Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cu(II)/DM-Segphos catalyzed asymmetric 1,3-dipolar cycloaddition of benzoisothiazole-2,2-dioxide-3-ylidenes and azomethine ylides†
Feifei Li,
Guorui Cao,
Yanfeng Gao and
Dawei Teng *
*
College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao 266042, China
First published on 9th February 2017
Abstract
Cu(OTf)2/DM-Segphos catalyzed asymmetric 1,3-dipolar cycloaddition between benzoisothiazole-2,2-dioxide-3-ylidenes and azomethine ylides was studied. The spiropyrrolidinyl-benzoisothiazolines were obtained in high yields with up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 99% ee. The enantioselective cycloaddition could be explained by the coordination of the imino esters 2 and chiral ligand DM-Segphos to the metallic center. The exo-selective cycloaddition course was attributed to the steric repulsion between the dipolarophiles and the 3,5-dimethylphenylphosphine group of the ligand.
1 dr and 99% ee. The enantioselective cycloaddition could be explained by the coordination of the imino esters 2 and chiral ligand DM-Segphos to the metallic center. The exo-selective cycloaddition course was attributed to the steric repulsion between the dipolarophiles and the 3,5-dimethylphenylphosphine group of the ligand.
Benzosultams are an important privileged class of structures in drug discovery, and are attractive synthetic targets due to their biological activities and intermediates for constructing molecular complexity and diversity.1 The catalytic asymmetric 1,3-dipolar cycloaddition reaction plays a crucial role in the enantioselective preparation of five-membered heterocycles.2 More specifically, the asymmetric 1,3-dipolar cycloaddition of azomethine ylides with activated olefins has become one of the most powerful methods for the construction of chiral spirocyclic pyrrolidines containing spiro quaternary stereogenic centers,3 which represent the key structural moiety widely present in a myriad of natural products and biologically active compounds.4 Since the pioneering work of Gong5 employing stoichiometric amounts of chiral organocatalyst, much attention has been paid to develop a catalytic asymmetric approach to synthesize spirocyclic pyrrolidines.6 Although various methods are developed for this transformation, most dipolarophiles applied in these reactions are 2-oxoindolin-3-ylidene,6a–e α-methylene-γ-butyrolactone,6f,g ethyl cyclopropylidene acetate,6h 2-alkylidene-cycloketone,6i 5-alkylidene thia(oxa)zolidine-2,4-dione,6j and 3-alkylidene-4-chromanone.6k
In the past several years, our research has been focused on developing spiro benzoisothiazole dioxide derivatives, which have been known to exhibit a variety of biological activities.7,8 To the best of our knowledge, there have no reports on catalytic asymmetric 1,3-dipolar cycloaddition of benzoisothiazole-2,2-dioxide-3-ylidene as the dipolarophile. Herein, we reported the first example of Cu(II)/DM-Segphos catalyzed asymmetric 1,3-dipolar cycloaddition of benzoisothiazole-2,2-dioxide-3-ylidene derivatives with azomethine ylides to give spiropyrrolidinyl-benzoisothiazoline derivatives in high diastereo- and enantioselectivity.
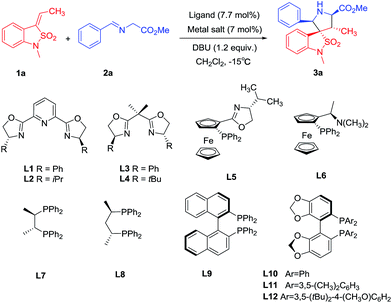
Our investigations began with a set of experiments directed at the identification of an optimal chiral Lewis acid system for the cycloaddition of benzoisothiazole-2,2-dioxide-3-ylidene 1a with azomethine ylide precursor 2a (Table 1). The initial reactions were performed with the combination of copper(II) trifluoromethanesulfonate and various chiral ligands as the chiral Lewis acid systems in dichloromethane at −15 °C. Under all reaction conditions, the exo-cycloadduct 3a was obtained in excellent diastereoselectivities (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Reactions with PyBox ligand L1, L2 led to the exo-cycloadduct 3a in low yields with moderate enantioselectivities, while the use of bisoxazoline ligands L3, L4 did not produce significant improvement (entry 1–4). Chiral ferrocene ligands L5 and L6 catalyzed the reaction efficiently to give exo-3a in good yields but also with moderate enantioselectivities (entry 5, 6). Chiral P,P-bidentate ligands L7 and L8 also could catalyse the reaction efficiently but with even lower enantioselectivities (entry 7, 8). Chiral P,P-axially ligands catalyzed the reaction efficiently with much better enantioselectivities (entry 9–12). Gratifyingly, we find that the bulky and electron-donating DM-Segphos ligand9 L11 catalyzed the reaction efficiently with excellent enantioselectivity. The spiropyrrolidinyl-benzoisothiazoline 3a was obtained in 95% yield and 96% ee (entry 11). The other Cu(I/II) salts, such as Cu(CH3CN)4ClO4 or Cu(CH3CN)4BF4 and Cu(OAc)2, produced exo-3a in slightly lower yields and enantioselectivities in combination with ligand L11 (entry 13–15).
1). Reactions with PyBox ligand L1, L2 led to the exo-cycloadduct 3a in low yields with moderate enantioselectivities, while the use of bisoxazoline ligands L3, L4 did not produce significant improvement (entry 1–4). Chiral ferrocene ligands L5 and L6 catalyzed the reaction efficiently to give exo-3a in good yields but also with moderate enantioselectivities (entry 5, 6). Chiral P,P-bidentate ligands L7 and L8 also could catalyse the reaction efficiently but with even lower enantioselectivities (entry 7, 8). Chiral P,P-axially ligands catalyzed the reaction efficiently with much better enantioselectivities (entry 9–12). Gratifyingly, we find that the bulky and electron-donating DM-Segphos ligand9 L11 catalyzed the reaction efficiently with excellent enantioselectivity. The spiropyrrolidinyl-benzoisothiazoline 3a was obtained in 95% yield and 96% ee (entry 11). The other Cu(I/II) salts, such as Cu(CH3CN)4ClO4 or Cu(CH3CN)4BF4 and Cu(OAc)2, produced exo-3a in slightly lower yields and enantioselectivities in combination with ligand L11 (entry 13–15).
| Entry | Ligand | Lewis acid | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|---|
| a Reaction condition: dipolarophile 1a (0.1 mmol), imino ester 2a (0.12 mmol), ligand (0.0077 mmol), Lewis acid (0.007 mmol), DBU (0.12 mmol), CH2Cl2 (0.4 mL), −15 °C, 2 h.b Isolated yield.c Determined by HPLC analysis.d Determined by chiral HPLC analysis after purification. | |||||
| 1 | L1 | Cu(OTf)2 | 21 | >99/1 | 55 |
| 2 | L2 | Cu(OTf)2 | 11 | >99/1 | 56 |
| 3 | L3 | Cu(OTf)2 | 17 | >99/1 | 40 |
| 4 | L4 | Cu(OTf)2 | 15 | >99/1 | 60 |
| 5 | L5 | Cu(OTf)2 | 94 | >99/1 | 53 |
| 6 | L6 | Cu(OTf)2 | 92 | >99/1 | 54 |
| 7 | L7 | Cu(OTf)2 | 92 | >99/1 | 35 |
| 8 | L8 | Cu(OTf)2 | 93 | >99/1 | 25 |
| 9 | L9 | Cu(OTf)2 | 93 | >99/1 | 66 |
| 10 | L10 | Cu(OTf)2 | 95 | >99/1 | 70 |
| 11 | L11 | Cu(OTf)2 | 95 | >99/1 | 96 |
| 12 | L12 | Cu(OTf)2 | 94 | >99/1 | 58 |
| 13 | L11 | Cu(CH3CN)4ClO4 | 83 | >99/1 | 90 |
| 14 | L11 | Cu(CH3CN)4BF4 | 89 | >99/1 | 92 |
| 15 | L11 | Cu(OAc)2 | 92 | >99/1 | 94 |
Having identified a promising chiral Lewis acid system for the asymmetric 1,3-dipolar cycloaddition, we examined the effects of bases, solvents, and temperature on the reaction yield, diastereoselectivity, and enantioselectivity (Table 2). The reactions using triethylamine, DBU, DIPEA, and DABCO all afforded exo-3a with good diastereoselectivities and enantioselectivities (entry 1–4). High reaction yield was achieved when DBU was used, while the other bases gave low yields. Solvents have much effect on the reaction yields and enantioselectivities, but little on the diastereoselectivities. It was shown that dichloromethane is the best solvent of choice (entry 1 vs. 5–7). Investigation on the effect of temperature showed that the reaction yields could be slightly improved but the enantioselectivities were decreased when the reaction temperature was increased (entry 1, 8, 9). It was noted that higher enantiomeric excess (99% ee, entry 10 vs. 1) was achieved when the temperature was decreased from −15 °C to −25 °C. Even lower temperature caused adverse effect on both reactivity and enantioselectivity (entry 11). Optimization on the catalyst loading showed that both diastereoselectivity and enantioselectivity were reduced when lowering the catalytic loading from 7 mol% to 5 mol% (entry 12). Examination of different reaction times disclosed that 2 h was the best choice (entry 13, 14).
| Entry | Solvent | Base | Temp. (°C) | Time (h) | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|---|---|---|
| a Reaction condition: dipolarophile 1a (0.1 mmol), imino ester 2a (0.12 mmol), DM-Segphos (0.0077 mmol), Cu(OTf)2 (0.007 mmol), base (0.12 mmol), solvent (0.4 mL).b Isolated yield.c Determined by HPLC analysis.d Determined by chiral HPLC analysis after purification.e Reaction performed with a 5 mol% of catalyst. | |||||||
| 1 | CH2Cl2 | DBU | −15 | 2 | 95 | >99/1 | 96 |
| 2 | CH2Cl2 | Et3N | −15 | 2 | 49 | >99/1 | 91 |
| 3 | CH2Cl2 | DIPEA | −15 | 2 | 42 | >99/1 | 94 |
| 4 | CH2Cl2 | DBACO | −15 | 2 | 35 | >99/1 | 94 |
| 5 | Toluene | DBU | −15 | 2 | 80 | >99/1 | 86 |
| 6 | THF | DBU | −15 | 2 | 79 | >99/1 | 56 |
| 7 | CH3CN | DBU | −15 | 2 | 89 | 99/1 | 45 |
| 8 | CH2Cl2 | DBU | 0 | 2 | 96 | >99/1 | 93 |
| 9 | CH2Cl2 | DBU | r.t. | 2 | 96 | >99/1 | 91 |
| 10 | CH2Cl2 | DBU | −25 | 2 | 95 | >99/1 | 99 |
| 11 | CH2Cl2 | DBU | −40 | 2 | 73 | >99/1 | 97 |
| 12e | CH2Cl2 | DBU | −25 | 2 | 95 | 97/3 | 96 |
| 13 | CH2Cl2 | DBU | −25 | 1.5 | 89 | >99/1 | 99 |
| 14 | CH2Cl2 | DBU | −25 | 2.5 | 95 | >99/1 | 99 |
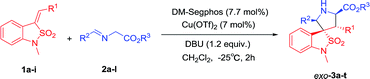
Under the optimized conditions, we next studied the Cu(II)/DM-Segphos catalyzed 1,3-dipolar cycloaddition of benzoisothiazole-2,2-dioxide-3-ylidene derivative 1a with a variety of azemothine ylides. As summarized in Table 3, a wide array of imino esters 2a–l derived from aromatic aldehyde reacted smoothly with 1a affording the desired exo-adducts 3a–l in good diastereoselectivities and enantioselectivities. The yields of the cycloadducts were sensitive to the position of the substituent on the phenyl group. Substrate with a para-substituent (4-Me) gave higher yield than that with a meta-substituent (3-Me) (3b vs. 3c). It appeared that the electronic property of the benzene ring had very limited effect on the reaction yields and enantioselectivities. Both substrates with electron-donating substituents (Me, OMe) and those with electron-withdrawing ones (F, Cl, Br, CF3) gave the cycloadducts in high yields and enantioselectivities (3b and 3d vs. 3e–h). The imino ester 2i derived from heteroaromatic aldehyde also gave 3i in 87% yield and 94% ee. The imino ester 2j–l with a much bulkier group (R3 = tBu) led to exo-cycloadducts 3j–l in good diastereoselectivity and enantioselectivity.
| a General procedure: after a suspension of the DM-Segphos (0.0077 mmol) and Cu(OTf)2 (0.007 mmol) in CH2Cl2 (0.2 mL) was stirred for 1 h at room temperature, a solution of the imino ester 2 (0.12 mmol) in CH2Cl2 (0.2 mL) was added. After being stirred at −25 °C for 10 min, DBU (0.12 mmol) and dipolarophile 1 (0.1 mmol) was added and the resulting solution was stirred at −25 °C for 2 h. |
|---|
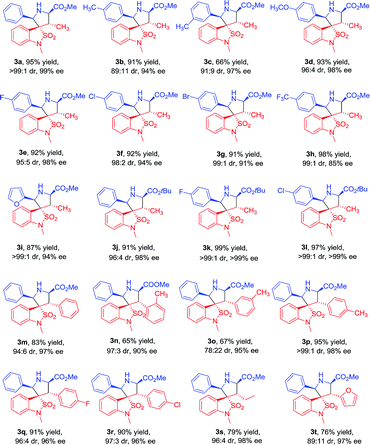 |
To further investigate the scope of the reaction, various benzoisothiazole-2,2-dioxide-3-ylidene derivatives 1b–i were then examined under the optimal conditions. Regardless of the electronic properties, the electron-donating and electron-withdrawing substituents had no major effect on the reaction and led to exo-cycloadducts 3m–t in moderate to good yields (65–95%), high diastereoselectivities (78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 to >99
22 to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
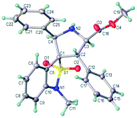
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr), and enantioselectivities (90% to 98% ee). The position of substituents on the benzyl ring had somewhat effect on the yields and enantiomeric excesses. Substrate with a para-substituent (4-Me, 95% yield, 98% ee) gave higher yield and enantioselectivity than that with an ortho- or meta-substituent (2-Me and 3-Me, 65–67% yield, 90–95% ee) (3p vs. 3n–o). The substrates with alkyl and heteroaromatic substituents also gave the cycloadducts in good yields, high diastereoselectivities, and enantioselectivities (3s and 3t). The absolute configuration of the product 3m was determined by crystal structure analysis (Fig. 1). The absolute configurations of the other cycloadducts were determined by analogy.10
1 dr), and enantioselectivities (90% to 98% ee). The position of substituents on the benzyl ring had somewhat effect on the yields and enantiomeric excesses. Substrate with a para-substituent (4-Me, 95% yield, 98% ee) gave higher yield and enantioselectivity than that with an ortho- or meta-substituent (2-Me and 3-Me, 65–67% yield, 90–95% ee) (3p vs. 3n–o). The substrates with alkyl and heteroaromatic substituents also gave the cycloadducts in good yields, high diastereoselectivities, and enantioselectivities (3s and 3t). The absolute configuration of the product 3m was determined by crystal structure analysis (Fig. 1). The absolute configurations of the other cycloadducts were determined by analogy.10
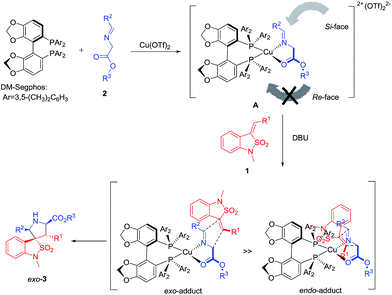
The stereochemical course of the reaction can be rationalized by means of the model proposed in Scheme 1.11 Firstly, the complex A is generated by coordination of the imino esters 2 and the bidentate chiral ligand DM-Segphos to Cu(II) in a tetrahedral arrangement. After the abstraction of a proton by the amine base, the attack of benzoisothiazole-2,2-dioxide-3-ylidenes 1 is favored to the Si-face avoiding the steric interaction with two 3,5-dimethylphenyl groups of the ligand. Finally, the steric repulsion between the sulfuryl group of dipolarophile and the substituent of the ligand inhibits the endo-orientation leading to high exo-selectivity for the Cu(II)/DM-Segphos catalyzed cycloaddition.
Conclusions
In summary, we have developed a highly enantioselective Cu(II)/DM-Segphos catalytic system for the asymmetric 1,3-dipolar cycloaddition of azomethine ylides with benzoisothiazole-2,2-dioxide-3-ylidenes. The corresponding spiropyrrolidinyl-benzoisothiazoline derivatives were afforded in good yields with high diastereo- and enantioselectivities under mild reaction conditions. Further investigations on the evaluation of alternative azomethine ylides that carry electron withdrawing groups other than esters, and the substituent tolerance of the benzoisothiazol-2,2-dioxide on the benzene ring are underway and will be reported in due course.Notes and references
- (a) K. C. Majumdar and S. Mondal, Chem. Rev., 2011, 111, 7749 CrossRef CAS PubMed; (b) T. Mezei, N. Mesterházy, T. Bakó, M. Porcs-Makkay, G. Simig and B. Volk, Org. Process Res. Dev., 2009, 13, 567 CrossRef CAS; (c) L. N. Tumey, M. J. Robarge, E. Gleason, J. Song, S. M. Murphy, G. Ekema, C. Doucette, D. Hanniford, M. Palmer, G. Pawlowski, J. Danzig, M. Loftus, K. Hunady, B. Sherf, R. W. Mays, A. Stricker-Krongrad, K. R. Brunden, Y. L. Bennani and J. J. Harrington, Bioorg. Med. Chem. Lett., 2010, 20, 3287 CrossRef CAS PubMed; (d) I. Fujita, T. Okumura, A. Sakakibara and Y. Kita, J. Pharm. Pharmacol., 2012, 64, 747 CrossRef CAS PubMed; (e) S. H. Kim, R. Amu, S. W. Kwon, S.-H. Lee, C. H. Kim, S. K. Kang, S. D. Rhee, M. A. Bae, S. H. Ahn, D. C. Ha, H. G. Cheon, K. Y. Kim and J. H. Ahn, Bioorg. Med. Chem. Lett., 2010, 20, 1065 CrossRef CAS PubMed; (f) M. Inagaki, T. Tsuri, H. Jyoyama, T. Ono, K. Yamada, M. Kobayashi, Y. Hori, A. Arimura, K. Yasui, K. Ohno, S. Kakudo, K. Koizumi, R. Suzuki, M. Kato, S. Kawai and S. Matsumoto, J. Med. Chem., 2000, 43, 2040 CrossRef CAS PubMed; (g) Z. Brzozowski, F. Saczewiski and N. Neamati, Bioorg. Med. Chem. Lett., 2006, 16, 5298 CrossRef CAS PubMed; (h) Y. Misu and H. Togo, Org. Biomol. Chem., 2003, 1, 1342 RSC; (i) G. J. Wells, M. Tao, K. A. Josef and R. Bihovski, J. Med. Chem., 2001, 44, 3488 CrossRef CAS PubMed; (j) R. J. Cherney, R. Mo, D. T. Meyer, K. D. Hardman, R.-Q. Liu, M. B. Covington, M. Qian, Z. R. Wasserman, D. D. Christ, J. M. Trzasko, R. C. Newton and C. P. Decicco, J. Med. Chem., 2004, 47, 2981 CrossRef CAS PubMed.
- For reviews, see: (a) K. V. Gothelf, in Cycloaddition Reactions in Organic Synthesis, ed. S. Kobayashi and K. A. Jørgensen, Wiley-VCH, Weinheim, Germany, 2002, p. 211 Search PubMed; (b) K. V. Gothelf and K. A. Jørgensen, in Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products, ed. A. Padwa and W. Pearson, Wiley & Sons, New York, 2002, ch. 12, p. 817 Search PubMed; (c) K. V. Gothelf and K. A. Jorgensen, Chem. Rev., 1998, 98, 863 CrossRef CAS PubMed; (d) S. Kanemasa, Synlett, 2002, 1371 CrossRef CAS; (e) H. Pellissier, Tetrahedron, 2007, 63, 2887 Search PubMed; (f) L. M. Stanley and M. P. Sibi, Chem. Rev., 2008, 108, 5366 Search PubMed; (g) A. Bădoiu, Y. Brinkmann, F. Viton and E. P. Kündig, Pure Appl. Chem., 2008, 80, 1013 CrossRef; (h) W. M. Golebiewski and M. Gucma, J. Heterocycl. Chem., 2008, 45, 1687 CrossRef CAS; (i) S. Kanemasa, Heterocycles, 2010, 82, 87 CrossRef CAS; (j) M. Kissane and A. R. Maguire, Chem. Soc. Rev., 2010, 39, 845 RSC; (k) C. Nájera, J. M. Sansano and M. Yus, J. Braz. Chem. Soc., 2010, 21, 377 CrossRef; (l) Y. Xing and N.-X. Wang, Coord. Chem. Rev., 2012, 256, 938 CrossRef CAS; (m) T. Hashimoto and K. Maruoka, Chem. Rev., 2015, 115, 5366 CrossRef CAS PubMed.
- (a) P. R. Sebahar and R. M. Williams, J. Am. Chem. Soc., 2000, 122, 5666 CrossRef CAS; (b) P. R. Sebahar, H. Osada, T. Usui and R. M. Williams, Tetrahedron, 2002, 58, 6311 CrossRef CAS; (c) K. Ding, Y. Lu, Z. Nikolovska-Coleska, S. Qiu, Y. Ding, W. Gao, J. Stuckey, K. Krajewski, P. P. Roller, Y. Tomita, D. A. Parrish, J. R. Deschamps and S. Wang, J. Am. Chem. Soc., 2005, 127, 10130 CrossRef CAS PubMed; (d) K. Ding, Y. Lu, Z. Nikolovska-Coleska, G. Wang, S. Qiu, S. Shangary, W. Gao, D. Qin, J. Stuckey, K. Krajewski, P. P. Roller and S. Wang, J. Med. Chem., 2006, 49, 3432 CrossRef CAS PubMed; (e) K. Ding, G. Wang, J. R. Deschamps, D. A. Parrish and S. Wang, Tetrahedron Lett., 2005, 46, 5949 CrossRef CAS; (f) P. R. Sebahar and R. M. Williams, Heterocycles, 2002, 58, 563 CrossRef CAS.
- For examples, (a) M. M. M. Santos, Tetrahedron, 2014, 70, 9735 CrossRef CAS; (b) Z. Zhang, X.-J. Chu, J.-J. Liu, Q. Ding, J. Zhang, D. Bartkovitz, N. Jiang, P. Karnachi, S.-S. So, C. Tovar, Z. M. Filipovic, B. Higgins, K. Glenn, K. Packman, L. Vassilev and B. Graves, ACS Med. Chem. Lett., 2014, 5, 124 CrossRef CAS PubMed; (c) A. I. Almansour, R. S. Kumar, F. Beevi, A. N. Shirazi, H. Osman, R. Ismail, T. S. Choon, B. Sullivan, K. McCaffrey, A. Nahhas, K. Parang and M. A. Ali, Molecules, 2014, 19, 10033 CrossRef PubMed; (d) S. Hati, S. Tripathy, P. K. Dutta, R. Agarwal, R. Srinivasan, A. Singh, S. Singh and S. Sen, Sci. Rep., 2016, 6, 32213 CrossRef PubMed; (e) A. P. Antonchick, C. Gerding-Reimers, M. Catarinella, M. Schürmann, H. Preut, S. Ziegler, D. Rauh and H. Waldmann, Nat. Chem., 2010, 2, 735 CrossRef CAS PubMed; (f) S. M. Rajesh, S. Perumal, J. C. Menendez, P. Yogeeswari and D. Sriram, Med. Chem. Commun., 2011, 2, 626 RSC; (g) S. Shangary, D. Qin, D. McEachern, M. Liu, R. S. Miller, S. Qiu, Z. Nikolovska-Coleska, K. Ding, G. Wang, J. Chen, D. Bernard, J. Zhang, Y. Lu, Q. Gu, R. B. Shah, K. J. Pienta, X. Ling, S. Kang, M. Guo, Y. Sun, D. Yang and S. Wang, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 3933 CrossRef CAS PubMed; (h) R. S. Kumar, A. I. Almansour, N. Arumugam, A. Basiri, Y. Kia and R. R. Kumar, Aust. J. Chem., 2014, 68, 863 CrossRef.
- X.-H. Chen, Q. Wei, S.-W. Luo, H. Xiao and L.-Z. Gong, J. Am. Chem. Soc., 2009, 131, 13819 CrossRef CAS PubMed.
- For catalytic asymmetric 1,3-dipolar cycloaddition of azomethine ylides, see: (a) A. P. Antonchick, C. Gerding-Reimers, M. Catarinella, M. Schürmann, H. Preut, S. Ziegler, D. Rauh and H. Waldmann, Nat. Chem., 2010, 2, 735 CrossRef CAS PubMed; (b) T.-L. Liu, Z.-Y. Xue, H.-Y. Tao and C.-J. Wang, Org. Biomol. Chem., 2011, 9, 1980 RSC; (c) L. Wang, X.-M. Shi, W.-P. Dong, L.-P. Zhu and R. Wang, Chem. Commun., 2013, 49, 3458 RSC; (d) W. Dai, X.-L. Jiang, Q. Wu, F. Shi and S.-J. Tu, J. Org. Chem., 2015, 80, 5737 CrossRef CAS PubMed; (e) J.-X. Zhang, H.-Y. Wang, Q.-W. Jin, C.-W. Zheng, G. Zhao and Y.-J. Shang, Org. Lett., 2016, 18, 4774 CrossRef CAS PubMed; (f) Q.-H. Li, T.-L. Liu, L. Wei, X. Zhou, H.-Y. Tao and C.-J. Wang, Chem. Commun., 2013, 49, 9642 RSC; (g) J. Castulik, J. Marek and C. Mazal, Tetrahedron, 2001, 57, 8339 CrossRef CAS; (h) T.-L. Liu, Z.-L. He, H.-Y. Tao, Y.-P. Cai and C.-J. Wang, Chem. Commun., 2011, 47, 2616 RSC; (i) T.-L. Liu, Z.-L. He, Q.-H. Li, H.-Y. Tao and C.-J. Wang, Adv. Synth. Catal., 2011, 353, 1713 CrossRef CAS; (j) W.-L. Yang, F.-F. Tang, F.-S. He, C.-Y. Li, X. Yu and W.-P. Deng, Org. Lett., 2015, 17, 4822 CrossRef CAS PubMed; (k) T.-L. Liu, Z.-L. He and C.-J. Wang, Chem. Commun., 2011, 47, 9600 RSC; (l) T.-L. Liu, Z.-L. He, H.-Y. Tao and C.-J. Wang, Chem.–Eur. J., 2012, 18, 8042 CrossRef CAS PubMed; (m) K. Liu, H.-L. Teng, L. Yao, H.-Y. Tao and C.-J. Wang, Org. Lett., 2013, 15, 2250 CrossRef CAS PubMed; (n) F. Shi, Z.-L. Tao, S.-W. Luo, S.-J. Tu and L.-Z. Gong, Chem.–Eur. J., 2012, 18, 6885 CrossRef CAS PubMed; (o) X. Ma, Y. Zhu, Q. Sun, X. Li, J. Su, L. Zhao, Y. Zhao, S. Qiu, W. Yan, K. Wang and R. Wang, Chem. Commun., 2015, 51, 8789 RSC; (p) G. Zhu, B. Wang, X. Bao, H. Zhang, Q. Wei and J. Qu, Chem. Commun., 2015, 51, 15510 RSC.
- (a) G. Cao, F. Long, Y. Zhao, Y. Wang, L. Huang and D. Teng, Tetrahedron, 2014, 70, 9359 CrossRef CAS; (b) G. Cao, Y. Wang, T. Cui, L. Huang and D. Teng, RSC Adv., 2016, 6, 22519 RSC.
- (a) S. Petry, K.-H. Baringhaus, N. Tennagels and G. Mueller, US 20,050,070,533 A1, 2005; (b) M. A. Collins, V. A. Mackner, J. E. Wrobel, J. P. Edwards, T. K. Jones, C. M. Tegley and L. Zhi, US 6,339,098, 2002; (c) H. Masako, M. Sachiko, N. Hiroshi, K. Hiroki and M. Yukari, WO 2,005,092,858 A2, 2005.
- For the use of Segphos ligands in catalytic asymmetric 1,3-dipolar cycloadditions of azomethine ylides, see: (a) R. Joseph, C. Murray and P. Garner, Org. Lett., 2014, 16, 1550 CrossRef CAS PubMed; (b) M. González-Esguevillas, J. Adrio and J. C. Carretero, Chem. Commun., 2013, 49, 4649 RSC; (c) E. E. Maroto, S. Filippone, A. Martín-Domenech, M. Suarez and N. Martín, J. Am. Chem. Soc., 2012, 134, 12936 CrossRef CAS PubMed; (d) J. Hernández-Toribio, S. Padilla, J. Adrio and J. C. Carretero, Angew. Chem., Int. Ed., 2012, 51, 8854 CrossRef PubMed; (e) M. González-Esguevillas, J. Adrio and J. C. Carretero, Chem. Commun., 2012, 48, 2149 RSC; (f) Y. Yamashita, T. Imaizumi and S. Kobayashi, Angew. Chem., Int. Ed., 2011, 50, 4893 CrossRef CAS PubMed; (g) A. López-Pérez, J. Adrio and J. C. Carretero, Angew. Chem., Int. Ed., 2009, 48, 340 CrossRef PubMed; (h) Y. Oderaotoshi, W. Cheng, S. Fujitomi, Y. Kasano, S. Minakata and M. Komatsu, Org. Lett., 2003, 5, 5043 CrossRef CAS PubMed.
- CCDC 1515086 (3m) contains the supplementary crystallographic data for this paper.†.
- M. Potowski, A. P. Antonchick and H. Waldmann, Chem. Commun., 2013, 49, 7800 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, full spectroscopic data for all new compounds, and crystal data for 3m (CIF). CCDC 1515086. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra26543j |
| This journal is © The Royal Society of Chemistry 2017 |