Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of 2-substituted benzo[b]thiophene via a Pd-catalyzed coupling of 2-iodothiophenol with phenylacetylene†
Jingwen Chena,
Haifeng Xiang *a,
Li Yangab and
Xiangge Zhou
*a,
Li Yangab and
Xiangge Zhou *a
*a
aCollege of Chemistry, Sichuan University, Chengdu 610064, P. R. China. E-mail: zhouxiangge@scu.edu.cn; Fax: +86-28-85412904
bCollege of Chemistry & Chemical Engineering, Yibin University, Yibin 644000, P. R. China
First published on 23rd January 2017
Abstract
A Pd(II)-catalyzed Sonogashira type cross-coupling reaction between 2-iodothiophenol and phenylacetylene has been developed. A series of 2-substituted benzo[b]thiophenes were obtained in moderate to good yield (up to 87%). The application of this method was demonstrated by the synthesis of 2-(4-(tert-butyl)phenyl)benzo[b]thiophene 1,1-dioxide and (4-methoxyphenyl)(2-(4-methoxyphenyl)benzo[b]thiophen-3-yl)methanone, which exhibit a fluorescence quantum yield of up to 1 and can be used as a cannabinoid receptor ligand, respectively.
Introduction
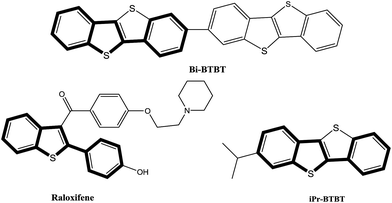
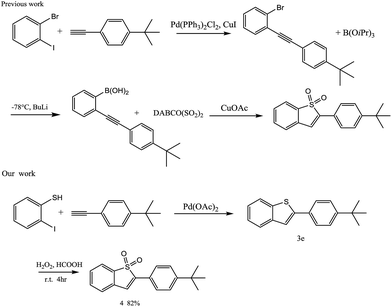
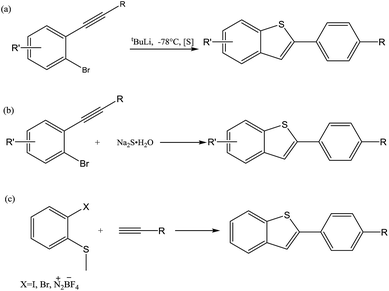
As a crucial class of heterocyclic compounds, 2-substituted benzo[b]thiophenes have broad biological properties1 and diversified applications in the field of materials science.2 They are usually considered as important structural motifs in pharmaceuticals and biologically active molecules. For example, as shown in Fig. 1, Bi-BTBT, raloxifene, and iPr-BTBT are examples of commercial drugs and organic semiconductors containing benzothiophene cores.3The normal approaches to synthesize 2-substituted benzo[b]thiophenes are normally focused on a coupling cyclization reaction of o-bromoalkynylbenzenes with various thiol surrogates upon lithium halogen exchange at −78 °C (Scheme 1a)4 or the annulation of alkynylbenzenes (Scheme 1b).5 While in the process of reporting this study, a similar study was reported by Fu and co-workers using the electrophilic cyclization of o-alkynyl thioanisole (Scheme 1c).6 However, the major obstacles of these methods are a result of the harsh reaction conditions or the limitation of the starting materials used.
 | ||
| Scheme 1 Selected examples of the commonly used synthetic methods to prepare 2-substituted benzo[b]thiophenes. | ||
On the other hand, the Sonogashira cross-coupling reaction7 between aryl or alkenyl halides with terminal alkynes in the presence of a transition-metal catalyst has become one of the most powerful methods to prepare alkyl-aryl and diarylsubstituted acetylenes.8 In a continuation of our study on catalytic Sonogashira cross-coupling reaction and synthesis of sulfur-containing heterocyclic compounds,9 herein we report the palladium-catalyzed synthesis of 2-substituted benzo[b]thiophenes using 2-halothiophenols and phenylacetylenes as starting materials.
Results and discussion
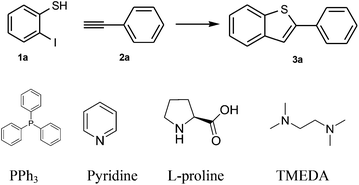
Our investigation started with the model substrates 2-iodothiophenol 1a and phenylacetylene 2a. As shown in Table 1, a variety of transition metal salts were tested and palladium acetate exhibited the best catalytic ability with a yield of 34% (Entries 1–8). Moreover, other metals including nickel, cobalt, and iron salts gave much less yields of 4%, 8%, and 5%, respectively (Entries 3, 4, and 5). In the case of copper salt, the coupling product between the alkyne was found to be the major product (Entries 1 and 2).10 The blank experiment further confirmed that no reaction occurred in the absence of a catalyst and ligand (Entry 9). Furthermore, silver salts were found to be beneficial to the reaction. In addition, AgTFA was shown to be the best one with a yield of 71% (Entries 10–12). Moreover, the ligand was also proved to promote the catalysis by up to 81% yield in the case of TMEDA (Entries 13–16). Lastly, screening the reaction temperature and catalyst loading indicated that 110 °C and 15 mol% catalyst were optimal for the reaction with yields up to 87% (Entries 17–23). Hence, it was concluded that the best conditions were 15 mol% Pd(OAc)2, 20 mol% TMEDA, and 1.1 equiv. AgTFA in DMF at 110 °C for 24 h.| Entry | Catalyst | Ligand | Additive | T/°C | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 2-iodothiophenol 1a (0.5 mmol), phenylacetylene 2a (4 equiv.), catalyst (10 mol%), ligand (20 mol%), and additive (1.1 equiv.) in DMF (2 mL) under N2 for 24 h.b Isolated yields.c Pd(OAc)2 (15 mol%).d Pd(OAc)2 (20 mol%).e Pd(OAc)2 (25 mol%). | |||||
| 1 | CuI | — | — | 100 | 14 |
| 2 | CuCl | — | — | 100 | Trace |
| 3 | NiCl2 | — | — | 100 | 4 |
| 4 | CoCl2·6H2O | — | — | 100 | 8 |
| 5 | FeSO4 | — | — | 100 | 5 |
| 6 | Pd(PPh3)Cl2 | — | — | 100 | 28 |
| 7 | Pd(PPh3)4 | — | — | 100 | 21 |
| 8 | Pd(OAc)2 | — | — | 100 | 34 |
| 9 | — | — | — | 100 | Trace |
| 10 | Pd(OAc)2 | — | AgOAc | 100 | 68 |
| 11 | Pd(OAc)2 | — | Ag2CO3 | 100 | 66 |
| 12 | Pd(OAc)2 | — | AgTFA | 100 | 71 |
| 13 | Pd(OAc)2 | PPh3 | AgTFA | 100 | 75 |
| 14 | Pd(OAc)2 | TMEDA | AgTFA | 100 | 81 |
| 15 | Pd(OAc)2 | L-Proline | AgTFA | 100 | 69 |
| 16 | Pd(OAc)2 | Pyridine | AgTFA | 100 | 72 |
| 17 | Pd(OAc)2 | TMEDA | AgTFA | 105 | 82 |
| 18 | Pd(OAc)2 | TMEDA | AgTFA | 110 | 85 |
| 19 | Pd(OAc)2 | TMEDA | AgTFA | 115 | 84 |
| 20 | Pd(OAc)2 | TMEDA | AgTFA | 120 | 84 |
| 21c | Pd(OAc)2 | TMEDA | AgTFA | 110 | 87 |
| 22d | Pd(OAc)2 | TMEDA | AgTFA | 110 | 87 |
| 23e | Pd(OAc)2 | TMEDA | AgTFA | 110 | 86 |
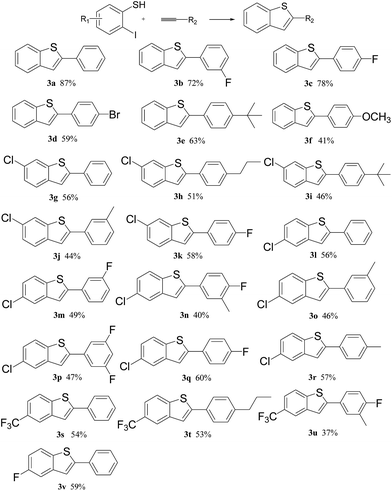
With the optimal reaction conditions in hand, we then explored the scope of 2-iodothiophenols and alkynes. As shown in Scheme 2, different alkynes with either electron-withdrawing groups (–F, –Br) or electron-donating groups (–tBu, –OCH3) can generate the desired products in yields from 41 to 78% under the standard conditions (3b–3e). Moreover, 2-iodothiophenols with various functional groups (such as –F, –Cl, and –CF3) can also be successfully applied in this method and novel compounds such as 3g, 3h, and 3i were also obtained in around 50% yield, which have great potential, especially in pharmaceutical compounds and materials synthesis.
To further explore the potential application of this method, the reaction of 1a and 2a was scaled up to 10.0 mmol in a 50 mL one-necked flask and the same efficiency was maintained (Scheme 3). The desired product can be obtained in 75% yield, which confirms its suitability for large-scale reaction.
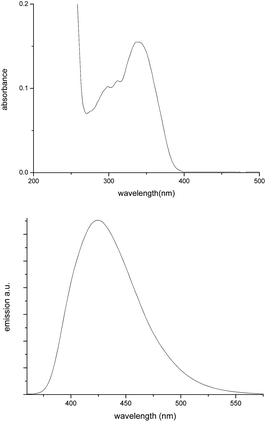
Furthermore, 2-(4-(tert-butyl)phenyl)benzo[b]thiophene 1,1-dioxide 4 can be easily obtained after adding H2O2 into 3e at room temperature, which could shorten one step and uses milder reaction conditions when compared with those reported in the literature (Scheme 4).11 Furthermore, Fig. 2 shows images of the compound 4 in MeCN and the solid state under sunlight (left) and under 360 nm UV light (right). Fig. 3 displays the absorption and emission spectra of compound 4 in MeCN (1.0 × 10−5 mol L−1). Note that compound 4 in MeCN exhibited an unexpectedly high fluorescence quantum yield of up to 1 that was measured using quinine sulfate as a standard (quinine in 5.0 × 10−5 mol L−1 sulfuric acid), which would shows broad prospects for use in organic light-emitting diodes (OLEDS).
 | ||
| Fig. 2 Images of compound 4 in MeCN (1.0 × 10−5 mol L−1) and the solid state under sunlight (left) and under 360 nm UV light (right). | ||
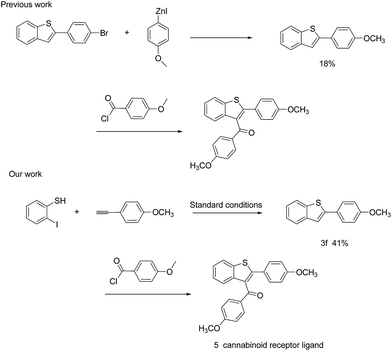
Besides this, we also tried to synthesize the benzothiophene derivative (4-methoxyphenyl)(2-(4-methoxyphenyl)benzo[b]thiophen-3-yl)methanone 5 using product 3f as the starting material in a higher yield than that reported in the literature. Compound 5 has been reported as a new cannabinoid receptor ligand and an intermediate of thrombin inhibitor.12
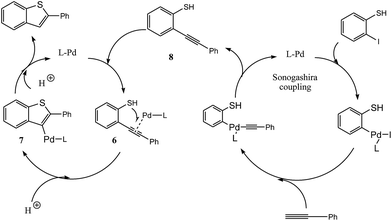
To explore the reaction pathway, a radical trapping experiment was carried out by the addition of a typical radical scavenger TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl). Almost the same yield (80%) indicated that the reaction did not involve a radical intermediate (Scheme 5). Furthermore, the intermediate 2-(phenylethynyl)benzenethiol 8 was observed by GC/MS in the reaction between 2-iodothiophenol and phenylacetylene after 3 hours.
Based on the experimental and literature data, we proposed a reaction pathway for the palladium-catalyzed synthesis of 2-substituted benzo[b]thiophenes from 2-halophenols and alkynes, which consists of two steps: the Sonogashira coupling of 2-halothiophenol with the alkyne and the subsequent cyclization of 2-alkynylthiophenol (Scheme 6). First, the Pd-catalyzed Sonogashira coupling of 2-halothiophenol with the alkyne affords intermediate 8. Then, coordination of Pd with intermediate 8 may provide complex 6, whose subsequent addition to the C–C triple bond gave intermediate 7. Protonation of intermediate 7 results in the formation of benzo[b]thiophene and the regenerated Pd-catalyst.
Conclusions
In summary, we developed an efficient catalytic system using 2-iodothiophenols as the starting material for the synthesis of a variety of 2-substituted benzo[b]thiophenes. This protocol involves the following advantages: easily available starting materials and simple operations with moderate to good yields, and will contribute a new optional route for the construction the benzo[b]thiophene ring. Moreover, the application of this method was considered as an example by the synthesis of 2-(4-(tert-butyl)phenyl)benzo[b]thiophene 1,1-dioxide and (4-methoxyphenyl)(2-(4-methoxyphenyl)benzo[b]thiophen-3-yl)methanone, which exhibit a fluorescence quantum yield up to 1 and use as a cannabinoid receptor ligand, respectively.Acknowledgements
We are grateful to the Natural Science Foundation of China (grant no. 21372169, 21472128, J1310008). We also acknowledge the comprehensive training platform of the specialized laboratory, College of Chemistry, Sichuan University for HRMS analysis.Notes and references
- For selected recent examples, see: (a) L. Berrade, B. Aisa, M. Ramirez, S. Galiano, S. Guccione, L. Moltzau, F. Levy, F. Nicoletti, G. Battaglia, G. Molinaro, I. Aldana, A. Monge and S. Perez-Silanes, J. Med. Chem., 2007, 50, 5644 CrossRef PubMed; (b) A. Venturelli, D. Tondi, L. Cancian, F. Morandi, G. Cannazza, B. Segatore, F. Prati, G. Amicosante, B. Shoichet and M. Costi, J. Med. Chem., 2011, 54, 3086 CrossRef PubMed; (c) R. Romagnoli, P. Baraldi, M. Carrion, C. Cara, D. Preti, F. Fruttarolo, M. Pavani, M. Tabrizi, M. Tolomeo, S. Grimaudo, A. Cristina, J. Balzarini, J. Hadfield, A. Brancale and E. Hamel, J. Med. Chem., 2007, 50, 2273 CrossRef CAS PubMed; (d) J. Chabert, B. Marquez, L. Neville, L. Joucla, S. Broussous, P. Bouhours, E. David, S. Pellet-Rostaing, B. Marquet, N. Moreau and M. Lemaire, Bioorg. Med. Chem., 2007, 15, 4482 CrossRef PubMed.
- (a) J. Gao, R. Li, L. Li, Q. Meng, H. Jiang, H. Li and W. Hu, Adv. Mater., 2007, 19, 3008 CrossRef CAS; (b) V. Bren, A. Dubonosov, V. Minkin, A. Tsukanov, T. Gribanova, E. Shepelenko, Y. Revinsky and V. Rybalkin, J. Phys. Org. Chem., 2007, 20, 917 CrossRef CAS; (c) T. Zhang, J. O'toole and C. Proctor, J. Sulfur Chem., 1999, 22, 1 CAS.
- (a) M. Abe, T. Mori, I. Osaka, K. Sugimoto and K. Takimiya, Chem. Mater., 2015, 27, 5049 CrossRef CAS; (b) B. Fox and S. Olson, J. Med. Chem., 2015, 58, 5256 CrossRef CAS PubMed; (c) G. Schweicher, V. Lemaur, Y. Geerts and Z. Bao, Adv. Mater., 2015, 27, 3066 CrossRef CAS PubMed; (d) E. Yamaguchi, C. Wang, A. Fukazawa, T. Higashiyama and S. Yamaguchi, Angew. Chem., Int. Ed., 2015, 54, 4539 CrossRef CAS PubMed; (e) E. Amir, M. Murai and C. Hawker, Chem. Sci., 2014, 5, 4483 RSC; (f) X. Peng, J. Deng and H. Xu, RSC Adv., 2013, 3, 24146 RSC; (g) S. Kawai, T. Nakashima and T. Kawai, J. Mater. Chem., 2009, 19, 3606 RSC; (h) S. Chen, W. Li, X. Li and W. Zhu, RSC Adv., 2015, 5, 87626 RSC.
- (a) H. Sashida, K. Sadamori and T. Tsuchiya, Synth. Commun., 1998, 28, 713 CrossRef CAS; (b) Y. Wang, S. Parkin and M. Watson, Org. Lett., 2008, 10, 4421 CrossRef CAS PubMed; (c) K. Takimiya, Y. Konda, H. Ebata, N. Niihara and T. Otsubo, J. Org. Chem., 2005, 70, 10569 CrossRef CAS PubMed.
- (a) T. Kashiki, S. Shinamura, M. Kohara, E. Miyazaki, K. Takimiya, M. Ikeda and H. Kuwabara, Org. Lett., 2009, 11, 2473 CrossRef CAS PubMed; (b) L. Sun, C. Den, R. Tang and X. Zhang, J. Org. Chem., 2011, 76, 7546 CrossRef CAS PubMed.
- L. Gao, B. Chang, W. Qiu, L. Wang and X. Fu, Adv. Synth. Catal., 2016, 358, 1202 CrossRef CAS.
- (a) K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 16, 4467 CrossRef; (b) H. Diek and F. Heck, Organomet. Chem., 1975, 93, 295 Search PubMed; (c) L. Cassar, Organomet. Chem., 1975, 93, 253 CrossRef CAS; (d) M. Miller and C. Johnson, J. Org. Chem., 1997, 62, 1582 CrossRef CAS; (e) S. Thorand and N. Krause, J. Org. Chem., 1998, 63, 8551 CrossRef CAS; (f) A. Chandra and B. Singh, Tetrahedron, 2008, 64, 11680 CrossRef CAS.
- (a) T. Magdesieva, O. Nikitin, A. Yakimansky, M. Goikhman and I. Podeshvo, Electrochim. Acta, 2011, 56, 3666 CrossRef CAS; (b) L. Wu, X. Shi, X. Xu, F. Liang and G. Huang, J. Chem. Sci., 2011, 123, 697 CrossRef CAS; (c) Y. Liang, S. Tang, X. Zhang, L. Mao, Y. Xie and J. Li, Org. Lett., 2006, 8, 3017 CrossRef CAS PubMed.
- (a) L. Yu and X. Zhou, Eur. J. Org. Chem., 2010, 29, 5560 CrossRef; (b) F. Ke, Z. Li and X. Zhou, Org. Lett., 2011, 13, 454 CrossRef CAS PubMed; (c) H. Deng, Z. Li and X. Zhou, Chem.–Eur. J., 2012, 18, 4840 CrossRef CAS PubMed; (d) R. Che, Z. Li and X. Zhou, Chem.–Eur. J., 2014, 20, 7258 CrossRef CAS PubMed.
- (a) C. Meng and R. Yuan, ACS Catal., 2015, 5, 3760 CrossRef CAS; (b) P. Röse, K. Harms and G. Hilt, J. Org. Chem., 2015, 80, 7311 CrossRef PubMed; (c) Y. Liu and J. Wan, Tetrahedron Lett., 2013, 54, 3953 CrossRef CAS.
- (a) J. Liu, A. Narita, S. Osella, W. Zhang, D. Schollmeyer, D. Beljonne, X. Feng and K. Müllen, J. Am. Chem. Soc., 2016, 138, 2602 CrossRef CAS PubMed; (b) R. Mao, D. Zheng, H. Xia and J. Wu, Org. Chem. Front., 2016, 3, 693 RSC.
- (a) J. Romero-Parra, J. Mella-Raipa, M. Torres, R. Escobar, M. Faúndez and C. David Pessoa-Mahana, Eur. J. Med. Chem., 2016, 124, 17 CrossRef CAS PubMed; (b) D. J. Sall, D. L. Bailey, J. A. Bastian and M. Zhang, J. Med. Chem., 2000, 43, 649 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra26611h |
| This journal is © The Royal Society of Chemistry 2017 |