Open Access Article
Open Access ArticleScavenging ability of dendritic PAMAM bridged hindered phenolic antioxidants towards DPPH˙ and ROO˙ free radicals
Cuiqin Li ,
Peng Sun,
Hongyang Yu,
Na Zhang and
Jun Wang*
,
Peng Sun,
Hongyang Yu,
Na Zhang and
Jun Wang*
Provincial Key Laboratory of Oil & Gas Chemical Technology, College of Chemistry & Chemical Engineering, Northeast Petroleum University, Daqing, 163318, China. E-mail: dqpilicuiqin@126.com; Fax: +86 0459 6504224; Tel: +86 0459 6504224
First published on 12th January 2017
Abstract
Antioxidant activities of first generation and the second generation dendritic antioxidants (1.0G and 2.0G dendritic antioxidants) with a hindered phenolic group and a tertiary amine group at the same molecule were evaluated using the DPPH˙ method and the oxygen uptake method. It was found that two dendritic antioxidants had good scavenging abilities on DPPH˙ and ROO˙ free radicals and their scavenging abilities were superior to Irganox 3114 with only hindered phenol groups. The antioxidant abilities of dendritic skeletons which only contained the tertiary amine groups were evaluated and they might scavenge DPPH˙ and ROO˙ free radical. The results of dendritic skeletons showed that dendritic antioxidants were the intramolecular complex primary antioxidants with hindered phenolic group and tertiary amine group, and they have good synergistic effect.
1. Introduction
Antioxidants are widely added to organic materials to prevent the compounds from degrading and losing their properties as they are exposed to oxygen, heat and light.1–3 The most primary antioxidants are hindered phenolic antioxidants and amine antioxidants which could terminate free radicals produced in the process of thermal oxidation.4 Nowadays it is well known that phenolic compounds play a key role as antioxidants due to the presence of hydroxyl substituents and their aromatic structure, which enables them to scavenge free radical. And moreover many studies5–7 showed that combining different antioxidants gives a mutual protective effect. Shi and co-workers8 studied the antioxidant property of lycopene, β-carotene, vitamins C and E, individually or in combination. The results showed that in a homogenous solution of linoleic acid methyl ester the combination of lycopene and vitamin E exhibited a greater inhibitory effect than the additive effect of individual antioxidants, while the combination of β-carotene and vitamin E did not produce a similar synergistic effect. Liu and co-workers9 studied the antioxidant property of the combination of natural antioxidants using the 2,2-diphenyl-1-picrylhydrazyl (DPPH˙) free radical method and the results showed that the antioxidant property of the combination of natural antioxidants was substantially superior to the sum of the individual antioxidant effects, and indicated the combination of the antioxidants exhibited good synergistic effect.However, the combination between different antioxidants might result in the decrease of antioxidant ability. Compounds with different antioxidant groups in the same molecule act as potent antioxidants which have intramolecular synergistic effects by different antioxidant groups.10–12 Wang and co-workers13 synthesized a new intermolecular complex antioxidant [3-(3,5-di-tert-butyl-4-hydroxy-phenyl)] propionyl phosphoric acid bisoctadecanol ester with phenol and phosphite groups, and the results showed that the antioxidant ability of the intermolecular complex antioxidant in polyolefin was superior to that of n-octadecyl-β-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionate (Irganox 1076). Nishiyama and co-workers14 investigated intramolecular synergistic effects between chromanol and thiopropionate groups of a series of antioxidants in tetralin oxidation, and the results showed that there were good intramolecular synergistic effects between chromanol and thiopropionate groups. These antioxidants were the compounds with primary antioxidant and secondary antioxidant in the same molecule. However, little works has been done on hindered phenol and tertiary amine groups in the same molecule.
In a previous paper, we reported that two dendritic antioxidants with hindered phenol and tertiary amine groups at the same molecule were synthesized and their antioxidant activities were better than that of 1,3,5-tri(3,5-di-tert-butyl-4-hydroxybenzyl) isocyanurate (Irganox 3114) and pentaerythritol tetrakis 3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionate (Irganox 1010) in polyolefins.15 However, the intramolecular synergistic effects between hindered phenol group and tertiary amine group were not investigated. The aim of this work is trying to investigate the intramolecular synergistic effects and the scavenging ability on DPPH˙ and ROO˙ free radical of the dendritic antioxidants by DPPH˙ method and oxygen uptake method. And moreover the proposal antioxidant mechanism was analysized.
2. Experimental
2.1 Materials
1,1-Diphenyl-2-picrylhydrazyl radical (DPPH˙) was purchased from Beijing Jingkehongda Biotechnology Co. Ltd. (China). Irganox 3114 purchased from Ciba Specialty Chemicals (Shanghai, China) was chemical reagent grade. Styrene, toluene and ethanol purchased from Tianjin Kemiou Chemical regent development center (China) were all of analytical reagent grade. Half-generation dendritic skeletons (0.5G PAMAM and 1.5G PAMAM) with ammonia as core and the first generation and the second generation dendrimer bridged hindered phenolic antioxidants (1.0G dendritic antioxidant and 2.0G dendritic antioxidant) were synthesized in our laboratory.15,162.2 Scavenging capacity of dendritic antioxidants on DPPH˙
DPPH˙ scavenging capacity was carried out using a modified method as described by Brand-Williams.17 DPPH˙ solution in ethanol was prepared daily and stored in the dark. Briefly, 2 mL samples of antioxidants dissolved in ethanol were added directly to 2 mL of DPPH˙ solution in ethanol (2 × 10−4 mol L−1), and then immediately shaken thoroughly. Absorbance of the mixture at 517 nm was recorded at different time intervals. The percentage of DPPH˙ scavenging at the different time was calculated using eqn (1).
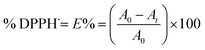 | (1) |
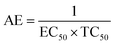 | (2) |
2.3 Inhibition of dendritic antioxidants on AIBN-induced oxidation of styrene
Briefly, the rate of oxygen uptake was measured under oxygen in a closed stainless steel autoclave of 50 mL. AIBN and antioxidant were dissolved directly in toluene. The process of AIBN-induced peroxidation of styrene was followed by an oxygen consumption apparatus equipped with an oxygen pressure gauge sensitive to oxygen pressure. Styrene in the toluene solution and antioxidant solution were put into a stainless steel autoclave which was charged with dry nitrogen three times and then was purged by oxygen, and the mixture was stirred for 10 min at 50 °C. The AIBN solution was injected to the mixture with a syringe in order to initiate the peroxidation of styrene. The initial concentrations of AIBN and styrene were 24 mM and 0.765 M, respectively. Every experiment was repeated at least three times with the standard deviation within 10%, and the final data were the average values from the three independent measurements.3. Result and discussion
3.1 Radical scavenging abilities of dendritic antioxidants towards DPPH˙
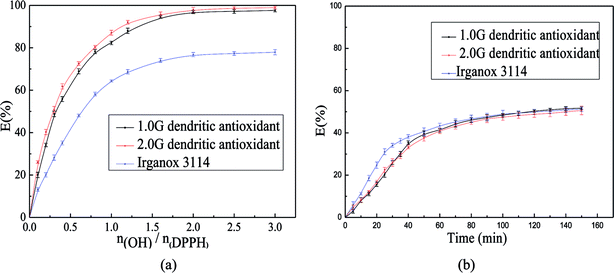
Scavenging ability of hindered phenolic antioxidants on DPPH˙ were affected not only by the concentration of phenolic hydroxyl group but also by the reaction time and the reaction temperature. Generally speaking, radical scavenging ability of hindered phenolic antioxidants increase with increasing of the concentration of phenolic hydroxyl group in the reaction system. Scavenging ability increases firstly with an increase of reaction time, and then keep constant when the reaction time reaches a certain value. The effect of reaction temperature on scavenging ability in the scavenging DPPH˙ system was little because the scavenging process was exothermic reaction.18 So the effects of the concentration of antioxidant and the reaction time on scavenging capacities at the temperature of 30 °C were investigated in this paper (Fig. 1).Due to its stable nitrogen radical, many hindered phenol antioxidants might react with different kinetics or might not react at all. Moreover, the reaction between DPPH˙ and antioxidant was reversible. Therefore, the antioxidant capacity of many antioxidants likely to be wrongly estimated when the scavenging reaction was based on fixed time ranging from 20–30 min instead of total reaction time that is required to attain steady state to complete this reaction. In this study, the effects of the molar ratio of antioxidant to DPPH˙ on the percentage of DPPH˙ scavenging were investigated at steady state reaction time (Fig. 1(a)) when the concentration of DPPH˙ was 1 × 10−4 mol L−1. And the percentage of DPPH˙ scavenging increased rapidly and then kept constant with increasing the molar ratio of antioxidant to DPPH˙ at steady state reaction time. This was due to that dendritic antioxidants with big steric hindrance were polyphenolic compounds and reversibility of reaction between antioxidant and DPPH˙. The scavenging effect of 1.0G dendritic antioxidant was superior to that of Irganox 3114 with the same number of phenolic hydroxyl group, and the scavenging effect of 2.0G dendritic antioxidant was superior to that of 1.0G dendritic antioxidant at the same molar ratio of antioxidant to DPPH˙. This could be caused by dendritic antioxidants with hindered phenolic groups and tertiary amine groups were intramolecular complex antioxidants, and they could terminate the DPPH radical not only by donating H-atoms but also by electron transfer. The EC50 values (Table 1) of dendritic antioxidants were obtained by the curve fitting to the Fig. 1(a).
| Antioxidant | EC50 (n(antioxidant)/n(DPPH˙)) | TC50 (min) | AE × 10−3 |
|---|---|---|---|
| 1.0G dendritic antioxidant | 0.32 | 116.98 | 2.67 |
| 2.0G dendritic antioxidant | 0.29 | 109.12 | 3.16 |
| Antioxidant 3114 | 0.64 | 125.47 | 1.24 |
The effects of reaction time on the E% value at the concentration EC50 were shown in Fig. 1(b). The E% values of the system with antioxidant increased quickly and then increased slowly with increasing of reaction time. Times reached the steady state for 1.0G dendritic antioxidant, 2.0G dendritic antioxidant and Irganox 3114 were 116.98 min, 109.12 min and 125.47 min, respectively. And the E% values of the three antioxidants at the time of the steady state were 50.01%, 48.17%, and 50.00%, respectively. The results showed that the reactions between dendritic antioxidants and DPPH˙ were slow, and the larger the steric hindrance is, the harder the reaction between antioxidant and DPPH˙.19 TC50 values of dendritic antioxidants were obtained by the curve fitting to the Fig. 1(b).
EC50 value is inversely related to the antioxidant capacity of the antioxidant, and the lower the EC50 value, the higher the antioxidant activity of the antioxidant is. TC50 value is related to the rate between antioxidant and DPPH˙ and the lower TC50 value, the faster the reaction rate is. The EC50 values of two dendritic antioxidants were lower than that of Irganox 3114, and this indicated that the antioxidant activities of two dendritic antioxidants were higher. Moreover, the EC50 and TC50 values of 2.0G dendritic antioxidant were lower than those of 1.0G dendritic antioxidant. The possible reason was that total H atom donating capacities of 2.0G dendritic antioxidant with six hindered phenolic groups in a molecule were higher, and the tertiary amine groups of 2.0G dendritic antioxidant might scavenge DPPH˙ radical by electron transfer.
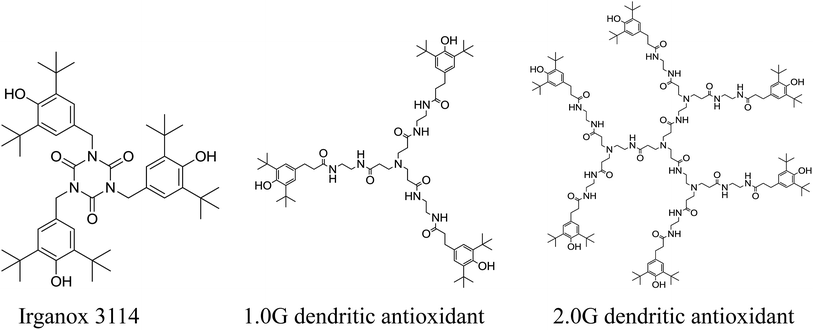
DPPH˙ method permits to evaluate not only the electron- or hydrogen atom-donating properties of antioxidants (EC50), but also the rate of their reaction towards the free radicals (TC50). Villaño and co-worker20 investigated radical scavenging ability of a series of polyphenolic compounds using DPPH˙ assay and the results showed that polyphenolic compounds with a high EC50 value had low TC50 value. However, dendritic antioxidant with a high EC50 value had a low reaction rate with DPPH˙. The AE values of two dendritic antioxidants were calculated and were listed in Table 1. The AE is a parameter that combined both EC50 and TC50. The larger the AE value is, the higher the scavenging ability of antioxidants on DPPH free radial is. The AE values of dendritic antioxidants were higher than that of Irganox 3114. Moreover, the AE value of 1.0G dendritic antioxidant was twice higher than that of Irganox 3114, and the AE value of 2.0G dendritic antioxidant was over twice higher than that of Irganox 3114. However, the structures of antioxidants (Fig. 2) showed that the number of phenolic group of 1.0G dendritic antioxidant was the same as that of Irganox 3114, and that of 2.0G dendritic antioxidant was as twice as that of 1.0G dendritic antioxidant (Fig. 2). The results indicated that not only the phenolic group of dendritic antioxidants might terminate free radicals, but also the tertiary amine group might terminate free radicals. Tertiary amine group connected with alkyl chain, and electron-donating ability made electron density of N atom in tertiary amine group increase and easy to terminate free radicals through electron transfer. 2.0G dendritic antioxidant had four tertiary group, while 1.0G dendritic antioxidant bears only one tertiary group. So the value of E% for 2.0G dendritic antioxidant was higher. Dendritic antioxidants are new bifunctional antioxidants which have intramolecular synergistic effects between phenol and tertiary amine groups.
3.2 Free scavenging abilities of dendritic antioxidants towards ROO˙
Though DPPH˙ method may evaluate antioxidant activity of hindered phenols, the DPPH˙ free radical was a stable radical and the antioxidant capacity of many antioxidants with lower activity likely to be wrongly estimated. In order to investigate the antioxidant ability of dendritic antioxidants, the high active peroxyl radicals in the process of the initiation of AIBN-induced oxidation of styrene in the oxygen system were used.21,22 Peroxyl radicals (ROO˙) generate in AIBN-induced oxidation of styrene. With an antioxidant adding to the reaction system, the antioxidant trap the peroxyl radical (ROO˙) to generation an antioxidant radical (A˙), and the antioxidant radical might couple rapidly with the peroxyl (ROO˙) to form the nonradical product. The radical-induced oxidation of styrene is inhibited until all the antioxidant is depleted. The oxygen consumption rate increases rapidly in the period of propagation in absence of antioxidant, and the oxygen consumption rate decreases with antioxidant adding. The oxygen uptake curves of the initiation of AIBN-induced oxidation of styrene in the presence of various concentrations of dendritic antioxidants were shown in Fig. 3.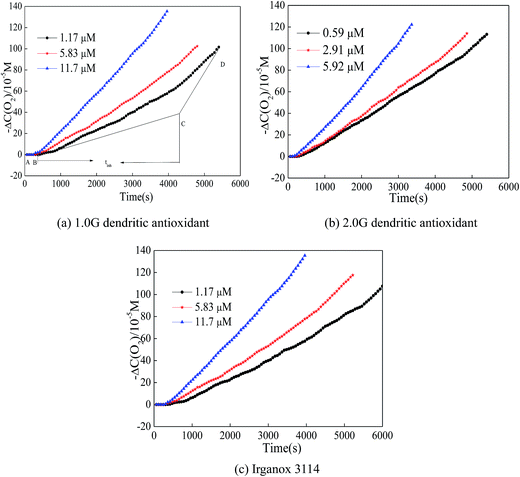 | ||
| Fig. 3 Oxygen consumption curve of the initiation of AIBN-induced oxidation of styrene for antioxidants. | ||
The initiation reaction, chain propagation reaction and chain termination reaction of styrene under oxygen system were treated on the basis of the steady-state hypothesis, in which the concentrations of the radicals remain constant. The rate of oxygen exhaustion during the oxidation of styrene (RH) in absence of antioxidant is described as Rp correlating with the rate of radical initiation (Ri) and the concentration of RH as shown in the following equation:
| Rp = −d[O2]/dt = (kp/(2kt)0.5)Ri0.5[LH] | (3) |
| −d[O2]/dt = Rinh = kp[LH]/(tinhkinh) | (4) |
| kclinh = Rinh/Ri | (5) |
| kclp = Rp/Ri | (6) |
The kinetic parameters in the presence of dendritic antioxidants or Irganox 3114 are collected in Table 2. The propagation kinetic parameters, such as Rp, kp/(2kt)0.5 and kclp decreased with the increase of the concentration of dendritic antioxidants, and the results showed that dendritic antioxidants scavenging the ROO˙ by donating H-atoms and inhibited the AIBN-induced peroxidation of styrene. The kinetic parameter of kinh, which indicated the antioxidant capacity of dendritic antioxidants, increased with increasing of the concentration of antioxidants, and the results demonstrated that the higher the concentrations of dendritic antioxidants or Irganox 3114, the more markedly the styrene is protected by antioxidants. As for kclp and kclinh, it can be found that the addition of various antioxidants shortens the cycles of radical propagation significantly. Moreover, the kclp and kclinh values decreased with increasing of the concentration of antioxidants. At the same concentration of phenolic hydroxyl group, the order of kclinh values for the three antioxidants was 2.0G dendritic antioxidant > 1.0G dendritic antioxidant > Irganox 3114, but the order of Rinh values for the three antioxidants was 2.0G dendritic antioxidant > Irganox 3114 > 1.0G dendritic antioxidant (at the two lower concentrations) when the concentration of phenolic hydroxyl groups was low, the phenolic hydroxyl through donating H atom as a main pathway to terminating free radicals. While the influence of phenolic hydroxyl did not changed significantly with the increase of concentration of antioxidant and tertiary amine played a vital role in terminating free radicals. This was the reason that the ranking matches with the data of Table 2 for the two lower concentrations, but not for the highest concentration.
| Antioxidant | Concentration (μM) | Rp (10−8 M s−1) | Rinh (10−8 M s−1) | kp/(2kt)0.5 (10−3) | kinh (105 M−1 s−1) | kclp | kclinh |
|---|---|---|---|---|---|---|---|
| a Ri = Rg = 2.64 × 10−6[AIBN] s−1 = 6.34 × 10−8 M s−1, [AIBN] = 24 mM, [LH] = 0.765 mol L−1. | |||||||
| 1.0G dendritic antioxidant | 1.17 | 18.7 | 16.0 | 0.97 | 7.55 | 2.95 | 2.52 |
| 5.83 | 12.43 | 7.52 | 0.65 | 14.18 | 1.96 | 1.19 | |
| 11.7 | 10.42 | 5.04 | 0.54 | 19.01 | 1.64 | 0.79 | |
| 2.0G dendritic antioxidant | 0.59 | 22.7 | 16.23 | 1.18 | 5.94 | 3.58 | 2.56 |
| 2.91 | 14.5 | 9.29 | 0.75 | 9.17 | 2.29 | 1.47 | |
| 5.92 | 12.4 | 7.12 | 0.64 | 10.37 | 1.96 | 1.44 | |
| Antioxidant 3114 | 1.17 | 19.87 | 15.88 | 1.03 | 7.46 | 3.13 | 2.35 |
| 5.83 | 12.97 | 7.67 | 0.67 | 13.96 | 2.04 | 0.97 | |
| 11.7 | 11.05 | 5.18 | 0.57 | 18.83 | 1.74 | 0.66 | |
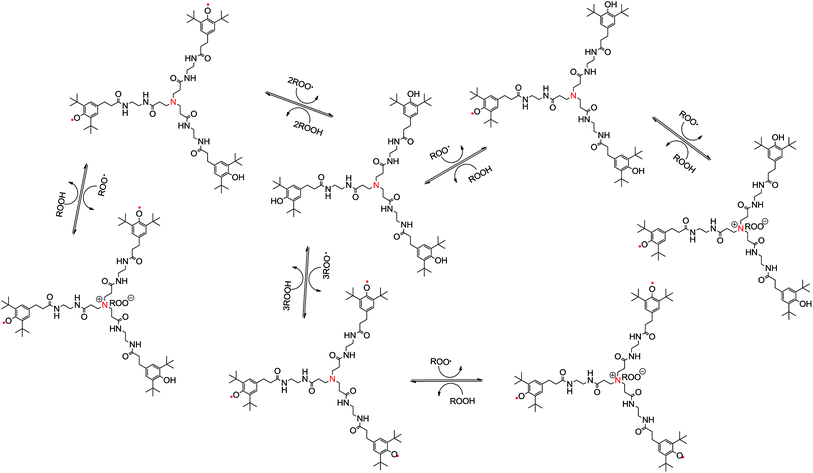
Besides the above kinetic parameters, the number of trapping ROO˙(n) is always applied to assess the antioxidant capacity. Table 3 involves tinh in the different concentration of antioxidants, and moreover, the relationships between tinh and the concentration of antioxidants are found and listed in Table 2. The n values of three antioxidants can be obtained by the relationships between tinh and the concentration of antioxidants and listed in Table 3 as well. The n value of 1.0G dendritic antioxidant was higher than that of Irganox 3114, and the n value of 2.0G dendritic antioxidant was highest of the three antioxidants. Moreover, the n value of 2.0G dendritic antioxidant was nearly three times than that of 1.0G dendritic antioxidant. However, the phenolic groups of 2.0G dendritic antioxidant was twice as many as that of 1.0G dendritic antioxidant. The fact reveals that dendritic antioxidants could terminate the ROO˙ not only by donating H-atoms of the phenolic groups but also by electron transfer of the tertiary amine groups (Fig. 4). 1.0G dendritic antioxidant has three hindered phenolic groups and a tertiary amine group, and 2.0G dendritic antioxidant has six hindered phenolic groups and four tertiary amine groups. But 2.0G dendritic antioxidant possesses bulkier branches caused a decrease in activity as compared to the 1.0G dendritic antioxidant. The above mentioned observation can be associated to the effect of the bulkier branches toward the reaction of tertiary amine groups and ROO˙: usually bulkier branches offer maximum resistance to ROO˙ coming into the tertiary amine groups.
| Antioxidant | tinh (s) = (n/Ri)[antioxidant (μM)] + constant | n |
|---|---|---|
| a Ri = Rg = 6.34 × 10−8 M s−1, n = coefficient × 6.34 × 10−8 M s−1. | ||
| 1.0G dendritic antioxidant | tinh = 39.9[antioxidant] + 1473.6 (r2 = 0.9874) | 2.5 |
| 2.0G dendritic antioxidant | tinh = 107.4[antioxidant] + 1799.1 (r2 = 0.9966) | 6.8 |
| Antioxidant 3114 | tinh = 35.1[antioxidant] + 1510.1 (r2 = 0.9910) | 2.2 |
3.3 Intramolecular synergistic effects of dendritic antioxidants
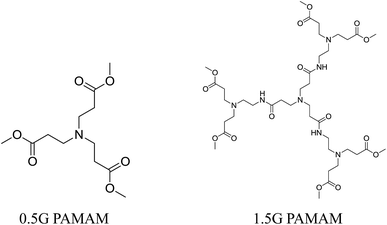
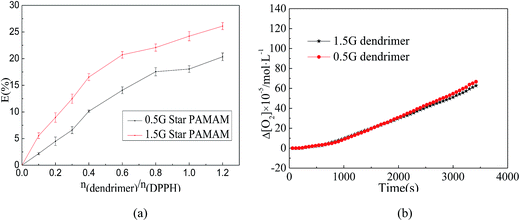
In order to verify the antioxidant ability of tertiary amine groups in dendritic antioxidants, we evaluated the antioxidant abilities of the skeletons (0.5G and 1.5G PAMAM) (Fig. 5) which only contained the tertiary amine group by the DPPH˙ method (the concentration of DPPH˙ was 2 × 10−4 mol L−1) and the oxygen uptake method (the concentration of 0.5G and 1.5G PAMAM were 11.7 μM and 5.92 μM, respectively). The results showed that 0.5G and 1.5G PAMAM had a certain scavenging abilities of DPPH˙ free radical, and the scavenging ability increased with increasing of the concentration of the skeletons (Fig. 6(a)). But the scavenging abilities of 0.5G and 1.5G PAMAM were lower. The results of the scavenging ROO˙ revealed that the kinh of 0.5G and 1.5G PAMAM were 8.09 × 10−5 M−1 s−1 and 8.62 × 10−5 M−1 s−1, respectively (Fig. 6(b)). Moreover, the antioxidant ability of 1.5G PAMAM was higher than that of 0.5G PAMAM, and this was because the number of tertiary amine antioxidant groups of 1.5G PAMAM was four times that of 0.5G PAMAM indicating that the tertiary amine group might terminate free radicals.23,24 The three alkyl chains of tertiary amine have better electron-donating ability, which made the electron density on the N atom abundant and easy to terminate free radicals through electron transfer. Besides, the more the number of tertiary amine, the higher the scavenging ability of antioxidant. The experimental results verified that dendritic antioxidants were intramolecular complex primary antioxidants and they had intramolecular synergistic antioxidant ability. This was the reason that the antioxidant ability of 1.0G dendritic antioxidant was higher than that of Irganox 3114, and the antioxidant ability of 2.0G dendritic antioxidant was higher than that of 1.0G dendritic antioxidant.4. Conclusion
Dendritic antioxidants with hindered phenolic group and tertiary amine groups have better scavenging abilities on DPPH˙ and ROO˙ free radical. The scavenging abilities of 2.0G dendritic antioxidant with six hindered phenol groups and four tertiary amine groups were greater than those of 1.0G dendritic antioxidant with three hindered phenol groups and one tertiary amine groups. In DPPH˙ and ROO˙ scavenging, antioxidant with dendritic structure has higher antioxidant ability than Irganox 3114 and when hindered phenol groups and tertiary amine groups exist in the molecule together, dendritic antioxidants will give out a highly synergetic effect. The scavenging abilities of 0.5G and 1.5G dendritic skeletons on the two free radicals showed that dendritic antioxidants might terminate free radicals not only by donating H-atoms but also by electron transfer, and dendritic antioxidants with hindered phenol group and tertiary amine group in the molecule together are intramolecular synergistic antioxidants.Acknowledgements
This work was supported by the financial foundation of National Nature Science Foundation of China (No. 51303020), Heilongjiang Education Department (No. 12521z005) and Northeast Petroleum University (No. ky120212).References
- R. K. Krishnaswamy, Polym. Eng. Sci., 2007, 47, 516–521 CAS.
- X. F. Wang, W. Y. Xing, G. Tang, N. N. Hong, W. Z. Hu, J. Zhan, L. Song, W. Yang and Y. Hu, Polym. Degrad. Stab., 2013, 98, 2391–2398 CrossRef CAS.
- Z. Q. Liu, Chem. Rev., 2010, 110, 5675–5691 CrossRef CAS PubMed.
- M. Lundbäck, M. S. Hedenqvist, A. Mattozzi and U. W. Gedde, Polym. Degrad. Stab., 2006, 91, 1571–1580 CrossRef.
- I. Kriston, Á. Orbán-Mester, G. Nagy, P. Staniek, E. Földes and B. Pukánszky, Polym. Degrad. Stab., 2009, 94, 719–729 CrossRef CAS.
- S. Yachigo, M. Sasaki and F. Kojima, Polym. Degrad. Stab., 1992, 35, 105–113 CrossRef CAS.
- C. S. Romano, K. Abadi, V. Repetto, A. A. Vojnov and S. Moreno, Food Chem., 2009, 115, 456–461 CrossRef CAS.
- J. Shi, Q. Qu, Y. Kakuda, S. J. Xue, Y. M. Jiang, S. Koide and Y. Y. Shim, J. Food Compos. Anal., 2007, 20, 603–608 CrossRef CAS.
- D. Liu, J. Shi, A. C. Ibarra, Y. Kakuda and S. J. Xue, LWT--Food Sci. Technol., 2008, 41, 1344–1349 CrossRef CAS.
- I. Bauer, W. D. Habicher, S. Korner and S. Al-Malaika, Polym. Degrad. Stab., 1997, 55, 217–224 CrossRef CAS.
- J. Pospisil, Polym. Degrad. Stab., 1993, 39, 103–109 CrossRef CAS.
- T. Nishiyama, T. Sugimoto and Y. Andoh, Polym. Degrad. Stab., 2001, 74, 189–193 CrossRef CAS.
- J. Wang, W. Ji and X. J. Zhang, Chem. Ind. Eng. Prog., 2008, 7, 1114–1118 Search PubMed.
- T. Nishiyama, Y. Andoh, T. Sugimoto and O. Tomoyuki, Polym. Degrad. Stab., 2003, 81, 409–413 CrossRef CAS.
- C. Q. Li, J. Wang, M. M. Ning and H. P. Zhang, J. Appl. Polym. Sci., 2012, 124, 4127–4135 CrossRef CAS.
- D. A. Tomalia, H. Baker, J. Dewaid, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder and P. Smith, Macromolecules, 1986, 19, 2466–2468 CrossRef CAS.
- K. Mishra, H. Ojha and N. K. Chaudhury, Food Chem., 2012, 130, 1036–1043 CrossRef CAS.
- C. Q. Li, Y. J. Wei, W. G. Shi, J. Wang and B. H. Wang, Prog. React. Kinet. Mech., 2015, 40, 279–290 CrossRef CAS.
- S. Roy, S. Mallick, T. Chakraborty, N. Ghosh, A. K. Singh, S. Manna and S. Majumdar, Food Chem., 2015, 173, 1172–1178 CrossRef CAS PubMed.
- D. Villaño, M. S. Fernández-Pachón, M. L. Moyá, A. M. Troncoso and M. C. García-Parrilla, Talanta, 2007, 71, 230–235 CrossRef PubMed.
- R. E. Hage, D. Perrin and N. Brosse, Nat. Resour., 2012, 3, 29–34 Search PubMed.
- M. Carocho and I. C. F. R. Ferreira, Food Chem. Toxicol., 2013, 51, 15–25 CrossRef CAS PubMed.
- J. Xie and K. M. Schaich, J. Agric. Food Chem., 2014, 62, 4251–4260 CrossRef CAS PubMed.
- M. C. Foti, J. Agric. Food Chem., 2015, 63, 8765–8776 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |