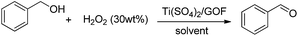Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and characterization of titanium(IV)/graphene oxide foam: a sustainable catalyst for the oxidation of benzyl alcohol to benzaldehyde†
Wenxi Ma,
Qiaoling Tong,
Jian Wang,
Huali Yang,
Meng Zhang,
Hailun Jiang,
Qinghe Wang*,
Yongxiang Liu* and
Maosheng Cheng*
Key Laboratory of Structure-Based Drug Design and Discovery (Shenyang Pharmaceutical University), Ministry of Education, Shenyang 110016, P. R. China. E-mail: qinghe-wang@163.com; yongxiang.liu@syphu.edu.cn; mscheng@syphu.edu.cn
First published on 20th January 2017
Abstract
A sustainable catalyst for the selective oxidation of benzyl alcohol (BnOH) to benzaldehyde (BzH) was developed by mineralizing Ti(SO4)2 on graphene oxide foam (GOF) surface. The Ti(SO4)2/GOF was characterized by scanning electron microscopy (SEM), energy disperse spectroscopy (EDS), X-ray diffraction (XRD), solid-state NMR (SSNMR), thermal gravimetric analysis (TGA), infrared (IR) and BET analysis. Recycling experiments proved that Ti(SO4)2/GOF possessed excellent reusability and durability. The industrial perspective of Ti(SO4)2/GOF was demonstrated by the preparation of BzH in a large-scale and solvent-free oxidation process.
The market demand of BzH as a valuable precursor for the production of pharmaceuticals, herbicides and spices, is huge every year, especially in developing countries. The oxidation of BnOH is one of major methods to obtain BzH. Traditionally, oxidation of BnOH to BzH was performed with stoichiometric amounts of chromium(VI) reagents, which were harmful to the environment due to the generation of copious amounts of heavy-metal and chlorine-containing wastes. In recent years, a lot of novel methods have been developed, such as photocatalysis,1 crystalline noble-metal oxide catalysis,2 electrocatalysis,3 metal-free method4 and noble metal nanocomposite catalysis.5 In addition, some green chemistry strategies such as oxidation processed in ionic liquids6 and under microwave irradiated conditions have also been developed.7 However, the methods mentioned above have several problems including low conversion or selectivity1a,d,8 complex synthesis processes of the catalysts,2,3 demanding a cocatalyst,4 unrecyclable metals,1c the high cost of noble metals5c,d,8 and serious environmental problems caused by heavy-metal wastes.
Graphene oxide (GO), was obtained by the oxidation of graphite and subsequent exfoliation of the carbon sheets by ultrasonic irradiation in water after dried in vacuo.9 Alternative freeze-drying treatment process would generate spongy graphene oxide foam (GOF).9 Various oxygen-containing groups may generate on GO or GOF surface and edge during oxidation process.4 Many astonishing chemical properties of GO are resulted from the functional groups, which can reduce the activation energy and increase the selectivity of reactions,5a,10 supply different chemical decoration sites,11 and also be reduced to reduced GO (rGO) leading to the reconstruction of sp2 hybridized carbon atoms.11c Many efforts have been made on the GO-based oxidation of BnOH into BzH, which include rGO–metal composites-catalyzed oxidation,5b photocatalysis oxidation based on GO skeleton,1a,d and GO-involved electrocatalysis oxidation.12 More sustainable and robust oxidation process is still urgent in the further development of GO-based methodologies.
Titanium sulfate [Ti(SO4)2] is a cheap water soluble inorganic titanium with low toxicity and abundant natural reserves. It has been widely used in the preparation of titanium dioxide an important semiconductor and photosensitive material.13 Many studies were focused on the use of Ti(SO4)2 as a precursor to prepare nano TiO2 and the exploration of its photocatalytic properties. However, there were fewer studies on the catalytic properties for its inherent instability to moisture and the insolubility in most organic solvents. To explore the inherent catalytic properties of Ti(SO4)2, we planned to mineralize Ti(SO4)2 on the surface of GOF to improve its ability in catalyzing reactions. We assumed that the use of GOF as a carrier to immobilize Ti(SO4)2 through the carboxyl groups on GOF edge could prevent metal ions to hydrolyze and could make the Ti(SO4)2 well dispersed on its matrix to ensure supplying more catalytic sites and recovering from reaction system. Moreover, the highly dispersed Ti4+ will contribute to adsorb BnOH on its surface by the interaction between hydroxyl group and Ti4+. On the other hand, various oxygen-containing groups on GO surface can interact with BnOH and H2O2 through intermolecular hydrogen bonds, which promote the oxidation of BnOH on the Ti(SO4)2 surface. Finally, we succeeded in mineralizing Ti(SO4)2 on the GOF surface, characterizing the new GOF–Ti(IV) complex by various spectroscopy and developing an environmentally friendly and sustainable method to oxidize BnOH to BzH. It is worthy to mention that the solvent and catalyst are recyclable and water is the only by-product for the process.
Our research began by the preparation of GO using modified Offeman's protocol.14 As-synthesized graphite oxide was dispersed in the aqueous solution of Ti(SO4)2 and put under the ultrasonic irradiation for 1 hour followed by freeze drying leading to Ti(SO4)2/GOF. Scanning electron microscope (SEM) and energy disperse spectroscopy (EDS) patterns demonstrated that Ti(SO4)2 was distributed on the surface of GOF with μM-size. Two types of crystal morphology were observed in the material, in which loosen structure was anchored on the edge and center depressed area and ‘branch-like’ structure was paved on GOF sheets (Fig. 1).
 | ||
| Fig. 1 The morphology of Ti(SO4)2/GOF. (a) SEM spectroscopy of fresh Ti(SO4)2/GOF; (b) EDS; (c) magnification of selected area in the left picture. | ||
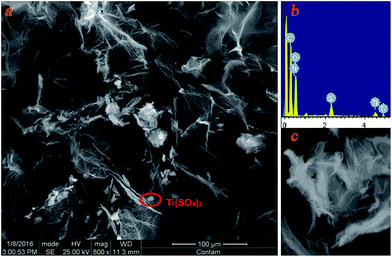
X-ray diffraction (XRD) pattern showed that the Ti(SO4)2 mineralized on GOF surface was amorphous crystal (Fig. 2). Diffraction peaks at 10° to 30° are amorphous peaks. What's more, no significant change was observed when it was reused 10 times for the oxidation, which will be introduced in details in the following applications. From the curves 1–3 in Fig. 2, it can be seen that pure amorphous Ti(SO4)2 showed only one peak, but the peak position shifted a little and split into two peaks in fresh Ti(SO4)2/GOF. We conjectured that this was caused by the interaction between Ti(SO4)2 and the different oxygen-containing groups on the surface of GOF during mineralization process.
 | ||
| Fig. 2 Powder XRD patterns. Curve 1: amorphous Ti(SO4)2. Curve 2: fresh Ti(SO4)2/GOF. Curve 3: recycled 10 times Ti(SO4)2/GOF. | ||
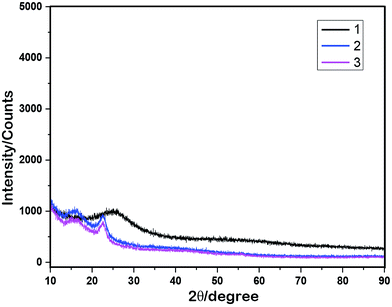
To prove the interaction between Ti(SO4)2 and GOF, the IR studies were performed. As indicated in Fig. 3, the absorption peak of ether/epoxide (C–O–C, green band) vanished in Ti(SO4)2/GOF, meanwhile, a new peak appeared in the fingerprint region (∼600 cm−1). This result directly proved the presence of titanium-oxygen coordination.15 Intriguingly, the absorption peak of phenolic hydroxyl (C–OH, 2000–3600 cm−1, blue bond) did not show any change at all. This phenomenon indicates that the Ti(SO4)2 prefers to interact with ether/epoxide, rather than hydroxyl. The ether/epoxide belongs to the electrophilic oxygen species on carbon matrix.10b Spin-trapping electron paramagnetic resonance (EPR) proved that the radicals generated from zigzag-edge could activate oxygen,16 meanwhile, the carbon skeleton exhibited the nature of ‘Frustrated Lewis Pairs (FLP)’.9 Moreover, the radicals can be delocalized through carbon matrix as ‘passageway’.4,16 These evidences indicate that the ether/epoxide can be activated by radicals. Therefore, we speculated that the radicals could immigrate from carbon skeleton to ether/epoxide, which activated the unshared pair electrons of oxygen to interact with titanium. Thus, the ether/epoxide seems likely ‘oxygen-containing solid carbene’.
 | ||
| Fig. 3 The IR spectra of GOF (black), fresh Ti(SO4)2/GOF (blue) and recycled Ti(SO4)2/GOF (pink) with KBr tablet. | ||
To gain an insight into the interaction between Ti(SO4)2 and GOF, the 13C-SSNMR measurement was performed (Fig. 4). Unlike IR spectrum, the 13C-SSNMR spectra revealed a series of significant decrease at 61.5 ppm (C–O–C), 71.0 ppm (C–OH) and 125.6 ppm (graphitic sp2-C) in freshly prepared Ti(SO4)2/GOF. In TiO2 crystal, Ti4+ defective sites will lead to generate ferromagnetism at room temperature.17 We inferred that this was caused by Ti4+ defective sites in Ti(SO4)2 crystal leading to ferromagnetism which depressed 13C-SSNMR signals. However, it was observed that the signals of C–O–C, graphitic sp2-C and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O were recovered again when the catalyst was reused for 10 times. SEM spectroscopy has shown that the Ti(SO4)2 will exfoliate off from the carbon skeleton after recycled 10 times, which will increase the 13C-SSNMR signals (see ESI†). Moreover, the intensity of O
C–O were recovered again when the catalyst was reused for 10 times. SEM spectroscopy has shown that the Ti(SO4)2 will exfoliate off from the carbon skeleton after recycled 10 times, which will increase the 13C-SSNMR signals (see ESI†). Moreover, the intensity of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O increased apparently, which may be caused by the formation of esters and anhydrides of the carboxyl groups on carbon sheets. In addition, the IR spectra indicated that the absorption peaks of COOH and C–OH became broader after 10 times reuses. Meanwhile, the BET analysis showed obvious decrease of the surface area in the catalyst recycled 10 times (Table 1). These results showed that the interlayer spacing among carbon sheets decreased apparently in the recycled catalyst. Besides, the thermal stabilities of fresh and reused Ti(SO4)2/GOF were affected by the amounts of esters and anhydrides.
C–O increased apparently, which may be caused by the formation of esters and anhydrides of the carboxyl groups on carbon sheets. In addition, the IR spectra indicated that the absorption peaks of COOH and C–OH became broader after 10 times reuses. Meanwhile, the BET analysis showed obvious decrease of the surface area in the catalyst recycled 10 times (Table 1). These results showed that the interlayer spacing among carbon sheets decreased apparently in the recycled catalyst. Besides, the thermal stabilities of fresh and reused Ti(SO4)2/GOF were affected by the amounts of esters and anhydrides.
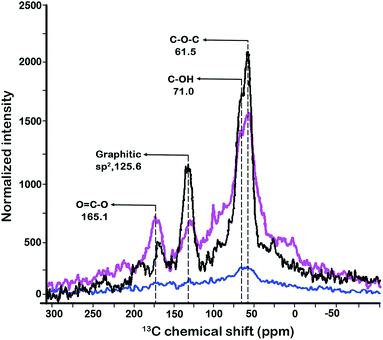 | ||
| Fig. 4 13C-SSNMR spectra of GOF (black), fresh Ti(SO4)2/GOF (blue) and recycled Ti(SO4)2/GOF (pink). (Direct 13C onepulse, ro = 4.0 kHz.) | ||
| Catalyst | Correlation coefficient | Surface area |
|---|---|---|
| Fresh | 0.9996 | 119.8 m2 g−1 |
| Recycled | 0.9995 | 19.8 m2 g−1 |
The thermal stability of the fresh and recycled Ti(SO4)2/GOF was studied on the basis of simple TGA experiments. Fig. 5 displayed the TGA thermograms of GOF, fresh and recycled for 10 times Ti(SO4)2/GOF for the oxidation of BnOH in nitrogen atmosphere. We found that Ti(SO4)2/GOF displayed different thermal stability below and beyond 200 °C. Our experiments showed that the thermal decomposition of GOF was fastest at 200 °C, which was consistent with previous report on the thermal stability of GO.18 The result indicated that the GOF and GO had the same thermal stability although they showed different physical forms. The rate of weight loss was different in fresh and recycled Ti(SO4)2/GOF. The rate of weight loss in fresh Ti(SO4)2/GOF was fastest at 150 °C, while that in reused Ti(SO4)2/GOF was fastest at 200 °C correspondingly. The carboxyl group would decompose from 150 °C to 200 °C and the esters and anhydrides would begin to decompose when the temperature was beyond 200 °C.18 Therefore, the dehydration of carboxyl and hydroxyl groups will occur along with the increase of use times.
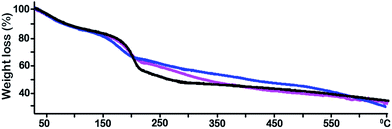 | ||
| Fig. 5 Thermal gravimetry of GOF (black), fresh Ti(SO4)2/GOF (blue) and recycled 10 times Ti(SO4)2/GOF (pink). | ||
Next, the obtained catalyst was examined in the oxidation of BnOH to BzH. The screening of reaction conditions was summarized in Table 2. Blank experiment showed that the conversion of BnOH was only 6.1% in the absence of catalyst (Table 2, entry 1). However, the conversion of BnOH rose to 70.2% and the selectivity to BzH was increased to 99% when Ti(SO4)2/GOF was used (Table 2, entry 2). Extension of reaction time had little influence on the conversion of BnOH (Table 2, entry 3). We failed to improve the conversion after tried different methods such as increase of the amount of H2O2 and Ti(SO4)2/GOF, addition of radical initiator, adjustion of the pH value and slow addition of H2O2 (Table 2, entries 4–8). Examination of solvent effect proved that THF was the optimal solvent, which afforded 88.9% conversion with 99% selectivity to BzH (Table 2, entries 9–11). It is important to notice that MeOH, the solvent we screened in the condition can't be oxidized in this mild condition. Precedent studies showed that the oxidation of MeOH was carried out at 300–400 °C using oxygen as the oxidant.19 We rationalized that THF as the optimal solvent can form intermolecular hydrogen bonds with H2O2, which prevent H2O2 to decompose by slow release of H2O2 into reaction system. Besides, aggregation of the catalyst was observed in acetonitrile and acetone. The influence of the amount of H2O2 was checked in THF and the best result was obtained when 3.0 eq. H2O2 was utilized (Table 2, entries 12–18). From the results we inferred that the rates of oxidation of BnOH and H2O2 decomposition were affected by the concentration of H2O2. Consequently, the conversion of BnOH is nonlinearly related with the amount of H2O2.
| Entrya | Reaction time (h) | Solvent | H2O2 | Con. (%) | Sel. (%) |
|---|---|---|---|---|---|
| a Reaction conditions: BnOH (720 mg, 6.66 mmol), Ti(SO4)2/GOF (400 mg), solvent (10 mL) and 30 wt% H2O2 (0.82 mL, 8.0 mmol) under reflux condition.b 2.0 eq. of H2O2 (30 wt%) was slowly dropped in 0.5 h.c 800 mg Ti(SO4)2/GOF was applied.d 0.1 mol% benzoyl peroxide was added into reaction system.e CH3COOH was added to adjust pH to 4. | |||||
| 1 | 1 | MeOH | 1.2 eq. | 6.1 | — |
| 2 | 1 | MeOH | 1.2 eq. | 70.2 | >99 |
| 3 | 3 | MeOH | 1.2 eq. | 74.3 | >99 |
| 4 | 3 | MeOH | 2.0 eq. | 74.8 | 98.8 |
| 5b | 3 | MeOH | 2.0 eq. | 74.8 | 98.6 |
| 6c | 3 | MeOH | 2.0 eq. | 76.7 | 98.6 |
| 7d | 3 | MeOH | 1.2 eq. | 75.1 | 97.6 |
| 8e | 3 | MeOH | 2.0 eq. | 75.9 | >99 |
| 9 | 3 | CH3CN | 1.2 eq. | 68.1 | >99 |
| 10 | 3 | THF | 1.2 eq. | 88.9 | >99 |
| 11 | 3 | CH3COCH3 | 1.2 eq. | 64.8 | — |
| 12 | 4 | THF | 1.0 eq. | 85.5 | >99 |
| 13 | 4 | THF | 2.0 eq. | 88.9 | >99 |
| 14 | 4 | THF | 3.0 eq. | 91.3 | 99.0 |
| 15 | 4 | THF | 3.5 eq. | 83.2 | 99.0 |
| 16 | 4 | THF | 4.0 eq. | 91.5 | 98.8 |
| 17 | 4 | THF | 5.0 eq. | 47.2 | — |
| 18 | 4 | THF | 6.0 eq. | 91.5 | 98.4 |
The reusability and durability of the catalyst are critical aspects to evaluate the value of industrial application in heterogeneous catalysis. We attempted to recycle Ti(SO4)2/GOF by filtration and use it in the oxidation of BnOH to BzH to examine the reusability and durability of the catalyst. To our delight, the result showed that the catalyst could be reused for more than ten times without significant loss of efficiency and selectivity (see Table S1†). In addition, the oxidation could also be performed under solvent-free condition in a large scale (see ESI†).
In conclusion, Ti(SO4)2/GOF showed unique catalytic activity and chemical selectivity for the oxidation of BnOH to BzH. The reusability and durability test of the catalyst proved that it possessed excellent chemical stability and could be recycled facilely, which demonstrated promising industrial respective. In addition, the preliminary studies on the interaction between Ti(SO4)2 and oxygen functional groups of GOF basal plane were performed, which contributed to understand the intrinsic characters of GOF deeply.
Notes and references
- (a) W. Song, A. K. Vannucci, B. H. Farnum, A. M. Lapides, M. K. Brennaman, B. Kalanyan, L. Alibabaei, J. J. Concepcion, M. D. Losego, G. N. Parsons and T. J. Meyer, J. Am. Chem. Soc., 2014, 136, 9773 CrossRef CAS PubMed; (b) W. Feng, G. Wu, L. Li and N. Guan, Green Chem., 2011, 13, 3265 RSC; (c) C. Meng, K. Yang, X. Fu and R. Yuan, ACS Catal., 2015, 5, 3760 CrossRef CAS; (d) J. Xu, L. Luo, G. Xiao, Z. Zhang, H. Lin, X. Wang and J. Long, ACS Catal., 2014, 4, 3302 CrossRef CAS; (e) M.-Q. Yang, N. Zhang and Y.-J. Xu, ACS Appl. Mater. Interfaces, 2013, 5, 1156 CrossRef CAS PubMed; (f) M. Alfè, D. Spasiano, V. Gargiulo, G. Vitiello, R. Di Capua and R. Marotta, Appl. Catal., A, 2014, 487, 91 CrossRef.
- K. Amakawa, Y. V. Kolen'ko, A. Villa, M. E. Schuster, L.-I. Csepei, G. Weinberg, S. Wrabetz, R. Naumann d'Alnoncourt, F. Girgsdies, L. Prati, R. Schlögl and A. Trunschke, ACS Catal., 2013, 3, 1103 CrossRef CAS.
- A. K. Vannucci, J. F. Hull, Z. Chen, R. A. Binstead, J. J. Concepcion and T. J. Meyer, J. Am. Chem. Soc., 2012, 134, 3972 CrossRef CAS PubMed.
- G. Lv, H. Wang, Y. Yang, T. Deng, C. Chen, Y. Zhu and X. Hou, ACS Catal., 2015, 5, 5636 CrossRef CAS.
- (a) L. Shao, X. Huang, D. Teschner and W. Zhang, ACS Catal., 2014, 4, 2369 CrossRef CAS; (b) J. Wang, S. A. Kondrat, Y. Wang, G. L. Brett, C. Giles, J. K. Bartley, L. Lu, Q. Liu, C. J. Kiely and G. J. Hutchings, ACS Catal., 2015, 5, 3575 CrossRef CAS; (c) V. R. Choudhary, A. Dhar, P. Jana, R. Jha and B. S. Uphade, Green Chem., 2005, 7, 768 RSC; (d) V. R. Choudhary, R. Jha and P. Jana, Green Chem., 2007, 9, 267 RSC.
- K. R. Seddon and A. Stark, Green Chem., 2002, 4, 119 RSC.
- (a) M. H. C. L. Dressen, J. E. Stumpel, B. H. P. van de Kruijs, J. Meuldijk, J. A. J. M. Vekemans and L. A. Hulshof, Green Chem., 2009, 11, 60 RSC; (b) H. Chen, Q. Tang, Y. Chen, Y. Yan, C. Zhou, Z. Guo, X. Jia and Y. Yang, Catal. Sci. Technol., 2013, 3, 328 RSC.
- Y. Hong, X. Jing, J. Huang, D. Sun, T. Odoom-Wubah, F. Yang, M. Du and Q. Li, ACS Sustainable Chem. Eng., 2014, 2, 1752 CrossRef CAS.
- S. Navalon, A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Chem. Rev., 2014, 114, 6179 CrossRef CAS PubMed.
- (a) B. Frank, J. Zhang, R. Blume, R. Schlögl and D. S. Su, Angew. Chem., Int. Ed., 2009, 48, 6913 CrossRef CAS PubMed; (b) B. Zhong, H. Liu, X. Gu and D. S. Su, ChemCatChem, 2014, 6, 1553 CrossRef CAS.
- (a) S. Eigler and A. Hirsch, Angew. Chem., Int. Ed., 2014, 53, 7720 CrossRef CAS PubMed; (b) V. Georgakilas, J. N. Tiwari, K. C. Kemp, J. A. Perman, A. B. Bourlinos, K. S. Kim and R. Zboril, Chem. Rev., 2016, 116, 5464 CrossRef CAS PubMed; (c) L. Liao, H. Peng and Z. Liu, J. Am. Chem. Soc., 2014, 136, 12194 CrossRef CAS PubMed.
- Y. Hao, X. Wang, Y. Zheng, J. Shen, J. Yuan, A.-j. Wang, L. Niu and S. Huang, Electrochim. Acta, 2016, 198, 127 CrossRef CAS.
- (a) J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Chem. Rev., 2014, 114, 9919 CrossRef CAS PubMed; (b) L. Wang and T. Sasaki, Chem. Rev., 2014, 114, 9455 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- O. A. Kholdeeva, T. A. Trubitsina, R. I. Maksimovskaya, A. V. Golovin, W. A. Neiwert, B. A. Kolesov, X. López and J. M. Poblet, Inorg. Chem., 2004, 43, 2284 CrossRef CAS PubMed.
- C. Su, M. Acik, K. Takai, J. Lu, S.-j. Hao, Y. Zheng, P. Wu, Q. Bao, T. Enoki, Y. J. Chabal and K. Ping Loh, Nat. Commun., 2012, 3, 1298 CrossRef PubMed.
- S. Wang, L. Pan, J.-J. Song, W. Mi, J.-J. Zou, L. Wang and X. Zhang, J. Am. Chem. Soc., 2015, 137, 2975 CrossRef CAS PubMed.
- J. H. Kang, T. Kim, J. Choi, J. Park, Y. S. Kim, M. S. Chang, H. Jung, K. T. Park, S. J. Yang and C. R. Park, Chem. Mater., 2016, 28, 756 CrossRef CAS.
- T. Waters, R. A. J. O'Hair and A. G. Wedd, J. Am. Chem. Soc., 2003, 125, 3384 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General procedures, large-scaled synthesis of BzH under solvent-free condition, recycling tests of Ti(SO4)2/GOF. See DOI: 10.1039/c6ra27690c |
| This journal is © The Royal Society of Chemistry 2017 |