Open Access Article
Open Access ArticleDirect synthesis of N-sulfinyl- and N-sulfonylimines via copper/L-proline-catalyzed aerobic oxidative cascade reaction of alcohols with sulfinamides or sulfonamides†
Guofu Zhang,
Shengjun Xu,
Xiaoqiang Xie,
Chengrong Ding * and
Shang Shan*
* and
Shang Shan*
College of Chemical Engineering, Zhejiang University of Technology, Hangzhou 310014, People's Republic of China. E-mail: dingcr@zjut.edu.cn; shans2001@163.com; Fax: +86-571-88320147; Tel: +86-571-88320147
First published on 30th January 2017
Abstract
An efficient one-pot synthetic method of N-sulfinyl- and N-sulfonylimines by the condensation of alcohols with sulfinamides or sulfonamides under mild and green conditions has been developed using a combination of CuI, L-proline and TEMPO. This system shows excellent functional group compatibility for a wide range of substrates and affords the corresponding products in good to excellent yields.
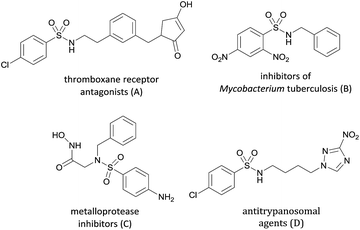
N-Sulfinyl- and N-sulfonylimines are versatile intermediates in organic synthesis.1 As active substrates, they can undergo various nucleophilic addition reactions,2 radical reactions,2j and hetero-Diels–Alder reactions3 to afford the expected N-sulfinyl- and N-sulfonylamide derivatives which are a class of important structure motifs prevalent in drugs, such as potent thromboxane receptor antagonists (A),4a inhibitors of Mycobacterium tuberculosis (B),4b metalloprotease inhibitors (C)4c and potential antitrypanosomal agents (D)4d (Scheme 1).
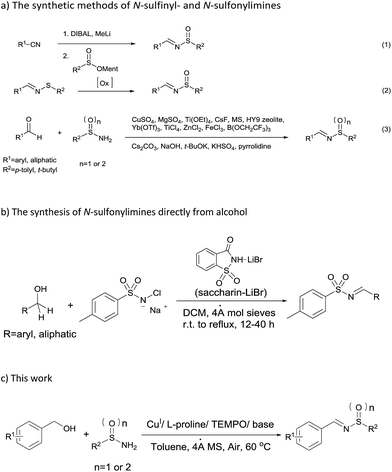
Due to the wide range of synthetic utility of these N-sulfinyl- and N-sulfonylaldimines, numerous synthetic methods have been developed. The main methods for the preparation of N- sulfinyl- and N-sulfonylimines include: (1) reaction of nitriles with an organometallic reagent (DIBAL, MeLi) and menthyl sulfinate;5 (2) asymmetric oxidation of sulfenimines6 and (3) condensation of sulfinamides or sulfonamides with aldehydes (Scheme 2a). The last method seems to be the most common and useful because the pure starting materials are now commercially available. Therefore, in the past decades, much attention have been paid to the direct condensation of sulfinamides or sulfonamides with aldehydes for the synthesis of N-sulfinyl- and N-sulfonylimines [Scheme 2a, eqn (3)].5e,7–18 Even though these reported protocols could be easier to obtain N-sulfinyl- or N-sulfonylimines, excess Lewis acid or stoichiometric base had to be used, which generated a large amount of environmentally unfriendly metallic waste. On the other hand, aldehydes can be obtained from the oxidation of alcohols.19 Therefore, the method that utilizing alcohol as one of the staring materials to afford the N-sulfinyl- or N-sulfonylimine was attractive. To the best of our knowledge, there was only one report on the formation of N-sulfonyl-imines starting directly from alcohols, which involved saccharin–lithium bromide-catalyzed oxidation of alcohols to aldehydes/ketones with chloramine-T followed by their condensation to afford N-tosylimines (Scheme 2b).20
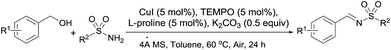
Recently, Stahl19a,b reported a copper/TEMPO-catalyzed alcohols oxidation to desired aldehydes efficiently under mild conditions with ambient air as the oxidant. Therefore, we sought to utilize the combination of copper salt, ligand and TEMPO as efficient catalysts for oxidation of alcohols to aldehydes followed by condensation with sulfin- or sulfonamides. Herein, we reported a copper-catalyzed one-pot multi-step reaction system for synthesis of N-sulfinyl- and N-sulfonylimines from alcohols with sulfin- or sulfonamides under air (Scheme 2c).
Initially, benzalcohol and 4-toluenesulfinamide were selected as the model substrates to determine the optimal conditions. First, the effect of solvent on this oxidative cascade transformation was examined (Table 1, entries 1–6). Good conversion of N-sulfinylimine was obtained, when the reaction was performed in CH2Cl2 or CH3OH with 5 mol% CuI and 5 mol% L-proline in the presence of 1.0 equiv. K2CO3 under air at 60 °C (Table 1, entries 1 and 4). The employment of THF, DMSO or DMF led to moderate conversions of the substrates (entries 2, 3, 6). To our delight, when switching the solvent to toluene, the conversion reached to 88% (entry 5). After then, the different copper salts were screened. As a result, the catalytic efficiency of Cu(I) salts were better than Cu(II) salts in this catalytic system (entries 5, 7–12), and CuI showed the better catalytic efficiency than other Cu(I) salts with the 88% conversion (entries 5, 7 and 8). Furthermore, the temperature also played a decisive role in this system. As shown in Table 1 entries 13 to 17, trace conversion of N-sulfinylimine was obtained at 120 °C or 100 °C, and the conversions at 80 °C, 40 °C and 25 °C were only 74%, 76% and 8%, respectively. Further investigation revealed that the ligand played a critical role in this copper-catalyzed transformation. Among the examined ligands such as L-proline, L-valine, β-alanine, L-histidine, sarcosine, glycine, phenprobamate and pyrrolidine, L-proline was the best (Table 1, entries 5, 18–24). Finally, control experiments showed that when CuI, TEMPO, L-proline or K2CO3 was omitted most substrates were recovered (entries 26–29), and quantitative conversion was obtained when 0.5 equiv. K2CO3 was used under the reaction conditions (Table 1, entry 25). Thus, the optimized reaction conditions (entry 25): substrates (1.0 mmol), TEMPO (5 mol%), K2CO3 (0.5 equiv.) at 60 °C with CuI (5 mol%) as catalyst and L-proline (5 mol%) as ligand were found.
| Entry | Solvent | Cu salt | Ligand | Conv.b (%) |
|---|---|---|---|---|
| a Reaction conditions: benzalcohol (1.0 mmol), 4-toluenesulfinamide (1.0 mmol), copper salt (5.0 mol%), TEMPO (5.0 mol%), ligand (5.0 mol%), K2CO3 (1.0 mmol), solvent (4 mL), 4 Å MS (700 mg), 60 °C, 12 h.b Determined by HPLC.c 120 °C.d 100 °C.e 80 °C.f 40 °C.g 25 °C.h K2CO3 (0.5 equiv.).i K2CO3 was omitted.j TEMPO was omitted.k Isolated yields. | ||||
| 1 | CH2Cl2 | CuI | L-Proline | 81 |
| 2 | THF | CuI | L-Proline | 76 |
| 3 | DMSO | CuI | L-Proline | 51 |
| 4 | CH3OH | CuI | L-Proline | 83 |
| 5 | Toluene | CuI | L-Proline | 88 |
| 6 | DMF | CuI | L-Proline | 54 |
| 7 | Toluene | CuCl | L-Proline | 82 |
| 8 | Toluene | CuBr | L-Proline | 83 |
| 9 | Toluene | CuCl2 | L-Proline | 73 |
| 10 | Toluene | CuBr2 | L-Proline | 74 |
| 11 | Toluene | CuSO4 | L-Proline | 45 |
| 12 | Toluene | Cu(OAc)2 | L-Proline | 68 |
| 13c | Toluene | CuI | L-Proline | Trace |
| 14d | Toluene | CuI | L-Proline | Trace |
| 15e | Toluene | CuI | L-Proline | 74 |
| 16f | Toluene | CuI | L-Proline | 76 |
| 17g | Toluene | CuI | L-Proline | 8 |
| 18 | Toluene | CuI | L-Valine | 77 |
| 19 | Toluene | CuI | β-Alanine | 40 |
| 20 | Toluene | CuI | L-Histidine | 61 |
| 21 | Toluene | CuI | Sarcosine | 57 |
| 22 | Toluene | CuI | Glycine | 68 |
| 23 | Toluene | CuI | Phenprobamate | 46 |
| 24 | Toluene | CuI | Pyrrolidine | 69 |
| 25h | Toluene | CuI | L-Proline | >99 (94k) |
| 26i | Toluene | CuI | L-Proline | 31 |
| 27j,h | Toluene | CuI | L-Proline | 11 |
| 28h | Toluene | — | L-Proline | 10 |
| 29h | Toluene | CuI | — | 20 |
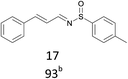
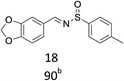
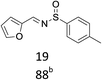
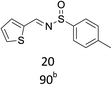
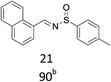
With the optimized conditions in hand, various aromatic alcohols were subjected to the standard reaction conditions. As shown in Table 2, various aromatic alcohols and 4-toluenesulfinamide were efficiently oxidative condensed into the corresponding N-sulfinylimines. The reaction was not only highly efficient but also showed excellent functional groups compatibility. A wide range of aromatic alcohols bearing electron-donating groups such as methyl and methoxy, or electron-withdrawing groups including halogen, nitro and trifluoromethyl substituents were converted into their corresponding N-sulfinylimines with good to excellent isolated yields (entries 1–16, 18, 21). Surprisingly, the efficient transformation of p-methylthiobenzyl alcohol and 4-toluenesulfinamide into the desired product was observed without transformation to sulfoxide or sulfone (entry 5). In addition, it was worth noting that the sterically hindered alcohols also provided the corresponding N-sulfinylimines in 85–90% yields (entries 11–15). Gratifyingly, heteroaryl alcohols such as 2-thienyl and 2-furyl methanol were also well tolerated to give the desire products in 88–90% yields (entries 19–20). Unsaturated alcohol (entries 17) also reacted to form the imine in 93% isolated yield. Unfortunately, less active aliphatic alcohols and secondary alcohols were not suitable in the reaction.
| Entry | Product | Yieldb (%) |
|---|---|---|
| a Reaction conditions: substrates (1.0 mmol), CuI (5 mol%), L-proline (5 mol%), TEMPO (5 mol%), K2CO3 (0.5 equiv.), toluene (4.0 mL), 4 Å MS (700 mg), 60 °C, 12 h.b Isolated yields. | ||
| 1 | R = H | 94 |
| 2 | R = 3,4-Me | 90 |
| 3 | R = p-OMe | 91 |
| 4 | R = 3,4,5-OMe | 91 |
| 5 | R = p-SMe | 89 |
| 6 | R = p-Cl | 89 |
| 7 | R = p-F | 92 |
| 8 | R = p-Br | 91 |
| 9 | R = p-NO2 | 93 |
| 10 | R = p-CF3 | 93 |
| 11 | R = o-Br | 88 |
| 12 | R = o-I | 85 |
| 13 | R = o-Cl | 86 |
| 14 | R = 2,4-Cl | 90 |
| 15 | R = o-Me | 88 |
| 16 | R = m-NO2 | 93 |
 |
 |
|
 |
 |
 |
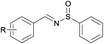
Next, the compatibility of a variety of other sulfinamides on this oxidative cascade reaction was examined, including tert-butanesulfinamide, bezenesulfinamide and 4-chloro-bezenesulfinamide, shown in Table 3. Good to excellent isolated yields were obtained for aromatic alcohols with electron-withdrawing (products 2, 4–6, 8, 10, 12, 15) and electron-donating (products 1, 3, 11, 13, 14) substituents. For aromatic alcohols bearing either ortho- (products 2, 3, 12) or meta- (product 14) substituents, the reactions proceeded smoothly and afforded N-sulfinylimines in good yields. In addition, allyl alcohols could also well tolerated under the optimal conditions in good isolated yields (entries 7, 8). However less active aliphatic alcohols and secondary alcohols could not afford the target products. In general, aromatic alcohols bearing electron-withdrawing substituents condensed more effectively with sulfinamides than those bearing electron-donating substituents (product 6 vs. 1, product 12 vs. 11, product 15 vs. 13).
| Product | Yieldb (%) | |
|---|---|---|
| a Reaction conditions: substrates (1.0 mmol), CuI (5 mol%), L-proline (5 mol%), TEMPO (5 mol%), K2CO3 (0.5 equiv.), toluene (4.0 mL), 4 Å MS (700 mg), 60 °C.b Isolated yields.c Reaction time 15 h.d Reaction time 14 h.e Reaction time 13 h. | ||
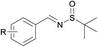 |
(1) R = 3,4,5-OMe | 86 |
| (2) R = o-I | 80 | |
| (3) R = o-OMe | 78 | |
| (4) R = p-Br | 96 | |
| (5) R = p-Cl | 95 | |
| (6) R = p-NO2 | 97 | |
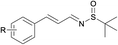 |
(7) R = H | 92 |
| (8) R = p-NO2 | 95 | |
 |
(9) R = H | 91 |
| (10) R = p-Cl | 92 | |
| (11) R = 3,4,5-OMe | 89 | |
| (12) R = o-NO2 | 95 | |
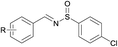 |
(13) R = 3,4-Me | 89c |
| (14) R = m-OMe | 90d | |
| (15) R = p-F | 91e | |
Finally, we turned our efforts to expand the scope of sulfonamides (Table 4). Although the strong electron-withdrawing character of the sulfonyl group leads to very low nucleophilicity of the RSO2NH2 nitrogen (much lower than of RSONH2), prolonging the reaction time to 24 h also obtained satisfying results. Here we investigated the oxidative cascade reaction of aryl alcohols with various kinds of sulfonamides. Electron-donating and -withdrawing substituents at the ortho-, para- and meta- positions of the aryl alcohol partners were well tolerated, providing the desired products in good yields. Surprisingly, the 2-pyridinesulfonamide was also toleranted in this system, giving the corresponding products in good isolated yields (Table 4, products 4–7). Beyond that, branched alkyl sulfonamides also underwent oxidative cascade condensation successfully to give the corresponding products in 87–90% isolated yields, such as tert-butylsulfonamide and cyclopropyl-sulfonamide (products 8–10).
| Product | Yieldb (%) | |
|---|---|---|
| a Reaction conditions: substrates (1.0 mmol), CuI (5 mol%), L-proline (5 mol%), TEMPO (5 mol%), K2CO3 (0.5 equiv.), toluene (4.0 mL), 4 Å MS (700 mg), 60 °C.b Isolated yields.c 1.2 equiv. of alcohol was used. | ||
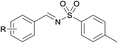 |
(1) R = H | 93 |
| (2) R = p-Br | 91 | |
| (3) R = 3,4,5-OMe | 89c | |
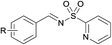 |
(4) R = H | 76 |
| (5) R = o-Me | 78 | |
| (6) R = m-OMe | 81 | |
| (7) R = p-NO2 | 80 | |
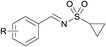 |
(8) R = H | 87 |
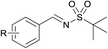 |
(9) R = p-NO2 | 90 |
| (10) R = p-F | 89c | |
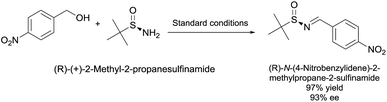
Given the synthetic importance of chiral sulfinamides in organic synthesis, we checked whether racemization occurred in the reaction using pure stereochemical tert-butylsulfin-amide as one of starting materials (Scheme 3). Satisfyingly, the imine product was obtained in 97% yield with 93% e.e. It was obvious that the chiral sulfinamide was also suitable in our system.
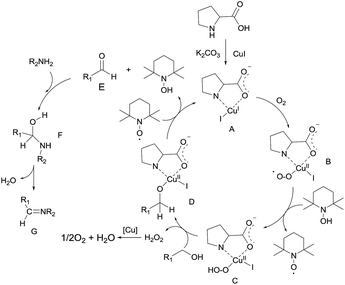
Based on the above promising results and related published research studies,17,19r,21 a possible mechanism was depicted in Scheme 4. The reaction of L-proline, K2CO3 and CuI gave the complex A, which reacted with O2 to afford a Cu(II)-superoxide species B. Species B abstracted a hydrogen from TEMPOH to form species C. Subsequent reaction of the species C with alcohol released H2O2 and afforded species D. β-Hydrogen elimination of the alkoxo moiety in D would occur to give a carbonyl product and TEMPOH, and regenerate A, followed by the insertion of a sulfinamide or sulfonamide to afford species F. Liberation of water from the F gave the product G.
Conclusions
In summary, an efficient and mild copper-catalyzed aerobic oxidative cascade system for the synthesis of N-sulfinyl- and N- sulfonylimines directly from aryl or allyl alcohols with sulfinamides or sulfonamides in one pot has been successfully developed. Under the optimized conditions, a wide range of arylalcohols and various sulfinamides (including chiral tert- sulfinamide) or sulfonamides were smoothly condensed into corresponding N-sulfinyl- or N-sulfonylimines with good to excellent isolated yields. Less active aliphatic alcohols and secondary alcohols were not suitable in the oxidative condensation system, unfortunately. What's more, this is the first example of copper-catalyzed aerobic oxidative cascade condensation for the formation of N-sulfinyl- and N-sulfonyl-imines from sulfinamides or sulfonamides with alcohols.Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (no. 20702051), the Natural Science Foundation of Zhejiang Province (LY13B020017) and the Key Innovation Team of Science and Technology in Zhejiang Province (no. 2010R50018).Notes and references
- (a) J. P. Begue, D. Bonnet-Delpon, B. Crousse and J. Legros, Chem. Soc. Rev., 2005, 34, 562 RSC; (b) S. M. Weinreb and R. K. Orr, Synthesis, 2005, 1205 CrossRef CAS; (c) C. H. Senananake, D. Krishnamurthy, Z. H. Lu, Z. Han and I. Gallou, Aldrichimica Acta, 2005, 38, 93 Search PubMed; (d) P. Zhou, B. C. Chen and F. A. Davis, Tetrahedron, 2004, 60, 8003 CrossRef CAS; (e) M. Gohain, Synlett, 2003, 2097 CrossRef CAS; (f) J. A. Ellman, T. D. Owens and T. P. Tang, Acc. Chem. Res., 2002, 35, 984 CrossRef CAS PubMed; (g) J. A. Ellman, Pure Appl. Chem., 2003, 75, 39 CrossRef CAS; (h) F. A. Davis and B. C. Chen, Chem. Soc. Rev., 1998, 27, 13 RSC; (i) R. Bloch, Chem. Rev., 1998, 98, 1407 CrossRef CAS PubMed; (j) D. Enders and U. Reinhold, Tetrahedron: Asymmetry, 1997, 8, 1895 CrossRef CAS.
- (a) T. Ooi, Y. Uematsu and K. Maruoka, J. Am. Chem. Soc., 2006, 128, 2548 CrossRef CAS PubMed; (b) H. F. Duan, Y. X. Jia, L. X. Wang and Q. L. Zhou, Org. Lett., 2006, 8, 2567 CrossRef CAS PubMed; (c) H. Fujisawa, E. Takahashi and T. Mukaiyama, Chem.–Eur. J., 2006, 12, 5082 CrossRef CAS PubMed; (d) M. Shi, L. H. Chen and C. Q. Li, J. Am. Chem. Soc., 2005, 127, 3790 CrossRef CAS PubMed; (e) T. Soeta, M. Kuriyama and K. Tomioka, J. Org. Chem., 2005, 70, 297 CrossRef CAS PubMed; (f) T. Hayashi, M. Kawai and N. Tokunaga, Angew. Chem., Int. Ed., 2004, 43, 6125 CrossRef CAS PubMed; (g) H. K. Yim and H. N. C. Wong, J. Org. Chem., 2004, 69, 2892 CrossRef CAS PubMed; (h) P. Wipf, C. Kendall and C. R. J. Stephenson, J. Am. Chem. Soc., 2003, 125, 761 CrossRef CAS PubMed; (i) V. K. Aggarwal, E. Alonso, M. Ferrara and S. E. Spey, J. Org. Chem., 2002, 67, 2335 CrossRef CAS PubMed; (j) K. I. Yamada, H. Fujihara, Y. Yamamoto, Y. Miwa, T. Taga and K. Tomioka, Org. Lett., 2002, 4, 3509 CrossRef CAS PubMed; (k) D. K. Wang, Y. G. Zhou, Y. Tang, X. L. Hou and L. X. Dai, J. Org. Chem., 1999, 64, 4233 CrossRef CAS.
- (a) O. G. Mancheno, R. G. Arrayas and J. C. Carretero, J. Am. Chem. Soc., 2004, 126, 456 CrossRef CAS PubMed; (b) P. E. Morgan, R. McCague and A. Whiting, J. Chem. Soc., Perkin Trans. 1, 2000, 515 RSC; (c) S. Yao, M. Johannsen, R. G. Hazell and K. A. Jørgensen, Angew. Chem., Int. Ed., 1998, 37, 3121 CrossRef CAS; (d) T. Bauer, S. Szymanski, A. Jezewski, P. Gluzinski and J. Jurczak, Tetrahedron: Asymmetry, 1997, 8, 2619 CrossRef CAS; (e) J. Sisko and S. M. Weinreb, Tetrahedron Lett., 1989, 30, 3037 CrossRef CAS; (f) D. L. Boger, W. L. Corbett, T. T. Curran and A. M. Kasper, J. Am. Chem. Soc., 1991, 113, 1713 CrossRef CAS.
- (a) C. Ballatore, J. H. Soper, F. Piscitelli, M. James, L. C. Huang, O. Atasoylu, D. M. Huryn, J. Q. Trojanowski, V. M. Y. Lee, K. R. Brunden and A. B. Smith, J. Med. Chem., 2011, 54, 6969 CrossRef CAS PubMed; (b) S. R. Malwal, D. Sriram, P. Yogeeswari, V. B. Konkimalla and H. Chakrapani, J. Med. Chem., 2012, 55, 553 CrossRef CAS PubMed; (c) C. T. Supuran, A. Scozzafava and B. W. Clare, Med. Res. Rev., 2002, 22, 329 CrossRef CAS PubMed; (d) M. V. Papadopoulou, W. D. Bloomer, H. S. Rosenzweig, E. Chatelain, M. Kaiser, S. R. Wilkinson, C. McKenzie and J. R. Ioset, J. Med. Chem., 2012, 55, 5554 CrossRef CAS PubMed.
- (a) R. Annunziata, M. Cinquini and F. Cozzi, J. Chem. Soc., Perkin Trans. 1, 1982, 339 RSC; (b) D. H. Hua, S. W. Miao, J. S. Chen and S. Iguchi, J. Org. Chem., 1991, 56, 4 CrossRef CAS; (c) P. Moreau, M. Essiz, J. Y. Merour and D. Bouzard, Tetrahedron: Asymmetry, 1997, 8, 591 CrossRef CAS; (d) T. K. Yang, R. Y. Chen, D. S. Lee, W. S. Peng, Y. Z. Jiang, A. Q. Mi and T. T. Jong, J. Org. Chem., 1994, 59, 914 CrossRef CAS; (e) G. C. Liu, D. A. Cogan and J. A. Ellman, J. Am. Chem. Soc., 1997, 119, 9913 CrossRef CAS; (f) F. A. Davis, R. E. Reddy, J. M. Szewczyk and P. S. Portonovo, Tetrahedron Lett., 1993, 34, 6229 CrossRef CAS.
- F. A. Davis, R. E. Reddy and R. T. Reddy, J. Org. Chem., 1992, 57, 6387 CrossRef CAS.
- (a) D. A. Cogan and J. A. Ellman, J. Am. Chem. Soc., 1999, 121, 268 CrossRef CAS; (b) G. C. Liu, D. A. Cogan, T. D. Owens, T. P. Tang and J. A. Ellman, J. Org. Chem., 1999, 64, 1278 CrossRef CAS.
- (a) F. A. Davis, R. E. Reddy, J. M. Szewczyk, G. V. Reddy, P. S. Portonovo, H. Zhang, D. Fanelli, R. T. Reddy, P. Zhou and P. J. Carroll, J. Org. Chem., 1997, 62, 2555 CrossRef CAS PubMed; (b) F. A. Davis, Y. Zhang, Y. Andemichael, T. Fang, D. L. Fanelli and H. Zhang, J. Org. Chem., 1999, 64, 1403 CrossRef CAS; (c) D. L. Fanelli, J. M. Szewczyk, Y. Zhang, G. V. Reddy, D. M. Burns and F. A. Davis, Org. Synth., 1999, 77, 50 Search PubMed.
- Z. Y. Jiang, W. H. Chan and A. W. M. Lee, J. Org. Chem., 2005, 70, 1081 CrossRef CAS PubMed.
- (a) W. A. White and H. Weingarten, J. Org. Chem., 1967, 32, 213 CrossRef CAS; (b) H. Weingarten, J. P. Chupp and W. A. White, J. Org. Chem., 1967, 32, 3246 CrossRef CAS; (c) I. Moretti and G. Torre, Synthesis, 1970, 141 CrossRef CAS; (d) W. B. Jennigs and C. J. Lovely, Tetrahedron Lett., 1988, 29, 3725 CrossRef.
- J. H. Billman and K. M. Tai, J. Org. Chem., 1958, 23, 535 CrossRef CAS.
- X. F. Wu, C. V. L. Bray, L. Bechki and C. Darcel, Tetrahedron, 2009, 65, 7380 CrossRef CAS.
- J. T. Reeves, M. D. Visco, M. A. Marsini, N. Grinberg, C. A. Busacca, A. E. Mattson and C. H. Senanayake, Org. Lett., 2015, 17, 2442 CrossRef CAS PubMed.
- S. Higashibayashi, H. Tohmiya, T. Mori, K. Hashimoto and M. Nakata, Synlett, 2004, 457 CAS.
- M. Ardej-Jakubisiak, R. Kawecki and A. Swietlinska, Tetrahedron: Asymmetry, 2007, 18, 2507 CrossRef CAS.
- Z. Huang, M. Zhang, Y. Wang and Y. Qin, Synlett, 2005, 1334 CAS.
- S. Morales, F. G. Guijarro, J. L. G. Ruano and M. B. Cid, J. Am. Chem. Soc., 2014, 136, 1082 CrossRef CAS PubMed.
- K. M. Wang, Z. G. Xing, Y. D. Ma and Q. L. Wang, Catal. Lett., 2008, 123, 129 CrossRef CAS.
- For examples, see: (a) J. M. Hoover and S. S. Stahl, J. Am. Chem. Soc., 2011, 133, 16901 CrossRef CAS PubMed; (b) J. E. Steves and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 15742 CrossRef CAS PubMed; (c) M. S. Sigman and D. R. Jensen, Acc. Chem. Res., 2006, 39, 221 CrossRef CAS PubMed; (d) G. F. Zhang, Y. Wang, X. Wen, C. R. Ding and Y. Li, Chem. Commun., 2012, 48, 2979 RSC; (e) C. Liu, S. Tang and A. W. Lei, Chem. Commun., 2013, 49, 1324 RSC; (f) B. T. Guan, D. Xing, G. X. Cai, X. B. Wan, N. Yu, Z. Fang, L. P. Yang and Z. J. Shi, J. Am. Chem. Soc., 2005, 127, 18004 CrossRef CAS PubMed; (g) H. Miyamura, R. Matsubara, Y. Miyazaki and S. Kobayashi, Angew. Chem., Int. Ed., 2007, 46, 4151 CrossRef CAS PubMed; (h) B. Karimi and F. K. Esfahani, Adv. Synth. Catal., 2012, 354, 1319 CrossRef CAS; (i) N. W. Wang, R. H. Liu, J. P. Chen and X. M. Liang, Chem. Commun., 2005, 5322 RSC; (j) W. L. Yin, C. H. Chu, Q. Q. Lu, J. W. Tao, X. M. Liang and R. H. Liu, Adv. Synth. Catal., 2010, 352, 113 CrossRef CAS; (k) N. Jiang and A. J. Ragauskas, Org. Lett., 2005, 7, 3689 CrossRef CAS PubMed; (l) G. Yang, W. Zhu, P. Zhang, H. Xue, W. Wang, J. Tian and M. Song, Adv. Synth. Catal., 2008, 350, 542 CrossRef CAS; (m) N. Jiang, D. Vinci, C. L. Liotta, C. A. Eckert and A. J. Ragauskas, Ind. Eng. Chem. Res., 2008, 47, 627 CrossRef CAS; (n) N. Jiang and A. J. Ragauskas, ChemSusChem, 2008, 1, 823 CrossRef CAS PubMed; (o) P. J. Figiel, A. M. Kirillov, Y. Y. Karabach, M. N. Kopylovich and A. J. L. Pombeiro, J. Mol. Catal. A: Chem., 2009, 305, 178 CrossRef CAS; (p) L. Liang, G. Rao, H. L. Sun and J. L. Zhang, Adv. Synth. Catal., 2010, 352, 2371 CrossRef CAS; (q) N. Mase, T. Mizumori and Y. Tatemoto, Chem. Commun., 2011, 47, 2086 RSC; (r) G. F. Zhang, X. W. Han, Y. Luan, Y. Wang, X. Wen and C. R. Ding, Chem. Commun., 2013, 49, 7908 RSC.
- R. Patel, V. P. Srivastava and L. D. S. Yadav, Adv. Synth. Catal., 2010, 352, 1610 CrossRef CAS.
- (a) J. M. Hoover, B. L. Ryland and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 2357 CrossRef CAS PubMed; (b) N. J. Hill, J. M. Hoover and S. S. Stahl, J. Chem. Educ., 2013, 90, 102 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, the optimization of copper-catalyzed oxidative cascade reaction and NMR data for products. See DOI: 10.1039/c6ra26490e |
| This journal is © The Royal Society of Chemistry 2017 |