Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A novel water soluble chemosensor based on carboxyl functionalized NDI derivatives for selective detection and facile removal of mercury(II)†
Qi Lin *a,
Peng-Peng Maoa,
Lu Liua,
Juan Liu*b,
You-Ming Zhanga,
Hong Yaoa and
Tai-Bao Wei
*a,
Peng-Peng Maoa,
Lu Liua,
Juan Liu*b,
You-Ming Zhanga,
Hong Yaoa and
Tai-Bao Wei *a
*a
aKey Laboratory of Eco-Environment-Related Polymer Materials, Ministry of Education of China, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu 730070, P. R. China. E-mail: linqi2004@126.com; weitaibao@126.com
bCollege of Chemical Engineering, Northwest University for Nationalities, Lanzhou, 730000, P. R. China. E-mail: liujuan656@126.com
First published on 13th February 2017
Abstract
Mercury(II) (Hg2+) has acute toxicity. It is still a challenge to design and synthesize chemosensors for selective detection and removal of Hg2+ in water solutions. By rationally combining the carboxyl group and naphthalene diimide moieties, we obtained a novel water-soluble Hg2+ chemosensor (M2). Interestingly, the sensor M2 showed a dramatic fluorescent “turn-on” response for Hg2+ in water. Moreover, the sensor M2 displayed a high specificity for Hg2+, other cations (including Fe3+, Ag+, Ca2+, Cu2+, Co2+, Ni2+, Cd2+, Pb2+, Zn2+, Cr3+, and Mg2+) had no influence on the Hg2+ detection process. Moreover, the sensor M2 showed high sensitivity for Hg2+, with detection limits of 1.18 × 10−6 M. Even more meaningfully, the sensor M2 can remove Hg2+ from water solutions effectively via the formation of a M2–Hg2+ coordination polymer, which can increase the possibility of M2 being used for practical applications.
Introduction
As we all know, ions play a significant role in life and technological processes.1–4 Moreover, heavy metal ions pose a big threat to human health and the environment due to their high toxicity and bioaccumulation.5,6 Among the heavy metals, mercury is one of the most toxic.7–11 However, large quantities of mercury salts are widely used in industrial chemicals, electricals, apparatus, dental amalgams, and batteries.12–15 Hence, the rational design and synthesis of efficient sensors to selectively detect and remove Hg2+ ions has attracted much attention.16–18 Moreover, due to most of the biological or environmental procedures being carried out in water systems, it is very important to develop a Hg2+ chemosensor, which can detect and remove Hg2+ in water.Thus far, some methods based on organic fluorophores19 or chromophores,20 semiconductor nanocrystalline materials,21 cyclic voltammetry,22 polymeric materials,23 and proteins24 have been established for the detection of Hg2+. However, most of these materials are difficult to be applied in the detection of mercury ions in water solutions due to their poor water solubility. Moreover, these chemosensors cannot remove Hg2+ in water simultaneously. Therefore, the search for effective sensing systems in a water environment is still a great challenge. At the same time, various absorbents, such as activated carbon,25 resins,26 silica,27 zeolites28 and metal sulfides29 have been applied to remove Hg2+ from wastewater; however, these absorbents cannot detect Hg2+ in water. The reports on simultaneous detection and removal of Hg2+ in water are still very scarce.30–33 Therefore, the development of water soluble Hg2+ chemosensors, which can synchronously detect and remove Hg2+ in water is an important task.
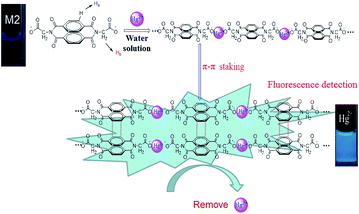
In view of this and based on our research in ion recognition,34,35 we report a water-soluble Hg2+ sensor M2 based on naphthalene diimide derivatives. In order to achieve fluorescent sensing and efficient removal of Hg2+ in water, we rationally introduced the carboxyl group into the sensor molecule as a hydrophilic group and Hg2+ binding site. Moreover, we introduced a naphthalene diimide moiety as the fluorescent signal group and π–π stacking site. As a result, the sensor M2 showed good solubility in water and could fluorescently “turn on” when it detected Hg2+ with high selectivity and sensitively in water solution. More interestingly, the sensor M2 could efficiently remove Hg2+ in water simultaneously.
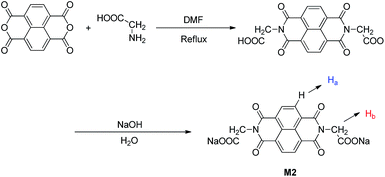
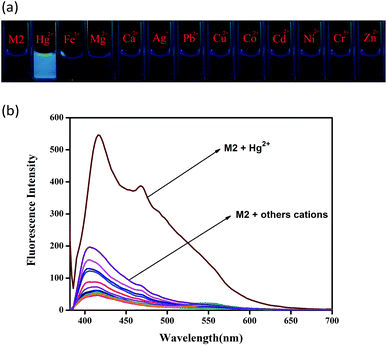
The sensor M2 was synthesized by a simple dehydration condensation procedure (Scheme 1) and was characterized by 1H NMR spectroscopy and ESI-MS (Fig. S1 and S2†). The recognition properties of the chemosensor M2 towards various metal cations, such as Fe3+, Hg2+, Ca2+, Cu2+, Co2+, Ni2+, Cd2+, Pb2+, Zn2+, Ag+, Cr3+, and Mg2+, were primarily investigated by fluorescence emission spectra in water solution (buffered with HEPES, pH = 7.2). The compound M2 alone displayed no fluorescence when it was excited at 365 nm (Fig. 1). However, when 10 equiv. of Hg2+ was added to the water solution of M2 (2.0 × 10−4 M), the solution emitted bright blue fluorescence emission. Simultaneously, in the fluorescence spectrum, an emission peak at 425 nm could be observed (Fig. S3†). To validate the selectivity of sensor M2 for Hg2+, the same tests were carried out for other cations (including Fe3+, Ca2+, Cu2+, Co2+, Ni2+, Cd2+, Pb2+, Zn2+, Cr3+, Ag+, and Mg2+); however, these cations could not induce any significant fluorescent changes in the fluorescence spectrum (Fig. 1). These results indicate that the sensor M2 can selectively fluorescently “turn-on” when sensing Hg2+ in water solution.
To further investigate the interaction between sensor M2 and Hg2+, the fluorescence emission spectral variation of sensor M2 (2.0 × 10−4 M) in water (buffered with HEPES, pH = 7.2) was recorded during titrations with different concentrations of Hg2+. As shown in Fig. 2, with the addition of increasing concentrations of Hg2+ from 0 to 3.26 equiv., the emission peak at 425 nm gradually enhanced. Furthermore, in order to determine the detection limits of M2 for Hg2+, the fluorescence spectra of blank tests were measured 20 times and the standard deviation of the blank measurements was determined (Fig. S4†). The fluorescence quantum yield (Φ) of sensor M2 in water is 0.03 with quinine hemisulfate salt as a reference while the Φ increased to 0.32 when sensor M2 reacts with Hg2+ (see ESI†). The linear fitting was performed according to the titration curves, and the mean intensity was calculated to determine the slope. The limit of detection (LOD) was determined from the equation LOD = K × σ/S,36 where K = 3, σ is the standard deviation of the emission intensity of M2 in the presence of Hg2+, and S is the slope of the calibration curve of the fluorescence emission. The detection limit of the fluorescence spectrum was 1.18 × 10−6 M. This data indicate that the sensor can detect Hg2+ at very low concentrations in the environment (Fig. S5†). Moreover, the binding constant (K) derived from the fluorescence titration data was found to be 2.65 × 109 M (see ESI†) using a Benesi–Hildebrand plot,37 which indicates a high detection sensitivity.
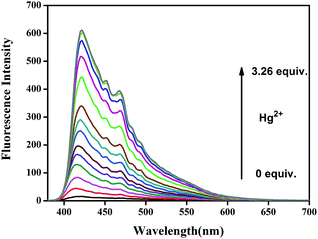 | ||
| Fig. 2 Fluorescence titration spectra of Hg2+ in water solution (buffered with HEPES, pH = 7.2) upon addition of increasing concentration of Hg2+. | ||
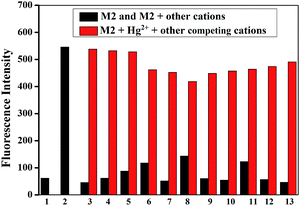
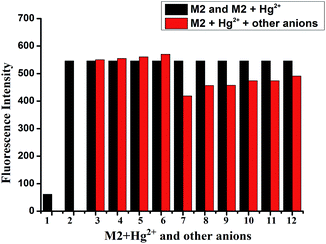
Then, the competition experiments were also measured by the addition of 10 equiv. of Hg2+ to the water solution of M2 in the presence of 10 equiv. of other metal ions, such as Fe3+, Ca2+, Cu2+, Co2+, Ni2+, Cd2+, Pb2+, Zn2+, Cr3+, Ag+ and Mg2+. As shown in Fig. 3, the effect on emission intensity of M2 upon the addition of higher concentrations of various cations was almost negligible. These results revealed that M2 had a remarkable selectivity towards Hg2+ over competitive ions, and moreover, the detection of Hg2+ by M2 was hardly affected by these common coexisting cations in water (buffered with HEPES, pH = 7.2). Moreover, in order to investigate the influence of coexisting anions on the Hg2+ sensing process, the competition experiments were also carried out by adding various anions into the M2–Hg2+ water solution (Fig. 4). As a result, coexisting anion ions, such as F−, Cl−, Br−, I−, Ac−, H2PO4−, HSO4−, HClO4−, SCN− and CN−, could not interfere in the Hg2+ sensing process of M2.
As we all know, the pH has a strong influence on the coordination properties of ligands and metal ions. Therefore, the effects of pH on the Hg2+ sensing process were also investigated (Fig. 5). Over the tested pH range, sensor M2 alone had no fluorescence and was stable in the pH range 4.0–13.0. However, the M2–Hg2+ complex showed a significant fluorescence response between pH 4.0 and 8.0. These results indicate that Hg2+ could be clearly detected by the fluorescence spectral measurement using M2 over a pH range from 4.0 to 8.0. In acidic conditions, carboxylate groups in M2 changed to carboxylic acids, whereas, in alkaline conditions, the Hg2+ could form Hg(OH)2. In either case, the coordination abilities of M2 and Hg2+ were restrained. Moreover, in strongly acidic (pH < 1) or strongly alkaline conditions (pH > 13), the sensor M2 showed unusual fluorescence, which indicated the M2 is unstable in these conditions.
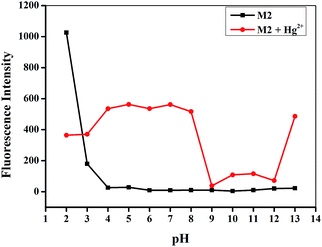 | ||
| Fig. 5 Effect of pH on the fluorescence spectra of M2 (20 μM) in response to Hg2+ (10 equiv.) from pH 2.0 to 13.0 in water solution. | ||
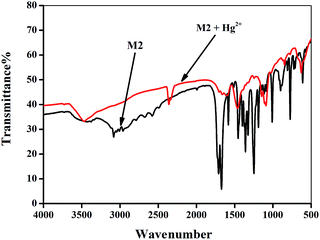
The recognition mechanism of the sensor M2 with Hg2+ was primarily investigated by IR spectroscopy. In the IR spectrum of M2 (Fig. 6), the carboxyl groups show the stretching vibrations absorption peak at 1715 cm−1. However, after the addition of Hg2+, this peak shifts to 1663 cm−1, which indicates that M2 bonds to Hg2+ via carboxyl groups. In addition, the mass spectroscopy results also supported this presumption. The ion peak at m/z 582.60 demonstrated the presence of [M2 + Hg2+] (Fig. S7†).
The recognition mechanism of the sensor M2 with Hg2+ was also investigated by 1H NMR titration. As shown in Fig. 7, sensor M2 shows two single peaks at 8.55 and 4.58 ppm in D2O solution, which correspond to the protons of the naphthalene rings (Ha) and –CH2 (Hb), respectively. With the addition of Hg2+, the signal of –CH2 (Hb) showed a slight upfield shift, indicating that M2 combined with Hg2+ via the carboxyl group. Moreover, the signal of the naphthalene rings (Ha) also showed a slight upfield shift, indicating that the π–π stacking interactions between the naphthalene rings were involved in the detection process.38 Moreover, the XRD patterns (Fig. S6†) of the M2–Hg2+ complex showed a peak at 2θ = 25.67° corresponding to d spacings of 3.53 Å, which also suggested that π–π stacking existed in the naphthyl groups.39 Interestingly, after adding 2.0 equiv. Hg2+, all the 1H NMR signal disappeared. According to these 1H NMR, XRD and IR experiments, we presumed that M2 formed a coordination polymer with Hg2+ and, simultaneously, each coordination polymer chain stacked together through the π–π interactions (Scheme 2).
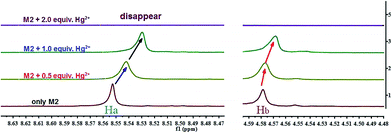 | ||
| Fig. 7 Partial 1H NMR spectra (600 MHz, D-H2O) of free M2 (0.1 M) and in the different concentration of Hg2+ respectively. | ||
Then, we further measured the capacity of the removal of Hg2+ by sensor M2 via inductively coupled plasma (ICP) analysis in aqueous solution (see ESI†). The ICP analysis identified an efficient removal of Hg2+. In order to assess the ingestion capacity of the sensor for Hg2+ in water for potential practical applications, M2 (0.19 mg) was suspended in a dilute aqueous solution of Hg(ClO4)2 (0.20 mg in 5.0 mL), and precipitation was found to occur. We separated the precipitate by centrifugation (20 min) and gained the supernatant liquor (5 mL). The ICP analysis verified that the concentration of the residual Hg2+ in water was less than 0.2 ppm, indicating that over 98% of Hg2+ could be effectively removed. This data indicated that Hg2+ could be effectively removed even in extremely dilute solutions.
In summary, a novel water-soluble Hg2+ sensor M2 was designed and synthesized via an easy to make method. The sensor M2 employs carboxyl groups as hydrophilic groups and Hg2+ binding sites, while NDI moieties act as signal groups and π–π stacking sites. Interestingly, the sensor M2 could fluorescently “turn-on” when it detected Hg2+ in water with high selectivity and sensitivity. Moreover and more meaningfully, the sensor M2 can remove Hg2+ from a water solution effectively via the formation of a M2–Hg2+ coordination polymer. Therefore, the sensor M2 can be used as an easy to make and efficient sensor for fluorescence detection as well as for the removal of Hg2+ in water.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (NSFC) (No. 21662031; 21661028; 21574104; 21262032), the Natural Science Foundation of Gansu Province (1506RJZA273) and the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (IRT 15R56).Notes and references
- C. Caltagirone and P. A. Gale, Chem. Soc. Rev., 2009, 38, 520 RSC.
- K. P. Carter, A. M. Young and A. E. Palmer, Chem. Rev., 2014, 114, 4564 CrossRef CAS PubMed.
- S. V. Krivovichev, O. Mentré, O. I. Siidra, M. Colmont and S. K. Filatov, Chem. Rev., 2013, 113, 6459 CrossRef CAS PubMed.
- N. Busschaert, C. Caltagirone, W. V. Rossom and P. A. Gale, Chem. Rev., 2015, 115, 8038 CrossRef CAS PubMed.
- C. T. Driscoll, R. P. Mason, H. M. Chan, D. J. Jacob and N. Pirrone, Environ. Sci. Technol., 2013, 47, 4967 CrossRef CAS PubMed.
- R. Zare-Dorabei, R. Rahimi, A. Koohi and S. Zargari, RSC Adv., 2015, 5, 93310 RSC.
- (a) L. Campbell, D. G. Dixon and R. E. Hecky, J. Toxicol. Environ. Health, Part B, 2003, 6, 325 CrossRef CAS PubMed; (b) D. G. Streets, M. K. Devane, Z. Lu, T. C. Bond, E. M. Sunderland and D. J. Jacob, Environ. Sci. Technol., 2011, 45, 10485 CrossRef CAS PubMed; (c) C. Jiang, Z. Guan, S. Y. Rachel Lim, L. Polavarapu and Q. H. Xu, Nanoscale, 2011, 3, 3316 RSC; (d) Y. Chen, L. Wu, Y. Chen, N. Bi, X. Zheng, H. Qi and M. Qin, Microchim. Acta, 2012, 177, 341 CrossRef CAS.
- (a) W. Ren, C. Zhu and E. Wang, Nanoscale, 2012, 4, 5902 RSC; (b) E. O. Ganbold, J. H. Park, K. S. Ock and S. W. Joo, Bull. Korean Chem. Soc., 2011, 32, 519 CrossRef CAS; (c) P. Li, H. Liu, L. Yang and J. Liu, Talanta, 2013, 106, 381 CrossRef CAS PubMed.
- J. Duan, M. Yang, Y. Lai, J. Yuan and J. Zhan, Anal. Chim. Acta, 2012, 723, 88 CrossRef CAS PubMed.
- H. H. Harris, I. J. Pickering and G. N. George, Science, 2003, 301 Search PubMed.
- P. B. Tchounwou, W. K. Ayensu, N. Ninashvili and D. Sutton, Environ. Toxicol., 2003, 18, 149 CrossRef CAS PubMed.
- A. Curley, V. A. Sedlak, E. F. Girling, R. E. Hawk, W. F. Barthel and P. E. Pierce, et al., Science, 1971, 2, 65 Search PubMed.
- I. Onyido, A. R. Norris and E. Buncel, Chem. Rev., 2004, 104, 5911 CrossRef CAS PubMed.
- I. Samb, J. Bell, P. Y. Toullec, V. Michelet and I. Leray, Org. Lett., 2011, 13, 1182 CrossRef CAS PubMed.
- X. Ma, J. Wang, Q. L. Shan, Z. W. Tan, G. H. Wei, D. B. Wei and Y. G. A. Du, Org. Lett., 2012, 14, 820 CrossRef CAS PubMed.
- (a) Z. S. Qian, X. Y. Shan, L. J. Chai, J. J. Ma, J. R. Chen and H. Feng, ACS Appl. Mater. Interfaces, 2014, 6, 6797 CrossRef CAS PubMed; (b) P. F. Wei, D. B. Li, B. B. Shi, Q. Wang and F. Huang, Chem. Commun., 2015, 51, 15169 RSC; (c) C. H. Yao, Y. H. Cao, Q. Wang, Y. Pan, J. L. Jiang and L. Y. Wang, Chem. Commun., 2016, 52, 8715 RSC; (d) C. Jin, M. Zhang, L. Wu, Y. F. Guan, Y. Pan, J. L. Jiang, C. Lin and L. Y. Wang, Chem. Commun., 2013, 49, 2015 Search PubMed.
- (a) M. Xue, Y. Yang, X. Chi, Z. Zhang and F. Huang, Acc. Chem. Res., 2012, 45, 1294 CrossRef CAS PubMed; (b) L. Y. Wang, L. H. Zhu and D. Cao, New J. Chem., 2015, 39, 7211 RSC; (c) X. Y. Hu, X. Wu, Q. Duan, T. Xiao, C. Lin and L. Wang, Org. Lett., 2012, 14, 4826 CrossRef CAS PubMed; (d) L. Y. Wang, X. G. Chen and D. Cao, RSC Adv., 2016, 6, 96676 RSC.
- (a) W. Ren, Y. Zhang, H. G. Chen, H. Z. F. Gao, N. B. Li and H. Q. Luo, Anal. Chem., 2016, 88, 1385 CrossRef CAS PubMed; (b) K. Johari, N. Saman, S. T. Song, S. C. Cheu, H. Kong and H. Mat, Chemosphere, 2016, 156, 56 CrossRef CAS PubMed; (c) S. Sun, X. Y. Hu, D. Chen, J. Shi, Y. Dong, C. Lin, Y. Pan and L. Wang, Polym. Chem., 2013, 4, 2224 RSC; (d) Y. Chen, M. He, B. Li, L. Wang, H. Meier and D. Cao, RSC Adv., 2013, 3, 21405 RSC.
- E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443 CrossRef CAS PubMed.
- D. T. Quang and J. S. Kim, Chem. Rev., 2010, 110, 6280 CrossRef CAS PubMed.
- C. Zhu, L. Li, F. Fang, J. Chen and Y. Wu, Chem. Lett., 2005, 34, 898 CrossRef CAS.
- M. A. Nolan and S. P. Kounaves, Anal. Chem., 1999, 71, 3567 CrossRef CAS.
- L. J. Fan, Y. Zhang and W. E. Jones, Macromolecules, 2005, 38, 2844 CrossRef CAS.
- P. Chen and C. He, J. Am. Chem. Soc., 2004, 126, 728 CrossRef CAS PubMed.
- A. S. K. Kumar and S. J. Jiang, RSC Adv., 2015, 5, 6294 RSC.
- C. T. Driscoll, R. P. Mason, H. M. Chan, D. J. Jacob and N. Pirrone, Environ. Sci. Technol., 2013, 47, 4967 CrossRef CAS PubMed.
- F. Di Natale, A. Erto, A. Lancia and D. Musmarra, J. Hazard. Mater., 2011, 192, 1842 CrossRef CAS PubMed.
- R. Qu, C. Wang, C. Sun, C. Ji, G. Cheng, X. Wang and G. Xu, Polym. Sci., 2004, 92, 1646 CAS.
- C. Wang, S. Tao, W. Wei, C. Meng, F. Liu and M. Han, J. Mater. Chem., 2010, 20, 4635 RSC.
- A. Chojnacki, K. Chojnacka, J. Hoffmann and H. Gorecki, Miner. Eng., 2004, 17, 933 CrossRef CAS.
- P. Martellaro, G. Moore, E. Peterson, E. Abbott and A. Gorenbain, Sep. Sci. Technol., 2001, 36, 1183 CrossRef CAS.
- S. Y. Ding, M. Dong, Y. W. Wang, Y. T. Chen, H. Z. Wang, C. Y. Su and W. Wang, J. Am. Chem. Soc., 2016, 138, 3031 CrossRef CAS PubMed.
- A. Tadjarodi, S. M. Ferdowsi and Z. D. Rouholah, Ultrason. Sonochem., 2016, 33, 118 CrossRef CAS PubMed.
- (a) Q. Lin, F. Zheng, L. Liu, P. P. Mao, Y. M. Zhang, H. Yao and T. B. Wei, RSC Adv., 2016, 6, 111928 RSC; (b) Q. Lin, T. T. Lu, X. Zhu, T. B. Wei, H. Li and Y. M. Zhang, Chem. Sci., 2016, 7, 5341 RSC; (c) Q. Lin, T. T. Lu, X. Zhu, B. Sun, Q. P. Yang, T. B. Wei and Y. M. Zhang, Chem. Commun., 2015, 51, 1635 RSC.
- (a) T. B. Wei, J. F. Chen, X. B. Cheng, H. Li, B. B. Han, Y. M. Zhang, H. Yao and Q. Lin, Org. Chem. Front., 2017, 4, 210 RSC; (b) Q. Lin, B. Sun, Q. P. Yang, Y. P. Fu, X. Zhu, T. B. Wei and Y. M. Zhang, Chem.–Eur. J., 2014, 20, 11457 CrossRef CAS PubMed; (c) X. B. Cheng, H. Li, F. Zheng, Q. Lin, H. Yao, Y. M. Zhang and T. B. Wei, RSC Adv., 2016, 6, 20987 RSC.
- Analytical Methods Committee, Analyst, 1987, 112, 199 RSC.
- A. A. Markeb and N. A. El-Maali, Talanta, 2014, 119, 417 CrossRef PubMed.
- L. Y. Wang, L. H. Zhu and D. R. Cao, New J. Chem., 2015, 39, 7211 RSC.
- C. Po, Z. Ke, A. Y. Y. Tam, H. F. Chow and V. W. W. Yam, Chem.–Eur. J., 2013, 19, 15735 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, synthesis of M2, NMR spectra, and other materials. See DOI: 10.1039/c6ra28419a |
| This journal is © The Royal Society of Chemistry 2017 |