 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Diaminodiacid-based synthesis of macrocyclic peptides using 1,2,3-triazole bridges as disulfide bond mimetics†
Ye Guo‡
ab,
Chao Liu‡a,
Hui Songc,
Feng-Liang Wangb,
Yan Zoua,
Qiu-Ye Wu*a and
Hong-Gang Hu *a
*a
aDepartment of Organic Chemistry, College of Pharmacy, Second Military Medical University, Shanghai 200433, China. E-mail: hhu66@smmu.edu.com; wuqysmmu@hotmail.com
bDepartment of Pharmacy, Baotou Medical College, Baotou, Inner Mongolia 014060, China
cCollege of Pharmacy, Weifang Medical University, Weifang, Shandong 261053, China
First published on 12th January 2017
Abstract
A new approach for the efficient construction of 1,2,3-triazole bridges as disulfide surrogates in peptides, utilizing the diaminodiacid strategy was established. Two thanatin derivatives were prepared with 1,5- and 1,4-disubstituted 1,2,3-triazole diaminodiacids as building blocks. The antibacterial activity studies are also described here.
Disulfide bonds have been shown to play widespread and essential roles in the maintenance of conformational stabilities and biological activities of peptides and proteins. In addition to this, they are indispensable for their thermal stability and their maintenance of protein integrity. As a result, disulfide bonds are called “the switches for protein function” due to their important status.1 However, disulfide bonds are prone to degradation in the physiological environment, mainly through reduction, polymerization or enzymatic cleavage, resulting in structural distortion and loss of activity.1c,1g,2
In recent years, great efforts have been made to replace disulfide bonds by synthetic disulfide surrogates, with improved redox stability and structural rigidity, in order to enhance their biological activity and pharmacokinetic properties in vivo. A number of disulfide-bond replacement strategies resulting in great metabolic stability have been employed, including thioether,3 olefin4 and other bridges.5 Nevertheless, tedious synthetic procedures and proper orthogonal protection is inevitable for cystathionine formation, and the ring-closing metathesis (RCM) usually leads to cis/trans isomers during dicarba bridging.3,4 On the other hand, additional purification or hydrogenation steps are usually required for the construction of an alkane-based structure with a defined configuration.5a
Benefiting from its chemical orthogonality and its stability against isomerases and proteases, the triazole-based method has attracted much attention in the past few years.6 It has already been extensively investigated and applied in peptide chemistry. For instance, the 1,4-disubstituted 1,2,3-triazole was used to properly mimic and rigidify the conformation of the amide backbone.7 Various macrocyclizations of peptides were successfully achieved through copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC), both in solution and on solid supports.8 Furthermore, the triazole motif has also been widely used as a linking group in carbohydrate chemistry and vaccine preparation.9 Therefore, the great chemical and biological compatibility of triazole renders it a wise choice for disulfide-bond replacement. In 2011, Meldal and co-workers described that 1,4-disubstituted 1,2,3-triazoles could be a proper surrogate of disulfide bridges of tachyplesin I, a naturally occurring peptide antibiotic.2b Soon afterwards, Kolmar et al. explored the utility of a 1,5-disubstituted 1,2,3-triazole group as a disulfide replacement in a monocyclic variant of the sunflower trypsin inhibitor-I (SFTI-1[1,14]) and found that “1,5” species could retain the biological activity more effectively compared with “1,4” variants.10
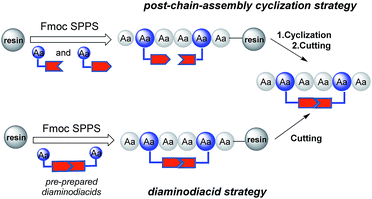
However, all of the peptide mimics in the abovementioned reports were synthesized via the post-chain assembly 1,2,3-triazole cyclization strategy (Fig. 1), which caused the potential difficulties of making nonpeptidic bridges on peptides.11 First of all, the ruthenium-catalyzed azide–alkyne cycloaddition (RuAAC) reaction, which was used to generate the 1,5-disubstituted 1,2,3-triazole bridge, was found to be either incompatible to solution-phase macrocylization of unprotected peptides or inefficient during SPPS even under harsh conditions (2.1% yield via microwave irradiation).10 Moreover, the post-chain strategy may have regioselectivity problems when multiple 1,2,3-triazole units need to be introduced.2b
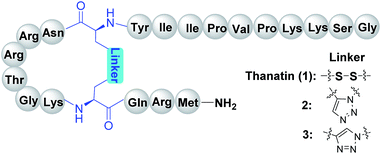
For these reasons, the construction of peptidomimetics containing single or multiple triazole bridges with both correct conformation and satisfactory output is still challenging. To overcome these shortcomings of the aforementioned synthetic strategy, we are interested in developing a novel strategy based on pre-prepared diaminodiacids for the synthesis of peptide mimics with 1,2,3-triazole as a disulfide surrogate (Fig. 1); this strategy has previously been successfully applied to prepare other peptide disulfide bond mimics.2a,11 Since every step is achieved via peptide coupling reactions, the difficulty in generating nonpeptidic structures on peptides can be avoided when using this strategy. To explore this new method, thanatin (GSKKPVPIIYCNRRTGKCQRM), a 21-residue macrocyclic antibiotic peptide, was selected as the model peptide.12 It has been reported that thanatin adopts a well-defined anti-parallel β-sheet structure stabilized by one cross-stand disulfide bond.13 In the present study, compounds 2 and 3 (Fig. 2) with 1,5- and 1,4-disubstituted 1,2,3-triazole bridges as disulfide replacements were designed and synthesized, respectively, and their anti-bacterial activities were evaluated to investigate the influence of different triazole motifs on thanatin.
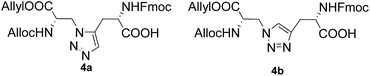
Previous studies have shown that diaminodiacids are useful for the synthesis of disulfide-bond surrogates and could be directly applied as building blocks just like mono amino acids.2a,11,14 To implement the synthesis of peptidomimetics containing two species of the 1,2,3-triazoles group, our efforts began by constructing diaminodiacids were then united into the peptide backbone directly via Fmoc/tBu SPPS (solid-phase peptide synthesis) to obtain the target thanatin derivatives. As shown in Fig. 3, the key diaminodiacids 4a and 4b were orthogonally protected with allyl/allyloxycarbonyl (Alloc) groups, which were compatible with the SPPS procedure and could be removed directly on resin using [Pd(PPh3)4]/PhSiH3.15
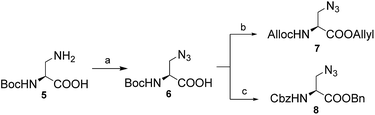
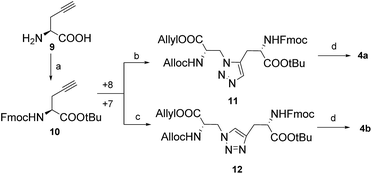
To synthesize the diaminodiacids 4a and 4b, as shown in Scheme 1, 3-amino-N-Boc-L-alanine (Boc-Dap-OH, 5) was selected as the starting material. First, 5 was transformed into 3-azido-N-Boc-L-alanine (6) via a Cu(II)-catalyzed diazo transfer reaction with a yield of 73.0%.16 Then, the tert-butoxycarbonyl (Boc) group of 6 was easily cleaved by trifluoroacetic acid, and the free amino acid was further protected with Alloc and allyl groups to afford 7 with a yield of 73.6%. Unfortunately, during the preparation of 4a, compound 7 was found to be incompatible with a ruthenium catalyst. Therefore, compound 8, a substitute with carbobenzyloxy (Cbz) and benzyl (Bn) groups, was generated for the 1,5-disubstituted 1,2,3-triazole building block with a yield of 62.0% in three steps.
Subsequently, installation of the tert-butyl (tBu) protective group, followed by capping of the Fmoc group on 2-propargyl-L-glycine (H-Pra-OH, 9) provided Fmoc-Pra-OtBu (10) with a 70.0% yield in two steps. On one hand, 10 was “clicked” through the Cp*RuCl(COD) reaction with 8, followed by replacement of Cbz and Bn by Alloc and allyl groups to achieve 11 with a yield of 54.7% in three steps.17 On the other hand, 12 could be obtained through the CuAAC reaction between 10 and 7 directly, with an 84.1% yield.18 Finally, the diaminodiacid building block 4a or 4b was prepared from 11 or 12, respectively, by removing the tBu protection group using trifluoroacetic acid; both reactions proceeded with a good yield of up to 95.0% and 96.3%, respectively (Scheme 2).
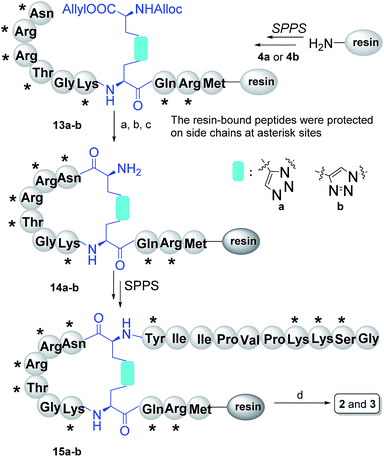
The synthesis of the peptidomimetics 2 and 3 was started from the Rink amide AM resin. The cyclization precursors, linear peptides 13a or 13b were assembled through standard Fmoc/tBu SPPS using HCTU (1-[bis(dimethylamino)methylene]-5-chlorobenzotriazolium 3-oxide hexafluorophosphate) as the coupling reagent.19 After the cleavage of the protective allyl and Alloc groups via [Pd(PPh3)4]/PhSiH3, macrocyclization was successfully accomplished with PyAOP ((7-azabenzotriazol-1-yloxy)tripyrroli-dino-phosphonium hexafluoro-phosphate), NMM (N-methylmorpholine) and HOAt (1-hydroxy-7-azabenzotriazole) to achieve the cyclic peptide 14a or 14b. The iterative process was continued by uniting the remaining amino acids to provide side chain protected peptides 15a or 15b on resin. Next, the one step global deprotection and cleavage under acidic conditions was successfully realized to obtain crude compounds 2 and 3 (Scheme 3). Finally, 2 and 3 were obtained following by further purification via reverse phase high-performance liquid chromatography, with a yield of 29.7% and 34.5%, respectively (according to initial resin load).
With thanatin and its analogues in hand, their antibacterial activities were measured via the MIC (minimal inhibitory concentration) test and summarized in Table 1. Two Gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus) and four Gram-negative bacteria (Escherichia coli, Salmonella typhimurum, Klebsiella pneumoniae and Pseudomonas aeruginosa) were grown in the presence of increasing concentrations of three peptides. It was found that the antibacterial activity of thanatin was stronger to the Gram negative bacteria than to the Gram positive bacteria, as previously reported.20 Neither of the peptidomimetics showed any anti-bacterial activity up to the peptide concentration of 20 μM against any of the bacterial strains except P. aeruginosa. Both of the 1,5- and 1,4-disubstituted patterns displayed an inhibitory potency at a concentration of 20 μM for P. aeruginosa with a reduction (50.0%) compared to thanatin. These results may suggest that a non-rigid and flexible linker is indispensable for antibiotic activity in thanatin. It is also important to note that the impact of structurally constrained disulfide replacements, such as 1,2,3-triazole bridges, may be dramatically detrimental for its activities in some cases.
| Peptides | Gram negative bacteria | Gram positive bacteria | ||||
|---|---|---|---|---|---|---|
| E. coli | S. typhimurum | K. pneumoniae | P. aeruginosa | B. subtilis | S. aureus | |
| a MIC values were measured by standard two-fold dilution protocol and reported in μM. | ||||||
| Thanatin | 5.0 | 10.0 | >20.0 | 10.0 | >20.0 | >20.0 |
| 2 | >20.0 | >20.0 | >20.0 | 20.0 | >20.0 | >20.0 |
| 3 | >20.0 | >20.0 | >20.0 | 20.0 | >20.0 | >20.0 |
To summarize, it was the first time that a diaminodiacid-based strategy was used to introduce 1,5- and 1,4-disubstituted 1,2,3-triazole bridges as disulfide mimics into peptides through SPPS to overcome the difficulties that often occur in post-chain assembly cyclization strategies. Thanatin, a well-studied antibiotic peptide containing a single disulfide bridge, was chosen as our model. With pre-made 1,5- and 1,4-disubstituted 1,2,3-triazole diaminodiacids as disulfide bond surrogates, we showed that these nonpeptidic units could be readily assembled into thanatin at desired sites with a 10-fold increased yield compared with the previously reported method, particularly for 1,5-disubstituted 1,2,3-triazole substitution.1e The antibacterial test measured via MIC with five bacterial strains indicated that the 1,2,3-triazole derivatives dramatically lost their antibiotic activities. One possible explanation may be that 1,5- and 1,4-disubstituted 1,2,3-triazole bridges, with their rigidity and constrained conformation, may change the bridge length compared with the original backbone. As a consequence, the anti-parallel β-sheet structure may be distorted, which is significant to its antibiotic activities. To further investigate this problem, experiments towards studying other 1,2,3-triazole bridges containing peptidomimetics, whose pharmaceutical activities are mainly based on β-hairpin rather than β-sheet structure, are currently under way. Moreover, the influence of polarity and charge change induced by 1,2,3-triazole bridges should also be investigated.
More significantly, although there was only one pair of 1,2,3-triazole bridges introduced into the peptide backbone in the present study, employing properly designed diaminodiacid building blocks with other SPPS compatible protection groups (such as p-nitrobenzyloxy carbonyl/p-nitrobenzyl, pNZ/pNB),21 tailor-made peptidomimetics containing multiple 1,2,3-triazole bridges can also be achieved through this synthetic approach with good regioselectivity. Profiting from this strategy, the fabrication of peptide mimics with prominent biological activities and pharmaceutical properties may be possible in the future.
Acknowledgements
We are grateful to the National Natural Science Foundation of China (No. 21402235, No. 21602121) and Instrumental Analysis Center of our university for NMR spectroscopic and mass spectrometric analyses.Notes and references
- (a) J. Gehrmann, P. F. Alewood and D. J. Craik, J. Mol. Biol., 1998, 278, 401–415 CrossRef CAS PubMed; (b) J. M. Thornton, J. Mol. Biol., 1981, 151, 261–287 CrossRef CAS PubMed; (c) D. L. Rabenstein and K. H. Weaver, J. Org. Chem., 1996, 61, 7391–7397 CrossRef CAS PubMed; (d) D. J. Craik, J. S. Mylne and N. L. Daly, Cell. Mol. Life Sci., 2010, 67, 9–16 CrossRef CAS PubMed; (e) H. M. Berman, J. Westbrook, Z. Feng, G. Gilliland, T. N. Bhat, H. Weissig, I. N. Shindyalov and P. E. Bourne, Nucleic Acids Res., 2000, 28, 235–242 CrossRef CAS PubMed; (f) P. J. Hogg, Trends Biochem. Sci., 2003, 28, 210–214 CrossRef CAS PubMed; (g) H. F. Gilbert, Methods Enzymol., 1995, 251, 8–28 CAS; (h) R. Wetzel, Trends Biochem. Sci., 1987, 12, 478–482 CrossRef CAS; (i) B. K. W. Chung and A. K. Yudin, Org. Biomol. Chem., 2015, 13, 8768–8779 RSC.
- (a) E. C. Gleeson, Z. J. Wang, S. D. Robinson, S. Chhabra, C. A. Macraild, W. R. Jackson, R. S. Norton and A. J. Robinson, Chem. Commun., 2016, 52, 4446–4449 RSC; (b) K. H. Nell and M. Meldal, Angew. Chem., 2011, 123, 5310–5312 CrossRef; (c) J. Gehrmann, P. F. Alewood and D. J. Craik, J. Mol. Biol., 1998, 278, 401–415 CrossRef CAS PubMed; (d) D. L. Rabenstein and K. H. Weaver, J. Org. Chem., 1996, 61, 7391–7397 CrossRef CAS PubMed; (e) M. C. A. Laboissiere, S. L. Sturley and R. T. Raines, J. Biol. Chem., 1995, 270, 28006–28009 CrossRef CAS PubMed.
- (a) Z. Dekan, I. Vetter, N. L. Daly, D. J. Craik, R. J. Lewis and P. F. Alewood, J. Am. Chem. Soc., 2011, 133, 15866–15869 CrossRef CAS PubMed; (b) J. Bondebjerg, M. Grunnet, T. Jespersen and M. Meldal, ChemBioChem, 2003, 4, 186–194 CrossRef CAS PubMed; (c) A. K. Galande, K. S. Bramlett, T. P. Burris, J. L. Wittliff and A. F. Spatola, J. Pept. Res., 2004, 63, 297–302 CrossRef CAS PubMed; (d) P. J. Knerr, A. Tzekou, D. Ricklin, H. C. Qu, H. Chen, W. A. Donk and J. D. Lambris, ACS Chem. Biol., 2011, 6, 753–760 CrossRef CAS PubMed.
- (a) R. F. Nutt, R. G. Strachan, D. F. Veber and F. W. Holly, J. Org. Chem., 1980, 45, 3078–3080 CrossRef CAS; (b) J. Elaridi, J. Patel, W. R. Jackson and A. J. Robinson, J. Org. Chem., 2006, 71, 7538–7545 CrossRef CAS PubMed; (c) D. J. Derksen, J. L. Stymiest and J. C. Vederas, J. Am. Chem. Soc., 2006, 128, 14252–14253 CrossRef CAS PubMed; (d) C. A. MacRaild, J. Illesinghe, B. J. Lierop, A. L. Townsend, M. Chebib, B. G. Livett, A. J. Robinson and R. S. Norton, J. Med. Chem., 2009, 52, 755–762 CrossRef CAS PubMed; (e) A. J. Robinson, B. J. Lierop, R. D. Garland, E. Teoh, J. Elaridi, J. P. Illesinghe and W. R. Jackson, Chem. Commun., 2009, 28, 4293–4295 RSC; (f) M. A. Hossain, L. M. Haugaard-Kedstrom, K. J. Rosengren, R. A. D. Bathgate and J. D. Wade, Org. Biomol. Chem., 2015, 13, 10895–10903 RSC.
- (a) A. K. Galande, J. O. Trent and A. F. Spatola, Biopolymers, 2003, 71, 534–551 CrossRef CAS PubMed; (b) M. A. Hossain, K. J. Rosengren, S. Zhang, R. A. Bathgate, G. W. Tregear, B. J. Van Lierop, A. J. Robinson and J. D. Wade, Org. Biomol. Chem., 2009, 7, 1547–1553 RSC; (c) T. Luhmann, S. K. Mong, M. D. Simon, L. Meinel and B. L. Pentelute, Org. Biomol. Chem., 2016, 14, 3345–3349 RSC; (d) C. M. B. K. Kourra and N. Cramer, Chem. Sci., 2016, 7, 7007–7012 RSC.
- C. W. Tornoe, S. J. Ssanderson, J. C. Mottram, G. H. Coombs and M. Meldal, J. Comb. Chem., 2004, 6, 312–324 CrossRef CAS PubMed.
- (a) Y. L. Angell and K. Burgess, Chem. Soc. Rev., 2007, 36, 1674–1689 RSC; (b) A. Brik, J. Alexandratos, Y. C. Lin, J. H. Elder, A. J. Olson, A. Wlodawer, D. S. Goodsell and C. H. Wong, ChemBioChem, 2005, 6, 1167–1169 CrossRef CAS PubMed; (c) K. Oh and Z. Guan, Chem. Commun., 2006, 29, 3069–3071 RSC; (d) G. M. Williams, K. Lee, X. Li, G. J. S. Copper and M. A. Brimble, Org. Biomol. Chem., 2015, 13, 4059–4063 RSC.
- (a) Y. Angell and K. Burgess, J. Org. Chem., 2005, 70, 9595–9598 CrossRef CAS PubMed; (b) V. D. Bock, R. Perciaccante, T. P. Jansen, H. Hiemstra and J. H. van Maarseveen, Org. Lett., 2006, 8, 919–922 CrossRef CAS PubMed; (c) R. A. Turner, A. G. Oliver and R. S. Lokey, Org. Lett., 2007, 9, 5011–5014 CrossRef CAS PubMed; (d) V. Goncalves, B. Gautier, A. Regazzetti, P. Coric, S. Bouaziz, C. Garbay, M. Vidal and N. Inguimbert, Bioorg. Med. Chem. Lett., 2007, 17, 5590–5594 CrossRef CAS PubMed; (e) V. D. Bock, D. Speijer, H. Hiemstra and J. H. van Maarseveen, Org. Biomol. Chem., 2007, 5, 971–975 RSC; (f) S. K. Agrawal, P. Panini, M. Sathe, D. Choprab and M. P. Kaushik, RSC Adv., 2014, 4, 10728–10730 RSC.
- (a) F. Pessel, I. Billault and M. C. Scherrmann, Green Chem., 2016, 18, 5558–5568 RSC; (b) M. Skwarczynski and I. Toth, Chem. Sci., 2016, 7, 842–854 RSC.
- M. Empting, O. Avrutina, R. Meusinger, S. Fabritz, M. Reinwarth, M. Biesalski, S. Voigt, G. Buntkowsky and H. Kolmar, Angew. Chem., Int. Ed., 2011, 50, 5207–5211 CrossRef CAS PubMed.
- H. K. Cui, Y. Guo, Y. He, F. L. Wang, H. N. Chang, Y. J. Wang, F. M. Wu, C. L. Tian and L. Liu, Angew. Chem., Int. Ed., 2013, 52, 9558–9562 CrossRef CAS PubMed.
- P. Fehlbaum, P. Bullet, S. Chernysh, J. P. Braind, J. P. Roussel, L. Letellier, C. Hetru and J. A. Hoffmann, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 1221–1225 CrossRef CAS.
- N. Mandard, P. Sodano, H. Labbe, J. M. Bonmatin, P. Bulet, C. Hetru, M. Ptak and F. Vovelle, Eur. J. Biochem., 1998, 256, 404–410 CAS.
- (a) F. L. Wang, Y. Guo, S. J. Li, Q. X. Guo, J. Shi and Y. M. Li, Org. Biomol. Chem., 2015, 13, 6286–6290 RSC; (b) Y. Guo, D. M. Sun, F. L. Wang, Y. He, L. Liu and C. L. Tian, Angew. Chem., Int. Ed., 2015, 54, 14276–14281 CrossRef CAS PubMed.
- Y. C. Huang, Y. M. Li, Y. Chen, M. Pan, Y. T. Li, L. Yu, Q. X. Guo and L. Liu, Angew. Chem., Int. Ed., 2013, 52, 4858–4862 CrossRef CAS PubMed.
- P. B. Alper, S. C. Hung and C. H. Wong, Tetrahedron Lett., 1996, 37, 6029–6032 CrossRef CAS.
- D. Tietze, M. Tischler, S. Voigt, D. Imhof, O. Ohlenschläger, M. Görlach and G. Buntkowsky, Chem.–Eur. J., 2010, 16, 7572–7578 CrossRef CAS PubMed.
- X. C. Chen, C. W. Lu, Y. M. Huang and D. V. McGrath, Tetrahedron, 2015, 71, 9154–9160 CrossRef CAS.
- (a) M. Pan, S. Gao, Y. Zheng, X. Tan, H. Lan, X. Tan, D. Sun, L. Lu, T. Wang, Q. Zheng, Y. Huang, J. Wang and L. Liu, J. Am. Chem. Soc., 2016, 138, 7429–7435 CrossRef CAS PubMed; (b) S. Tang, Y. Y. Si, Z. P. Wang, K. R. Mei, X. Chen, J. Y. Cheng, J. S. Zheng and L. Liu, Angew. Chem., Int. Ed., 2015, 54, 5713–5717 CrossRef CAS PubMed; (c) J. S. Zheng, S. Tang, Y. K. Qi, Z. P. Wang and L. Liu, Nat. Protoc., 2013, 8, 2483–2495 CrossRef CAS PubMed.
- M. K. Lee, L. Cha, S. H. Lee and K. S. Hahm, J. Biochem. Mol. Biol., 2002, 35, 291–296 CAS.
- A. I. Llobet, J. G. Camell, M. Álvarez and F. Albericio, Eur. J. Org. Chem., 2005, 14, 3031–3039 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra26617g |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: