Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An organocatalytic method for the synthesis of some novel xanthene derivatives by the intramolecular Friedel–Crafts reaction†
Tülay Yıldız * and
Hatice Başpinar Küçük
* and
Hatice Başpinar Küçük
Istanbul University, Faculty of Engineering, Department of Chemistry, 34320, Istanbul, Turkey. E-mail: tulayyil@istanbul.edu.tr
First published on 15th March 2017
Abstract
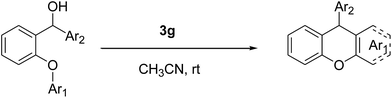
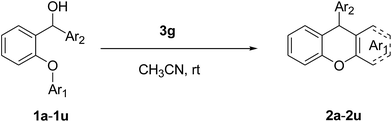
An efficient organocatalytic method for the synthesis of new substituted 9-arylxanthenes (2a–2u) starting from diarylcarbinol compounds with an arenoxy group (1a–1u) has been developed using the intramolecular Friedel–Crafts reaction. The substrates were prepared in two steps by Ullmann-type coupling and then Grignard reaction. Some organic Brønsted acids were studied as catalysts (3a–3g) in the intramolecular Friedel–Crafts alkylation reaction for the first time. N-triflylphosphoramide (3g) provided the synthesis of some novel substituted 9-arylxanthenes with excellent yields at room temperature within 15 minutes.
Introduction
Xanthenes and their derivatives have biological characteristics; they are used against inflammatory diseases and to treat illnesses caused by viruses and bacteria.1 Thus, xanthenes are pharmaceutically active and used in photodynamic therapy.2 They are used also as fluorescent materials or dyes and molecular switches, which occur in natural products.3 The structure of xanthenes being straight and strict is advantageous; they are used as linkers in peptide syntheses and in the synthesis of unnatural amino acids.4 The HIV-1 nucleocapsid protein has around 2000 small molecules (the NCI Diversity Set), in which 26 of them are active inhibitors and five of them were reported to have a xanthenyl ring.5Many synthetic routes to obtain xanthene derivatives have been reported.6 The most important examples are the iron-catalysed cascade benzylation–cyclisation of phenols,7 Pd-catalyzed cyclisation of polycyclic aryltriflate esters,8 Lewis acid-catalysed cyclisation of salicylaldehydes and cyclohexenones or tetralones,9 and the condensation of 2-naphthol and numerous aryl aldehydes.10 The synthesis of substituted 9-arylxanthenes using the tandem arylation/Friedel–Crafts reaction of O-hydroxy bisbenzylic alcohols with diaryliodonium salts was recently reported by Wang et al.11 Another study concerning the synthesis of substituted 9-arylxanthenes reported an Sc(OTf)3-catalyzed domino reaction.12 Additionally some nitrogen or sulphur-containing xanthene derivatives, which may be used especially as antitumor agents, were synthesized in previous works.13
We have determined that the most suitable method to obtain 9-arylxanthenes is the intramolecular Friedel–Crafts alkylation (FCA) of proper alcohols because the literature on arylation using an FCA protocol has recently started to see new developments. In particular, arylation that does not use any metals has became a very popular topic for avoiding the disadvantages of organometallic chemistry, which poses high cost, uses toxic materials, and poses difficulties during purification.14 These new methods have opened new doors using mild reaction conditions and employing cheap and less toxic catalysts.15
In this field, the development of new π-activated alcohols has increasing importance over the development of organohalides, which are more toxic and require harsh conditions.16 Thus, Lautens et al. reported an efficient synthesis of the targeted cis-hexahydrobenzophenanthridines from tetralins, which are very easy to obtain. The reaction included π-activated alcohols produced using intramolecular FCA in the presence of Lewis acids.17 An important work which realised cyclization of π-activated alcohols by Bi(OTf)3-catalyzed intramolecular FCA was reported by Rueping et al.18 To synthesise substituted 9-arylxanthenes, another Lewis acid-catalysed intramolecular FCA procedure was carried out by Panda and co-workers in 2009.19 In this study, they used well-known FCA catalysts and some Lewis acids like conc. H2SO4, anhyd. AlCl3, FeCl3, Sc(OTf)3 and TfOH. Among the catalysts studied, FeCl3 gave the best result with 91% yield in dry CH2Cl2 at room temperature.
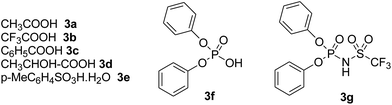
According to the literature, although there are reports of hydroarylation reactions of activated alkenes using FCA with Brønsted acids such as diphenyl hydrogen phosphate as the catalysts,20 there are no reports of intramolecular FCA using organic Brønsted acids as the catalysts instead of Lewis acids. Considering the recent reports, non-chiral, organic Brønsted “super-acids” are comparable or even better than Lewis or inorganic Brønsted acids.21 Furthermore, the organic Brønsted acids are similar to Lewis acids as they are usually stable against oxidizing ambience or humidity, and they can be easily handled and are stable over a long period of time.22 Recently, in order to create new types of super acids or super acid catalysts, new Brønsted acids including ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NSO2CF3 or
NSO2CF3 or ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NSO2F groups were synthesized because these groups increase the acidity substantially.23 It is understood that triflate groups in particular, among all acidic compounds, increase the acidity significantly. Due to N-triflate groups having lower pKa values than normal organic acids,24 the more acidic N-triflate groups can catalyze the reactions with generally better yields and in less time. As an example of an organic Brønsted acid, N-triflylphosphoramide (3g) (Fig. 1) was used in some previous reports. A study on this subject includes the preparation of 1,3-dioxolane-4-ones starting from α-hydroxy acids and aldehydes with the use of 3g.25 Another example reports a transformation, which has merged the Ugi reaction and Houben–Hoesch cyclisation in the presence of 3g.26 In that report, after several Brønsted and Lewis acids were examined, they found that the acid-promoted reaction is effective in the synthesis of many 3-aminoindole molecules using 3g with high yields and under mild conditions.
NSO2F groups were synthesized because these groups increase the acidity substantially.23 It is understood that triflate groups in particular, among all acidic compounds, increase the acidity significantly. Due to N-triflate groups having lower pKa values than normal organic acids,24 the more acidic N-triflate groups can catalyze the reactions with generally better yields and in less time. As an example of an organic Brønsted acid, N-triflylphosphoramide (3g) (Fig. 1) was used in some previous reports. A study on this subject includes the preparation of 1,3-dioxolane-4-ones starting from α-hydroxy acids and aldehydes with the use of 3g.25 Another example reports a transformation, which has merged the Ugi reaction and Houben–Hoesch cyclisation in the presence of 3g.26 In that report, after several Brønsted and Lewis acids were examined, they found that the acid-promoted reaction is effective in the synthesis of many 3-aminoindole molecules using 3g with high yields and under mild conditions.
In this study, to develop an effective and economic organocatalytic FCA protocol to synthesize some novel substituted arylxanthenes under mild conditions, we explored some organic Brønsted acids as catalysts (3a–3g) in the intramolecular FCA reaction for the first time.
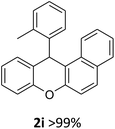
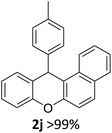
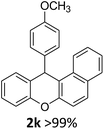
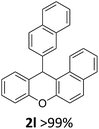
We obtained arylxanthenes with excellent yields using N-triflylphosphoramide (3g). Among all synthesised substituted arylxanthenes, seventeen of the compounds are novel, and the others, 2i,27 2j,27 and 2k,19 have been synthesised previously with different methods.
Results and discussion
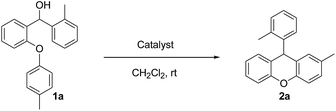
Our starting materials were synthesised in two steps involving metal-free Ullmann coupling28 and then Grignard reaction. Finally, we obtained diarylcarbinol compounds including an arenoxy group as the starting material with very high yields (95–100%). All starting compounds (diarylcarbinols) are novel except 1k.19We have chosen carbinol 1a in order to examine the catalysts and carry out initial optimisation experiments of the intramolecular FCA. Therefore, we explored the different organic Brønsted acids as catalysts for the intramolecular FCA reaction (Table 1). The catalysts we used were acetic acid (3a), trifluoroacetic acid (3b), benzoic acid (3c), L-lactic acid (3d), p-toluenesulfonic acid (3e), diphenyl hydrogen phosphate (3f), and N-triflylphosphoramide (3g). Firstly, the solution of 1a and acid catalyst (3a–3g) in dichloromethane was stirred at 25 °C until the starting material was consumed. In this way, the different Brønsted acids were compared against each other for the intramolecular FCA reaction.
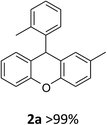
Moderate yields were obtained using 3b and 3e catalysts (Table 1, entries 3 and 6). 3a and 3f gave very low yields (Table 1, entries 2 and 7) despite the long reaction times. Benzoic acid (3c) and L-lactic acid (3d) did not react at all (Table 1, entries 4 and 5). As expected, no conversion was observed in the absence of the catalyst (Table 1, entry 1). Interestingly, the best result was achieved when 10 mol% of 3g was used to obtain the desired product 2a in 78% yield within 2 hours (Table 1, entry 8).
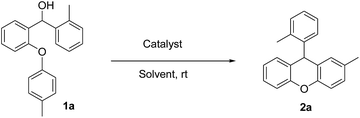
In the second phase, we examined the effect of the solvent on the intramolecular FCA reaction 1a using the best catalyst 3g (Table 2). Performing the reaction in several solvents gave interesting results. The intramolecular FCA reaction of 1a with CH2Cl2 and CHCl3 showed good yields with side products. While THF, benzene and toluene gave very low yields, CH3CN provided the best result with 99% yield and no side reaction when using 10 mol% of 3g (Table 2, entry 6). Also we observed a lower yield when using 5 mol% of the catalyst (Table 2, entry 7). Additionally, the other best catalysts 3b and 3e were also tested in CH3CN. However, lower yields of 85 and 87% were obtained in these reactions (Table 2, entries 8 and 9).
| Entry | Catalyst | Solvent | Cat. amount (mol%) | Time | Yieldb (%) |
|---|---|---|---|---|---|
| a Condition: 1a (0.1 mmol) and catalyst (10% mmol) in solvent (2.5 mL) were stirred at room temperature.b Yield of isolated product. | |||||
| 1 | 3g | CH2Cl2 | 10 | 2 h | 78 |
| 2 | 3g | Benzene | 10 | 2 h | 13 |
| 3 | 3g | Toluene | 10 | 2 h | 17 |
| 4 | 3g | CHCl3 | 10 | 2 h | 91 |
| 5 | 3g | THF | 10 | 2 h | 11 |
| 6 | 3g | CH3CN | 10 | 15 min | >99 |
| 7 | 3g | CH3CN | 5 | 15 min | 85 |
| 8 | 3b | CH3CN | 10 | 15 min | 85 |
| 9 | 3e | CH3CN | 10 | 15 min | 87 |
In parallel to our work, it was shown in previous studies that CH3CN is a good discriminating solvent for strong acids, because of its weak basicity and weak solvating power to anions.23 Therefore we found that performing the cyclisation by intramolecular FCA with 10 mol% 3g in CH3CN at room temperature for 15 min showed excellent results for obtaining substituted 9-arylxanthene derivatives. After these optimisation reactions, we looked at the differences when we used different aromatic hydroxy compounds (Ar1) in the Ullmann coupling reaction. We used different substituent aryl groups including Me, Br, Cl, F, CN, NO2 etc. (Ar1) during this reaction step and synthesized diaryl compounds that have these substituents on the arenoxy group in order to investigate the effect of the different functional groups on the intramolecular FCA. The other aryl group p-methoxy phenyl (Ar2) was chosen for use in all substrates for these experiments. The effect of the functional groups on Ar1 screening reactions are summarized in Table 3.
| Entry | Product | Ar1 | Ar2 | Yieldb (%) |
|---|---|---|---|---|
| a Condition: carbinol (0.1 mmol) and 3g in CH3CN (2.5 mL) were stirred at room temperature.b Yield of isolated product. | ||||
| 1 | 2e | 2-MeC6H4 | 4-MeOC6H4 | 96 |
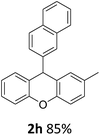
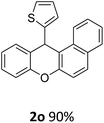
| 2 | 2k | 2-Np | 4-MeOC6H4 | >99 |
| 3 | 2p | 3-BrC6H4 | 4-MeOC6H4 | 98 |
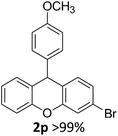
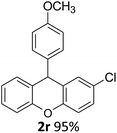
| 4 | 2r | 4-ClC6H4 | 4-MeOC6H4 | 95 |
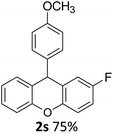
| 5 | 2s | 4-FC6H4 | 4-MeOC6H4 | 75 |
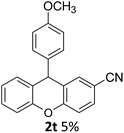
| 6 | 2t | 4-CNC6H4 | 4-MeOC6H4 | 5 |
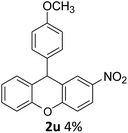
| 7 | 2u | 4-NO2C6H4 | 4-MeOC6H4 | 4 |
As can be seen in Table 3, electron donating groups like methyl and naphthyl groups (entries 1 and 2) increase the rate of the electrophilic substitution. Therefore, their yields are excellent during intramolecular FCA. When we look at halogen containing electron withdrawing groups, although they are weakly deactivating, their yields are very good, especially for the 3-Br substituent (Table 3, entry 3). As expected, the other electron withdrawing groups nitro and cyano gave very low yields (Table 3, entries 6 and 7), because of their strongly deactivating effect in the electrophilic aromatic substitution reactions.
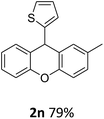
Following this, various arylmagnesium bromides (Ar2) were used in the Grignard reaction step. When Ar1 was kept as an Me group with different positions and Ar2 (or R) was changed with different groups and positions (Table 4), we observed that CH3 and CH3O groups showed almost the same yield with 99–95% (entries 1–5). In addition, the position of the Me group on the Ar1 did not significantly affect the reaction. The naphthyl and thienyl groups resulted in slightly lower yields of 88 and 79% (Table 4, entries 6 and 8). When a long chain alkyl group was used instead of Ar2, the yield was only 73% (Table 4, entry 7). Consequently, the Ar1 group is more effective than the Ar2 group in this reaction (FCA) because the Ar1 group is part of the mechanism of the reaction.
| Entry | Product | Ar1 | Ar2 (or R) | Yieldb (%) |
|---|---|---|---|---|
| a Condition: carbinol (0.1 mmol) and 3g (10% mmol) in CH3CN (2.5 mL) were stirred at room temperature.b Yield of isolated product. | ||||
| 1 | 2a | 4-MeC6H4 | 2-MeC6H4 | >99 |
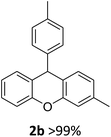
| 2 | 2b | 3-MeC6H4 | 4-MeC6H4 | >99 |
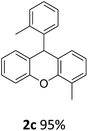
| 3 | 2c | 2-MeC6H4 | 2-MeC6H4 | 95 |
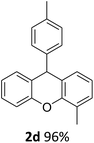
| 4 | 2d | 2-MeC6H4 | 4-MeC6H4 | 96 |
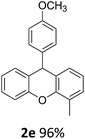
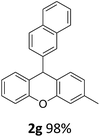
| 5 | 2e | 2-MeC6H4 | 4-MeOC6H4 | 96 |
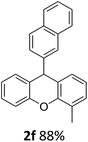
| 6 | 2f | 2-MeC6H4 | 2-Np | 88 |
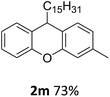
| 7 | 2m | 3-MeC6H4 | C15H31 | 73 |
| 8 | 2n | 4-MeC6H4 | 2-Thienyl | 79 |
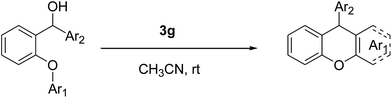
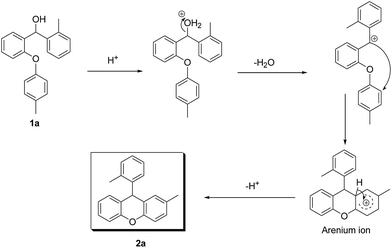
The well known SN1 type intramolecular Friedel–Crafts alkylation mechanism can be shown for our compounds (Scheme 1).
In the proposed mechanism, after protonation of the starting material 1a, a water molecule leaves and the carbocation anion is formed. Following this, the carbocation anion attacks the other aryl functional group and the arenium ion is produced. Finally, a proton is lost from the arenium ion and the cyclisation is completed to give the desired product 2a. The new ring formed is very stable because it is six membered.
In addition, although the mechanism that we have proposed is an SN1 type Friedel–Crafts mechanism, an ortho-quinonemethide (o-QM) intermediate may also be composed in the presence of the acidic media. Recently, Rueping et al. studied this mechanism with asymmetric Brønsted acids.29 In these works, a possible transition-state model was proposed in order to describe the mechanism of some Brønsted acid catalysed reactions via multiple hydrogen-bond interplay between the chiral catalyst and substrate.
Experimental
General information
The majority of the chemicals used in this work were commercially available from Merck or Aldrich. CH3CN was purchased from Merck with catalog number 114291. It has 99.8% purity and was not additionally dried. The starting carbinols 1a–1u were prepared by Ullmann coupling of 2-fluorobenzaldehyde and substituted phenols, and then by the Grignard reaction of 2-arenoxybenzaldehydes and some arylmagnesium bromides. All substrates were purified by crystallization or column chromatography and characterized by IR, 1H-NMR, and 13C-NMR spectroscopy, elemental analysis and GC-MS. All novel products were characterized by IR, 1H-NMR, and 13C-NMR spectroscopy, elemental analysis and GC-MS. The reactions were monitored by TLC using silica gel plates and the products were purified by flash column chromatography on silica gel (Merck; 230–400 mesh), eluting with hexane–ethyl acetate (v/v 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The NMR spectra were recorded at 500 MHz for 1H NMR and 125 MHz for 13C NMR using Me4Si as the internal standard in CDCl3. The GC-MS were recorded on a Shimadzu/QP2010 Plus. The IR spectra were recorded on a Mattson 1000 spectrometer. The melting points were determined with a Büchi Melting Point B-540.
1). The NMR spectra were recorded at 500 MHz for 1H NMR and 125 MHz for 13C NMR using Me4Si as the internal standard in CDCl3. The GC-MS were recorded on a Shimadzu/QP2010 Plus. The IR spectra were recorded on a Mattson 1000 spectrometer. The melting points were determined with a Büchi Melting Point B-540.
General procedure for intramolecular Friedel–Crafts cyclization
Under room temperature conditions, N-triflylphosphoramide (3g) (10 mol%) was added to a stirred solution of a starting alcohol compound (1a–1m) (0.1 mmol) in CH3CN (2.5 mL), and the reaction was stirred for 15 min. After the completion of the reaction, as observed with TLC, the mixture was concentrated in a vacuum and extracted with ethylacetate. After the usual reaction workup and concentration, the product was charged on silica gel.Conclusions
In conclusion, an efficient synthetic method was developed for the synthesis of new substituted 9-arylxanthenes via intramolecular Friedel–Crafts cyclisation, using non-transition metal organocatalysis, and starting from diarylcarbinol compounds. In this work, we looked for an optimisation of the reaction conditions to yield highly efficient 9-arylxanthene synthesis. Thus, we examined some organic Brønsted-acids as catalysts and determined the best catalyst for intramolecular FCA. We found that N-triflylphosphoramide (3g) as a catalyst is highly effective, inexpensive and non-toxic for the rapid synthesis of xanthenes in CH3CN at room temperature, when compared to previous intramolecular FCA reactions and the synthesis of 9-arylxanthenes.Finally, we determined a new organocatalytic FCA protocol, which does not require inert reaction conditions or any protection, to synthesise some novel substituted arylxanthenes using 3g with excellent yields. The twenty newly synthesised substituted 9-arylxanthenes 2a–2u (Table 5) may be used as natural and bio-active compounds, dyes or fluorescent materials, and their biochemical properties and activities will be investigated in the future.
Acknowledgements
This work was supported by Istanbul University, Scientific Research Projects, project numbers BYP 41636 and BYP 53627.Notes and references
- J. P. Poupelin, G. Saint-Ruf, O. Foussard-Blanpin, G. Narcisse, G. Uchida-Ernouf and R. Lacroix, Eur. J. Med. Chem., 1978, 13, 67 CAS; R. W. Lambert, J. A. Martin, J. H. Merrett, K. E. B. Parkes and G. J. Thomas, Int. Appl., WO 9706178, 1997.
- A. Banerjee and A. K. Mukherjee, Stain Technol., 1981, 56, 83 CrossRef CAS PubMed.
- M. S. T. Gonçalves, Chem. Rev., 2008, 109, 190 CrossRef CAS PubMed; B. L. Feringa, J. Org. Chem., 2007, 72, 6635 CrossRef PubMed.
- N. P. Buu-Hoi, G. Saint-Ruf, A. De and H. T. Hieu, Bull. Chim. Ther., 1972, 7, 83 CAS.
- A. G. Stephen, K. M. Worthy, E. Towler, J. A. Mikovits, S. Sei, P. Roberts, Q. Yang, R. K. Akee, P. Klausmeyer, T. G. McCloud, L. Henderson, A. Rein, D. G. Covell, M. Currens, R. H. Shoemaker and R. J. Fisher, Biochem. Biophys. Res. Commun., 2002, 296, 1228 CrossRef CAS PubMed.
- H. Li, J. Yang, Y. Liu and Y. Li, J. Org. Chem., 2009, 74, 6797 CrossRef CAS PubMed; K. Okuma, K. Nojima, N. Matsunaga and K. Shioji, Org. Lett., 2009, 11, 169 CrossRef PubMed; J. Zhao and R. C. Larock, J. Org. Chem., 2007, 72, 583 CrossRef PubMed; J. Zhao and R. C. Larock, Org. Lett., 2005, 7, 4273 CrossRef PubMed.
- X. Xu, X. Xu, H. Li, X. Xie and Y. Li, Org. Lett., 2010, 12, 100 CrossRef CAS PubMed.
- J.-Q. Wang and R. G. Harvey, Tetrahedron, 2002, 58, 5927 CrossRef CAS.
- E. Böβ, T. Hillringhaus, J. Nitsch and M. Klussmann, Org. Biomol. Chem., 2011, 9, 1744 Search PubMed.
- K. R. M. Naidu, B. S. Krishna, M. A. Kumar, P. Arulselvan, S. I. Khalivulla and O. Lasekan, Molecules, 2012, 17, 7543 CrossRef CAS PubMed; G. M. Ziarani, A.-R. Badiei and M. Azizi, Sci. Iran., Trans. C, 2011, 18, 453 CrossRef.
- S. Mao, Z. Hua, X. Wu, Y. Yang, J. Han and L. Wang, Chemistry Select, 2016, 3, 403 Search PubMed.
- R. Singh and G. Panda, Org. Biomol. Chem., 2010, 8, 1097 CAS.
- R. A. Al-Qawasmeh, Y. Lee, M.-Y. Cao, X. Gu, S. Viau, J. Lightfoot, J. A. Wright and A. H. Young, Bioorg. Med. Chem. Lett., 2009, 19, 104 CrossRef CAS PubMed; S. Ohno, H. Shimizu, T. Kataoka and M. Hori, Chem. Pharm. Bull., 1984, 32, 3471 CrossRef.
- E. Shirakawa, K. Itoh, T. Higashino and T. Hayashi, J. Am. Chem. Soc., 2010, 132, 15537 CrossRef CAS PubMed; S. Yanagisawa, K. Ueda, T. Taniguchi and K. Itami, Org. Lett., 2008, 10, 4673 CrossRef PubMed.
- X. Zhang, W. Rao and S. Wai Hong Chan, Org. Biomol. Chem., 2009, 7, 4186 Search PubMed; X. Deng, J. T. Liang, J. Liu, H. McAllister, C. Schubert and N. S. Mani, Org. Process Res. Dev., 2007, 11, 1043 CrossRef CAS; A. S. K. Hashmi, L. Schwarz, P. Rubenbauer and M. C. Blanco, Adv. Synth. Catal., 2006, 348, 705 CrossRef.
- E. Emer, R. Sinisi, M. G. Capdevila, D. Petruzziello, F. De Vincentis and P. Cozzi, Eur. J. Org. Chem., 2011, 4, 647 CrossRef CAS; M. Bandini and M. Tragni, Org. Biomol. Chem., 2009, 7, 1501 Search PubMed; J. Muzart, Tetrahedron, 2008, 64, 5815 CrossRef.
- C. Liebert, M. K. Brinks, A. G. Capacci, M. J. Fleming and M. Lautens, Org. Lett., 2011, 13, 3000 CrossRef CAS PubMed.
- M. Rueping, B. J. Nachtsheim and W. Ieawsuwan, Adv. Synth. Catal., 2006, 348, 1033 CrossRef CAS.
- S. K. Das, R. Singh and G. Panda, Eur. J. Org. Chem., 2009, 28, 4757 CrossRef.
- I. Fleischer and J. Pospech, RSC Adv., 2015, 5, 493 RSC.
- M. Rueping, B. J. Nachtsheim, W. Ieawsuwan and I. Atodiresei, Angew. Chem., Int. Ed., 2011, 50, 6706 CrossRef CAS PubMed; K. Kaupmees, N. Tolstoluzhsky, S. Raja, M. Rueping and I. Leito, Angew. Chem., Int. Ed., 2013, 52, 11569 CrossRef PubMed; M. Rueping, B. J. Nachtsheim, S. A. Moreth and M. Bolte, Angew. Chem., Int. Ed., 2008, 47, 593 CrossRef PubMed.
- T. Akiyama, Chem. Rev., 2007, 107, 5744 CrossRef CAS PubMed.
- L. M. Yagupolskii, V. N. Petrik, N. V. Kondratenko, L. Sooväli, I. Kaljurand, I. Leito and I. A. Koppel, J. Chem. Soc., Perkin Trans. 2, 2002, 2, 1950 RSC.
- M. Rueping, B. J. Nachtshe, R. M. Koenigs and W. Ieawsuwan, Chem.–Eur. J., 2010, 16, 13116 CrossRef CAS PubMed.
- H. B. Küçük, Tetrahedron Lett., 2015, 56, 5583 CrossRef.
- J. S. Schneekloth Jr, J. Kim and E. J. Sorensen, Tetrahedron, 2009, 65, 3096 CrossRef PubMed.
- R. Dada, G. Singh, A. Pareek, S. Kausar and S. Yaragorla, Tetrahedron Lett., 2016, 57, 3739 CrossRef CAS.
- G. W. Yeager and D. N. Schissel, Synthesis, 1995, 1, 28 CrossRef.
- C.-C. Hsiao, S. Raja, H.-H. Liao, I. Atodiresei and M. Rueping, Angew. Chem., Int. Ed., 2015, 54, 576 CrossRef CAS PubMed; C.-C. Hsiao, H.-H. Liao and M. Rueping, Angew. Chem., Int. Ed., 2014, 53, 13258 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra27094h |
| This journal is © The Royal Society of Chemistry 2017 |