resulting from differences in both origin and treatment of the lignin substrates. Laccase treatments and laccase-mediator treatments induced changes in the molar area distribution of S and G residues in pyrograms obtained from Py-GC/MS. The overall S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratio was not affected by LMTs, but grouping of Py-GC/MS residues provided results indicative of overall oxidation and reduction of double bonds in lignin residues (alkene oxidation). Selective changes of different residues within the S and G distribution, suggested that LMTs also introduced some selectivity towards lignin subunits. The data obtained open up for new uses of lignin by laccase-assisted modification.
G ratio was not affected by LMTs, but grouping of Py-GC/MS residues provided results indicative of overall oxidation and reduction of double bonds in lignin residues (alkene oxidation). Selective changes of different residues within the S and G distribution, suggested that LMTs also introduced some selectivity towards lignin subunits. The data obtained open up for new uses of lignin by laccase-assisted modification.
Experimental
Materials
After treatment, the suspension was centrifuged for 20 min at 5350g, the pellet obtained was washed twice with deionized water; this procedure was repeated twice. After the treatments, the pellet was washed, dried at 70 °C, ground and sieved to a max. particle size of 355 μm. Beech organosolv lignin (BOL) was produced at Thünen Institute of Wood Research (Hamburg, Germany) by ethanol water pulping 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (wt%) of beech wood chips. The pulping conditions were 90 min at 170 °C with liquor to wood ratio of 4
1 (wt%) of beech wood chips. The pulping conditions were 90 min at 170 °C with liquor to wood ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in presence of 0.5 wt% H2SO4 based on dry wood mass. Compositional analyses of the two lignin biomasses according to NREL Standard Procedures for Biomass Compositional Analysis54 showed that WSL comprised 46.9 wt% of carbohydrates and 43.7 wt% of lignin. The composition of BOL was 4.5 wt% carbohydrate with a lignin content of 87.4 wt%.
1 in presence of 0.5 wt% H2SO4 based on dry wood mass. Compositional analyses of the two lignin biomasses according to NREL Standard Procedures for Biomass Compositional Analysis54 showed that WSL comprised 46.9 wt% of carbohydrates and 43.7 wt% of lignin. The composition of BOL was 4.5 wt% carbohydrate with a lignin content of 87.4 wt%.
Laccase activity assay
Laccase activity was determined by monitoring the oxidation of syringaldazine at 530 nm (ε = 6.5 × 104 M−1 cm−1). The assay reaction mixture contained 0.1 mM syringaldazine, 3.3 vol% methanol, 25 mM sodium acetate (pH 5.0), 25 °C, and a suitable amount of enzyme. Oxidation of syringaldazine was monitored by measuring the increase in absorbance at 530 nm. Enzyme activity was expressed in units: one International Unit (IU) is defined as 1 μmol of substrate (syringaldazine) converted per minute at 25 °C, pH 5.0.Laccase-mediator treatment (LMT)
Treatments of the WSL and BOL were carried out at a substrate consistency of 5 wt% (dry matter (DM)), in 1 ml reaction volume at pH 4.8, 50 °C, and 1250 rpm shaking for 24 h. Each laccase was added in a dosage of 88 U g−1 DM (units as determined from the syringaldazine assay, described above). Mediator concentrations were 16 mM and samples with HPI had continuously small amounts of NaOH added to keep pH at 4.8. Untreated, laccase-treated, and mediator-treated samples were used as controls. After treatments, laccase activity was terminated by heating at 99 °C in 10 min. After 10 min of centrifugation at 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, the samples were divided into liquid and solid fractions. The solid fraction was washed twice (30 min at 1250 rpm shaking) in 1.5 ml milliQ water and freeze dried.
000g, the samples were divided into liquid and solid fractions. The solid fraction was washed twice (30 min at 1250 rpm shaking) in 1.5 ml milliQ water and freeze dried.
Monitoring of laccase activity during treatments
The activity of each laccase with and without lignin biomass present was monitored over 24 h at conditions identical to those of the laccase-mediator treatment (all run in duplicates). Samples were taken at 0, 1, 3, 7, 14 and 24 h of reaction, centrifuged for 1 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, and activity was measured immediately in the syringaldazine assay.
000g, and activity was measured immediately in the syringaldazine assay.
Lignin composition by Py-GC/MS analysis
The composition of the insoluble part of each lignin substrate after treatments with and without laccase and mediators was determined in triplicate by pyrolysis gas chromatography/mass spectrometry analysis (Py-GC/MS). Estimation of the distribution of monomeric lignin- and lignin-derived residues from Py-GC/MS was done according to the following steps according to del Río et al.55 and Jurak et al.37• Obtainment of exact retention times and peak areas from signals of known levels of pure standard compounds; then, calculation of the molar peak area from the relevant pure compound signal by dividing the peak area signal for an amount of a standard compound by the molecular mass of that compound.
• In the lignin sample runs specification of a cut-off of 1% molar area of total for consideration.
• Normalization of relevant peaks in lignin sample runs by summation of the areas for the identified molar peaks (i.e. sum = 100%); data for triplicate runs were averaged.
• Estimation of relative occurrence of each monomeric moiety was estimated by dividing the molar peak area of the monomeric residue by the total weigh of the lignin sample weighed in for Py-GC/MS analysis (last mentioned, was done for a few of the comparisons (Table 2), were the molar areas were compared per unit amount of sample weighed in for the Py-GC/MS analysis).
Acknowledgements
This work was supported by the Danish National Advanced Technology Foundation via the Technology Platform “Biomass for the 21st century – B21st”.References
- T. Kudanga and M. Le Roes-Hill, Appl. Microbiol. Biotechnol., 2014, 98, 6525–6542 CrossRef CAS PubMed.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344, 709–719 CrossRef CAS PubMed.
- R. Rinaldi, R. Jastrzebski, M. T. Clough, J. Ralph, M. Kennema, P. C. A. Bruijnincx and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2016, 55, 8164–8215 CrossRef CAS PubMed.
- W. Boerjan, J. Ralph and M. Baucher, Annu. Rev. Plant Biol., 2003, 54, 519–546 CrossRef CAS PubMed.
- L. P. Christopher, B. Yao and Y. Ji, Front. Energy Res., 2014, 2, 1–13 Search PubMed.
- C. A. Gasser, G. Hommes, A. Schaffer and P. F. Corvini, Appl. Microbiol. Biotechnol., 2012, 95, 1115–1134 CrossRef CAS PubMed.
- G. Nyanhongo, T. Kudanga, E. Prasetyo and G. Guebitz, Biotechnol. Genet. Eng. Rev., 2010, 27, 305–330 CrossRef CAS PubMed.
- T. Kudanga, G. S. Nyanhongo, G. M. Guebitz and S. Burton, Enzyme Microb. Technol., 2011, 48, 195–208 CrossRef CAS PubMed.
- S. M. Jones and E. I. Solomon, Cell. Mol. Life Sci., 2015, 72, 869–883 CrossRef CAS PubMed.
- A. K. Sitarz, J. D. Mikkelsen and A. S. Meyer, Crit. Rev. Biotechnol., 2016, 36, 70–86 CrossRef CAS PubMed.
- F. d'Acunzo, C. Galli, P. Gentili and F. Sergi, New J. Chem., 2006, 30, 583–591 RSC.
- K. Lundquist and J. Parkås, BioResources, 2011, 6, 920–926 CAS.
- L. Munk, A. K. Sitarz, D. C. Kalyani, J. D. Mikkelsen and A. S. Meyer, Biotechnol. Adv., 2015, 33, 13–24 CrossRef CAS PubMed.
- C. Crestini and D. S. Argyropoulos, Bioorg. Med. Chem., 1998, 6, 2161–2169 CrossRef CAS PubMed.
- C. Galli and P. Gentili, J. Phys. Org. Chem., 2004, 17, 973–977 CrossRef CAS.
- R. Bourbonnais and M. G. Paice, FEBS Lett., 1990, 267, 99–102 CrossRef CAS PubMed.
- C. Eggert, U. Temp, J. F. D. Dean and K.-E. L. Eriksson, FEBS Lett., 1996, 391, 144–148 CrossRef CAS PubMed.
- P. Baiocco, A. M. Barreca, M. Fabbrini, C. Galli and P. Gentili, Org. Biomol. Chem., 2003, 1, 191–197 CAS.
- S. Coseri, Catal. Rev., 2009, 51, 218–292 CAS.
- M. Fabbrini, C. Galli and P. Gentili, J. Mol. Catal. B: Enzym., 2002, 16, 231–240 CrossRef CAS.
- G. Cantarella, C. Galli and P. Gentili, New J. Chem., 2004, 28, 366–372 RSC.
- J. L. Hodgson, M. Namazian, S. E. Bottle and M. L. Coote, J. Phys. Chem. A, 2007, 111, 13595–13605 CrossRef CAS PubMed.
- D. Ibarra, I. Chávez María, J. Rencoret, C. del Río José, A. Gutiérrez, J. Romero, S. Camarero, J. M. Martínez, J. Jiménez-Barbero and A. T. Martínez, Holzforschung, 2007, 61, 634–646 CrossRef CAS.
- A. Rico, J. Rencoret, J. del Río, Á. T. Martinez and A. Gutierrez, Biotechnol. Biofuels, 2014, 7, 6 CrossRef PubMed.
- X. Du, J. Li, G. R. Gellerstedt, J. Rencoret, J. C. Del Río, A. T. Martínez and A. Gutiérrez, Biomacromolecules, 2013, 14, 3073–3080 CrossRef CAS PubMed.
- F. Hollmann and I. W. C. E. Arends, Polymers, 2012, 4, 759–793 CrossRef.
- M. Alcalde, Ind. Enzymes, 2007, 461–476 Search PubMed.
- A. Garzillo, M. Colao, V. Buonocore, R. Oliva, L. Falcigno, M. Saviano, A. Santoro, R. Zappala, R. Bonomo, C. Bianco, P. Giardina, G. Palmieri and G. Sannia, J. Protein Chem., 2001, 20, 191–201 CrossRef CAS PubMed.
- O. V. Morozova, G. P. Shumakovich, M. A. Gorbacheva, S. V. Shleev and A. I. Yaropolov, Biochemistry (Moscow), 2007, 72, 1136–1150 CrossRef CAS.
- J. C. del Río, J. Rencoret, P. Prinsen, Á. T. Martínez, J. Ralph and A. Gutiérrez, J. Agric. Food Chem., 2012, 60, 5922–5935 CrossRef PubMed.
- R. J. Sammons, D. P. Harper, N. Labbé, J. J. Bozell, T. Elder and T. G. Rials, BioResources, 2013, 8, 2752–2767 CrossRef.
- K. L. Nielsen, C. Indiani, A. Henriksen, A. Feis, M. Becucci, M. Gajhede, G. Smulevich and K. G. Welinder, Biochemistry, 2001, 40, 11013–11021 CrossRef CAS PubMed.
- J. Ralph, S. Quideau, J. H. Grabber and R. D. Hatfield, J. Chem. Soc., Perkin Trans. 1, 1994, 23, 3485–3498 RSC.
- R. El Hage, N. Brosse, L. Chrusciel, C. Sanchez, P. Sannigrahi and A. Ragauskas, Polym. Degrad. Stab., 2009, 94, 1632–1638 CrossRef CAS.
- J.-Y. Kim, S. Oh, H. Hwang, U.-J. Kim and J. W. Choi, Polym. Degrad. Stab., 2013, 98, 1671–1678 CrossRef CAS.
- T. D. H. Bugg and R. Rahmanpour, Curr. Opin. Chem. Biol., 2015, 29, 10–17 CrossRef CAS PubMed.
- E. Jurak, A. M. Punt, W. Arts, M. A. Kabel and H. Gruppen, PLoS One, 2015, 10, e0138909 Search PubMed.
- L. Melone and C. Punta, Beilstein J. Org. Chem., 2013, 9, 1296–1310 CrossRef CAS PubMed.
- D. Rochefort, D. Leech and R. Bourbonnais, Green Chem., 2004, 6, 14–24 RSC.
- F. d'Acunzo, P. Baiocco and C. Galli, New J. Chem., 2003, 27, 329–332 RSC.
- G. Cantarella, C. Galli and P. Gentili, J. Mol. Catal. B: Enzym., 2003, 22, 135–144 CrossRef CAS.
- C. Galli, P. Gentili, O. Lanzalunga, M. Lucarini and G. F. Pedulli, Chem. Commun., 2004, 20, 2356–2357 RSC.
- N. Koshino, B. Saha and J. H. Espenson, J. Org. Chem., 2003, 68, 9364–9370 CrossRef CAS PubMed.
- P. Astolfi, P. Brandi, C. Galli, P. Gentili, M. F. Gerini, L. Greci and O. Lanzalunga, New J. Chem., 2005, 29, 1308–1317 RSC.
- Y. Pu, F. Hu, F. Huang and A. J. Ragauskas, BioEnergy Res., 2015, 8, 992–1003 CrossRef CAS.
- R. Bag, D. Sar and T. Punniyamurthy, Org. Lett., 2015, 17, 2010–2013 CrossRef CAS PubMed.
- S. Witayakran and A. J. Ragauskas, Enzyme Microb. Technol., 2009, 44, 176–181 CrossRef CAS.
- A. Dong, X. Fan, Q. Wang, Y. Yu and A. Cavaco-Paulo, Int. J. Biol. Macromol., 2015, 79, 353–362 CrossRef CAS PubMed.
- C. Mai, O. Milstein and A. Huttermann, J. Biotechnol., 2000, 79, 173–183 CrossRef CAS PubMed.
- M. F. Fathalla and S. N. Khattab, J. Chem. Soc. Pak., 2011, 33, 324–332 CAS.
- S. Patil, L. Chen and J. M. Tanko, Eur. J. Org. Chem., 2014, 2014, 502–505 CrossRef CAS.
- A. A. Andia, M. R. Miner and K. Woerpel, Org. Lett., 2015, 17, 2704–2707 CrossRef CAS PubMed.
- M. Ambye-Jensen, S. T. Thomsen, Z. Kádár and A. S. Meyer, Biotechnol. Biofuels, 2013, 6, 116 CrossRef CAS PubMed.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter and D. Templeton, NREL Analytical Procedure, National Renewable Energy Laboratory, Golden, CO, USA, 2008 Search PubMed.
- J. C. del Río, A. Gutiérrez, I. M. Rodríguez, D. Ibarra and Á. T. Martínez, J. Anal. Appl. Pyrolysis, 2007, 79, 39–46 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Analytical data details of lignin residues identified by Py-GC/MS, laccase activity robustness during treatments. See DOI: 10.1039/c6ra26106j |
| This journal is © The Royal Society of Chemistry 2017 |