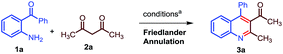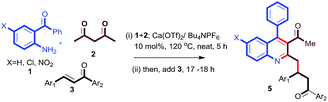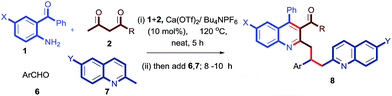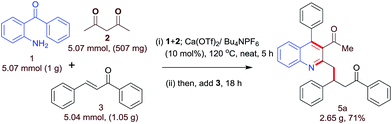DOI:
10.1039/C6RA28642A
(Paper)
RSC Adv., 2017,
7, 18874-18882
Highly efficient one-pot tandem Friedlander annulation and chemo-selective Csp3–H functionalization under calcium catalysis†
Received
24th December 2016
, Accepted 13th March 2017
First published on 28th March 2017
Abstract
A highly efficient and regioselective Friedlander synthesis of 2-methyl-3-acyl quinolines is described, which occurs under solvent-free conditions and employs calcium triflate as a sustainable catalyst. For the first time in the literature, these 2-methyl-3-acyl quinolines undergo an in situ chemoselective Csp3–H functionalization to furnish structurally enriched quinoline heterocycles in high yields and with atom and step economy.
Introduction
Rational drug design based on privileged scaffolds is one of the most powerful concepts in modern drug discovery.1 A quinoline moiety is one of these privileged scaffolds, and it has been extensively explored owing to its broad biological spectrum.2 For example, quinoline derivatives serve as anti-malarial drugs (quinine, chloroquine),2 anti-inflammatories,3 antibacterials, have antituberculosis properties,4 are multifunctional agents for Alzheimer's disease,5 and find uses as other therapeutic agents.6 In addition, these moieties are an integral part of many biologically active natural products (Fig. 1).7 Hence, the development of new synthetic methods for quinoline derivatives is an active area of organic synthesis. Among several synthetic methods available, functionalization of 2-methylquinolines has emerged as a new synthetic technique to address the synthesis of quinoline-based new chemical entities (NCE).8 However, most of these methods utilize 2-methylazaarenes as a starting point for functionalization to generate the new libraries. In general, 2-methyl quinolines can be synthesized starting from easily available o-acyl anilines and a suitable carbonyl compound through a Friedlander annulation under acidic or basic conditions.9 To the best of our knowledge, there are only a couple of reports available in which in situ-generated 2-methyl quinolines were functionalized.10,11 Nevertheless, both of these reports were limited to direct synthesis of styryl quinolines and no chemoselectivity was attained; moreover, the reaction could not even proceed with preformed 2-methyl, 3-acyl quinolines with In(OTf)3.11 Hence, it is highly desirable to explore the chemoselectivity of these 2-methyl, 3-acyl quinolines through functionalization of their methyl groups to generate useful quinoline derivatives.
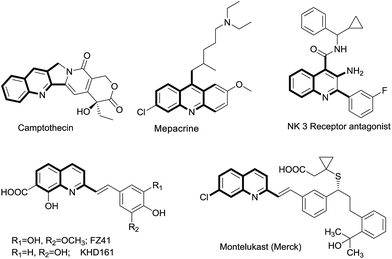 |
| | Fig. 1 Representative examples of 2-alkyl quinoline derivatives present in natural products and drug molecules. | |
On the other hand, one-pot multicomponent reactions (MCRs) have been proven to be efficient alternative green synthetic reactions for some existing classical stepwise reactions.12 MCRs are well known for the synthesis of complex molecules starting from simple starting materials. Looking at its importance, we have been working on a one-pot, solvent-free/in-water multicomponent approach using calcium triflate as an environmentally benign catalyst.8i–k,13 As a continuation of our interest in the facile, selective, and sustainable synthesis of biologically important heterocycles, we disclose here a highly efficient one-pot tandem calcium-catalysed Friedlander annulation followed by chemoselective C–H functionalization to generate quinoline-based new chemical entities.
Results and discussion
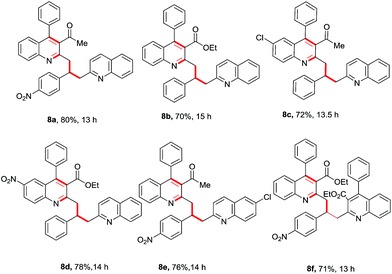
As depicted in Fig. 2, we designed a Friedlander synthesis of quinoline (3) which contains two activated methyl groups, the chemoselectivity of which could be differentiated by a suitable combination of reagents and conditions. Based on our expertise in Csp3–H functionalization, we decided to functionalize the methyl group (Csp3–H) on the 2nd position in a tandem one-pot multicomponent approach.8i–k Thus, compound (I) could be achieved by selecting a suitable electrophile (and this can be an aldehyde or isatin) which could accommodate two moles of methyl azaarene. Compound (II) was envisaged from the chemoselective and conjugate addition of compound 3 to the activated alkenes in one pot.
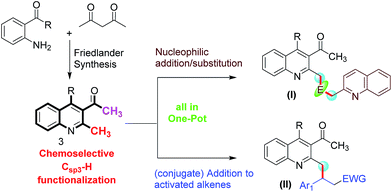 |
| | Fig. 2 Schematic representation of our synthetic plan for quinoline derivatives through a one-pot Friedlander annulation followed by chemoselective functionalization (E = electrophile; EWG = electron withdrawing group). | |
In order to implement our concept, initially we decided to look for better conditions for 2-methyl quinoline synthesis and its conjugate (Michael) addition to a chalcone derivative in one pot. 2-Aminobenzophenone (1a) and acetylacetone (2a) were chosen as starting materials for the Friedlander synthesis of 2-methyl, 3-acetyl, 4-phenyl quinoline (3a). As shown in Table 1, stoichiometric amounts of 1a and 2a were refluxed in water with 10 mol% of Ca(OTf)2/Bu4NPF6 and the reaction gave a positive result with 61% of 3a after 6 h (entry 1, Table 1). Toluene gave a better result compared to water (Table 1, entry 2) and DCE gave a lower yield of 3a. However, the reaction yielded excellent results under neat conditions (entry 4, Table 1). After a careful observation of the optimization studies, we found that the Friedlander synthesis was effective at 120 °C under neat conditions with 10 mol% of Ca(OTf)2/Bu4NPF6 to furnish a nearly quantitative yield of 3a.14
Table 1 Optimization of reaction conditions for the calcium-catalysed Friedlander synthesis of 2-methyl, 3-acyl, 4-phenyl quinoline (3a)a
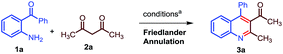
|
| Entry |
Catalyst (mol%) |
Reaction conditions |
Yield (%) |
| Reaction condition: 1a (0.50 mmol), 2a (0.50 mmol) were heated in a closed vessel under neat conditions at 120 °C for 5 h. |
| 1 |
Ca(OTf)2/Bu4NPF6 (10/10) |
Water, 6 h, 110 °C |
61 |
| 2 |
Ca(OTf)2/Bu4NPF6 (10/10) |
Toluene, 6 h, 120 °C |
73 |
| 3 |
Ca(OTf)2/Bu4NPF6 (10/10) |
1,2-Dichloroethane, 24 h, 80 °C |
45 |
| 4 |
Ca(OTf)2/Bu4NPF6 (10/10) |
Neat, 5 h, 120 °C |
98 |
| 5 |
Ca(OTf)2/Bu4NPF6 (10/10) |
Neat, 9 h, 100 °C |
80 |
| 6 |
Ca(OTf)2/Bu4NPF6 (5/5) |
Neat, 11 h, 120 °C |
93 |
| 7 |
Ca(OTf)2/Bu4NPF6 (5/10) |
Neat, 10 h, 120 °C |
95 |
| 8 |
Ca(OTf)2/Bu4NPF6 (10/5) |
Neat, 10 h, 120 °C |
94 |
Encouraged by this observation, we proceeded further and added simple chalcone 4a to the above reaction to check the possibility of a conjugate addition reaction. Gratifyingly, the reaction gave Michael adduct 5a in 72% yield after 18 h. Encouraged by this observation, applicability of the reaction condition for the one-pot synthesis of 4-(3-acetyl-4-phenylquinolin-2-yl)-1,3-diphenylbutan-1-one (5a) was generalized with different enones bearing different electron-withdrawing and -donating groups. As shown in Table 2, ortho-amino benzophenone (1a) and acetylacetone (2a) were reacted with various chalcones in presence of 10 mol% Ca(OTf)2/Bu4NPF6 under neat conditions15 through a tandem Friedlander annulation followed by Michael addition to give the quinoline derivatives 5a–5d in good yields. 5-Chloro-2-aminobenzophenone derivative (1b) also reacted with acetylacetone and various chalcone derivatives under the same conditions and produced the quinoline derivatives 5e–5i in good yields. Interestingly, ortho-aminobenzophenone bearing an electron-withdrawing group (–NO2) also showed a similar reactivity with acetylacetone and chalcones, to furnish the quinoline derivatives 5j–5l in moderate to good yields as shown in Table 2.
Table 2 Substrate scope for the Ca(OTf)2-catalyzed tandem Friedlander annulation and Michael addition for the synthesis of substituted quinoline derivativesa
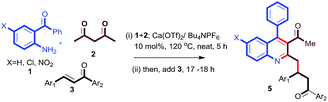
|
| Stoichiometry of reactants: 1 (0.50 mmol), 2 (0.50 mmol) & 3 (0.50 mmol); reaction was performed in a sealed vessel; isolated yields were reported. |
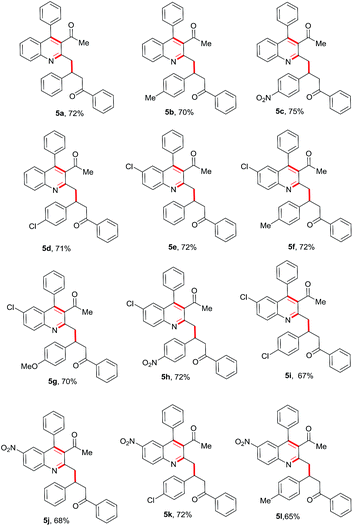 |
After a successful demonstration of a three-component calcium-catalyzed tandem Friedlander synthesis of 2-methyl, 3-acyl quinolines and their chemo-selective functionalization through a Michael addition to the chalcone compounds (Table 2), we decided to make dimeric quinoline derivatives. For this, aldehydes were taken as electrophilic partners instead of chalcones, as it is known that 2-methylquinoline adds to aldehydes to yield alcoholic compounds which may further undergo another nucleophilic substitution with a second mole of 2-methylquinoline in the presence of Ca(II).13f To implement this idea, we performed the Friedlander annulation and then added 1 equiv. of benzaldehyde and 2-methylquinoline (1 equiv.) in one pot; the reaction was continued for another 8 h to isolate the desired product 8a in 80% yield. Refreshed by this result, we extended this procedure for the synthesis of other dimeric quinolines 8b–8e in excellent yields as shown in Table 3. When 4-nitrobenzaldehyde was added alone after the Friedlander annulation, a homodimeric quinoline derivative 8f was isolated in 71% yield after 13 h.
Table 3 Substrate scope in the one-pot four-component Ca(II)-catalyzed Friedlander annulation and chemoselective Csp3–H functionalization for the synthesis of dimeric quinoline derivativesa
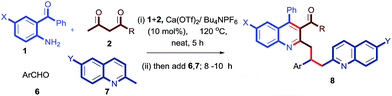
|
| Stoichiometry of reactants: 1 (0.50 mmol), 2 (0.50 mmol), 6 (0.50 mmol) & 7 (0.55 mmol); reaction was performed in a sealed vessel; isolated yields were reported. |
 |
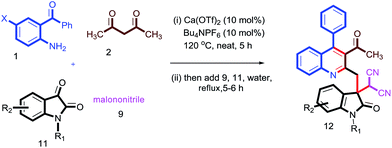
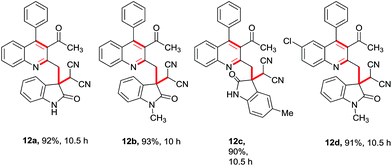
Having these fruitful results in hand, we investigated another four-component reaction for the synthesis of quinoline derivatives through a tandem Friedlander annulation and C–H functionalization as described in Table 4. Benzaldehyde (6) and malononitrile (9) were added to the substituted 2-methylquinoline (formed through Friedlander annulation) in one pot and the reaction was further refluxed in water for an additional 5 h to obtain the four-component adduct 2-(2-(3-acetyl-4-phenylquinolin-2-yl)-1-phenylethyl)malononitrile (10a) in 92% yield through a simple filtration (Table 4). This compound was so pure that no further recrystallization was required. The substrate scope of this one-pot four-component synthesis was demonstrated by the participation of a large number of aryl aldehydes bearing electron-donating/-withdrawing groups and substituted ortho-amino benzophenones to furnish the respective compounds 10a–10i with excellent yields, as depicted in Table 4. This idea was further extended for the synthesis of biologically important quaternary centered oxindolyl–quinoline derivatives by simply switching the electrophile from aldehyde to isatin (Table 5). Thus, quaternary-centered oxindolyl derivative 12a was isolated through simple filtration in 92% yield in 5.5 h under similar conditions. Similarly, 1-methylisatin with 3a yielded 12b and 12d in 93% and 91% yields, respectively, whereas 5-methylisatin furnished the product 12c in 91% yield.
Table 4 Substrate scope for the Csp3–H functionalisation of substituted quinolines with aldehyde and malononitrile in a one-pot four-component reactiona

|
| Reaction conditions: 1 (0.50 mmol), 2 (0.50 mmol), 6 (0.50 mmol) & 9 (0.50 mmol); isolated yields after filtration were reported. |
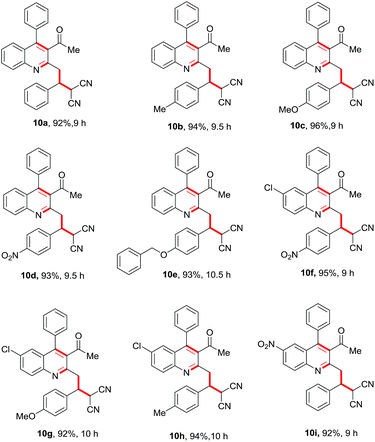 |
Table 5 Substrate scope for the synthesis of quaternary centred oxindolyl–quinolines through one-pot 4-component strategya
|
| Reaction conditions: 1 (0.50 mmol), 2 (0.50 mmol), 9 (0.50 mmol) & 11 (0.50 mmol); isolated yields after filtration were reported. |
 |
The synthetic utility of this protocol was demonstrated via a gram scale synthesis of 5a (2.65 g) through a tandem Friedlander annulation/C–H functionalization (chemoselective) and 71% yield of the desired product was obtained (Scheme 1).
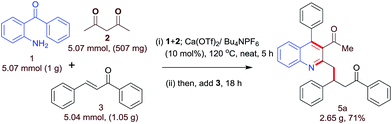 |
| | Scheme 1 Gram-scale demonstration of calcium-catalyzed three-component synthesis of 5a. | |
Conclusions
In summary, we described the first report of tandem Friedlander annulation and chemoselective Csp3–H functionalization of in situ-generated 2-methyl, 3-acyl quinolines under calcium catalysis. The wide substrate scope, high yields, and flexibility to extend to more varieties of quinoline derivatives under calcium catalysis, given the atom and step economy of the method, will attract attention from medicinal chemists wishing to explore further the biological utilities of quinoline derivatives.
Experimental section
General remarks
All chemicals were purchased from commercial sources and were used as received without further purification. 1H, 13C NMR spectra were recorded on an Avance Bruker 500 MHz spectrometer in CDCl3. Chemical shifts (δ) are given in ppm relative to tetramethylsilane (TMS) and calibrated to residual chloroform peaks. Coupling constants (J) are reported in Hz and coupling patterns are described as: s = singlet, d = doublet, t = triplet, q = quartet, quint = quintet, hept = heptet, m = multiplet. Melting points were measured with a Büchi Melting Point B-540 apparatus. Reactions were monitored by thin layer chromatography (TLC) with aluminium sheets silica gel 60 F254 from Merck with detection by UV light and charring with β-naphthol and ninhydrin stain.
Procedures
General experimental procedure for the synthesis of 4-(3-acetyl-4-phenylquinolin-2-yl)-1,3-diphenylbutan-1-one (5a)15. 2-Amino benzophenone (0.507 mmol, 100 mg), acetyl acetone (0.65 mmol, 50.7 mg), Ca(OTf)2 (10 mol%), and nBuNPF6 (10 mol%) were heated at 120 °C under solvent-free conditions for 4–5 h. After completion of the reaction, chalcone (0.507 mmol) was added to the reaction mixture. Then, heating of the reaction was continued for another 16–18 h at 120 °C. After completion, the reaction mass was diluted with water and extracted into ethyl acetate thrice. The solvent was evaporated under reduced pressure. The crude material obtained was purified with column chromatography to obtain pure 5a (72% yield) as a light brown solid; mp 132–133 °C; 1H NMR (500 MHz, CDCl3): δ 8.11 (d, J = 8.5 Hz, 1H), 7.88 (d, J = 7.5 Hz, 2H), 7.76–7.73 (m, 1H), 7.65–7.61 (m, 2H), 7.53–7.52 (m, 4H), 7.50–7.44 (m, 2H), 7.41–7.38 (m, 2H), 7.28–7.25 (m, 3H), 7.16 (t, J = 7 Hz, 2H), 4.32–4.26 (m, 1H), 3.67 (dd, J = 16.5 Hz, 17 Hz, 1H), 3.47–3.42 (m, 1H), 3.38–3.30 (m, 2H), 2.03 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.8, 198.7, 155.1, 147.4, 144.6, 137.2, 135.3, 135.2, 135.1, 134.8, 130.0, 128.9, 128.7, 128.4, 128.3, 128.0, 127.6, 126.6, 126.5, 126.1, 126.0, 125.1, 125.0, 44.1, 43.6, 40.6, 32.2; HRMS (ESI) m/z calcd for C33H28O2N [M + H]+ 470.2115; found 470.2118.
4-(3-Acetyl-4-phenylquinolin-2-yl)-1-phenyl-3-(p-tolyl)butan-1-one (5b). Yield 70%; brown solid; mp 198–200 °C; 1H NMR (500 MHz, CDCl3): δ 8.09 (d, J = 8 Hz, 1H), 8.02 (d, J = 8.5 Hz, 1H), 7.87 (d, J = 7.5 Hz, 2H), 7.53–7.49 (m, 4H), 7.40–7.37 (m, 4H), 7.31–7.29 (m, 2H), 7.21 (d, J = 8 Hz, 2H), 7.07 (d, J = 8 Hz, 2H), 4.24–4.21 (m, 1H), 3.64 (dd, J = 16.5 Hz, 16.5 Hz, 1H), 3.43–3.34 (m, 2H), 2.27 (s, 3H), 1.87 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.9, 198.8, 155.2, 147.4, 144.1, 141.5, 137.1, 135.9, 135.3, 135.1, 132.7, 130.3, 130.2, 130.1, 129.2, 129.1, 128.9, 128.8, 128.7, 128.5, 128.3, 128.1, 127.4, 126.6, 126.5, 126.2, 126.1, 125.0, 44.1, 43.4, 40.2, 32.2, 23.9, 21.0; HRMS (ESI) m/z calcd for C34H29O2N [M + H] 483.2198; found 483.2203.
4-(3-Acetyl-4-phenylquinolin-2-yl)-3-(4-nitrophenyl)-1-phenylbutan-1-one (5c). Yield 75%; brown solid; mp 205–206 °C; 1H NMR (500 MHz, CDCl3): δ 8.13 (d, J = 8.5 Hz, 2H), 8.03 (d, J = 8.5 Hz, 1H), 7.89 (d, J = 7 Hz, 2H), 7.74–7.71 (m, 1H), 7.64 (d, J = 8 Hz, 1H), 7.56–7.53 (m, 6H), 7.49–7.47 (m, 1H), 7.42 (t, J = 8 Hz, 2H), 7.37–7.33 (m, 2H), 4.50–4.45 (m, 1H), 3.69 (dd, J = 17.5 Hz, 17 Hz, 1H), 3.53–3.48 (m, 1H), 3.34–3.32 (m, 2H), 1.93 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.8, 197.2, 153.9, 152.6, 147.4, 146.5, 144.6, 136.7, 135.1, 134.7, 133.1, 130.1, 129.2, 129.1, 128.9, 128.8, 128.7, 128.5, 128.0, 126.9, 126.2, 125.0, 123.7, 43.7, 42.4, 40.0, 32.3; HRMS (ESI) m/z calcd for C33H26O4N2 [M + H] 514.1892; found 514.1896.
3-(3-Acetyl-4-phenylquinolin-2-yl)-2-(4-chlorophenyl)-1-phenylpropan-1-one (5d). Yield 71%; brown solid; mp 214–216 °C; 1H NMR (500 MHz, CDCl3): δ 8.03 (d, J = 8 Hz, 1H), 7.88 (d, J = 7.5 Hz, 2H), 7.71 (t, J = 8.5 Hz, 1H), 7.63 (d, J = 8 Hz, 1H), 7.53–7.46 (m, 5H), 7.42–7.39 (m, 1H), 7.32–7.27 (m, 5H), 7.23 (d, J = 8.5 Hz, 2H), 4.30–4.27 (m, 1H), 3.62 (dd, J = 16.5 Hz, 18.5 Hz, 1H), 3.43–3.39 (m, 1H), 3.34–3.32 (d, J = 6.5 Hz, 2H), 1.89 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.8, 198.4, 154.6, 143.1, 136.9, 135.2, 134.9, 132.9, 132.0, 130.1, 130.0, 129.2, 129.1, 129.0, 128.9, 128.8, 128.7, 128.6, 128.4, 128.0, 126.8, 126.1, 125.0, 44.0, 43.0, 39.8, 32.3; HRMS (ESI) m/z calcd for C33H24O2NCl [M]+ 489.1459; found 489.1463.
3-(3-Acetyl-6-chloro-4-phenylquinolin-2-yl)-1,2-diphenylpropan-1-one (5e). Yield 72%; brown solid; mp 220–222 °C; 1H NMR (500 MHz, CDCl3): δ 8.10 (d, J = 8 Hz, 1H), 8.04 (d, J = 8.5 Hz, 1H), 7.88 (d, J = 7.5 Hz, 2H), 7.75 (t, J = 7 Hz, 1H), 7.71 (t, J = 7 Hz, 1H), 7.66–7.62 (m, 2H), 7.54–7.52 (m, 4H), 7.32–7.25 (m, 5H), 7.18–7.15 (m, 1H), 4.31–4.25 (m, 1H), 3.67 (dd, J = 17 Hz, 17 Hz, 1H), 3.48–3.43 (m, 1H), 3.39–3.29 (m, 2H), 2.03 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.9, 198.7, 155.1, 153.5, 147.4, 144.5, 137.1, 132.7, 130.2, 130.1, 130.0, 129.1, 128.9, 128.8, 128.7, 128.6, 128.5, 128.4, 128.0, 127.6, 126.4, 126.2, 125.0, 44.0, 43.2, 40.6, 32.2; HRMS (ESI) m/z calcd for C33H24O2NCl [M]+ 489.1459; found 489.1463.
3-(3-Acetyl-6-chloro-4-phenylquinolin-2-yl)-1-phenyl-2-(p-tolyl)propan-1-one (5f). Yield 72%; light brown solid; mp 215–217 °C; 1H NMR (500 MHz, CDCl3): δ 7.94 (d, J = 8.5 Hz, 1H), 7.86 (d, J = 7.5 Hz, 2H), 7.61 (dd, J = 9 Hz, 9 Hz, 1H), 7.57–7.50 (m, 5H), 7.41–7.37 (m, 3H), 7.30–7.28 (m, 1H), 7.21 (d, J = 8 Hz, 2H), 7.08 (d, J = 8 Hz, 2H), 4.24–4.22 (m, 1H), 3.64 (dd, J = 17 Hz, 17 Hz, 1H), 3.42–3.37 (m, 1H), 3.32–3.29 (m, 2H), 2.28 (s, 3H), 1.88 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.4, 198.6, 155.7, 145.6, 143.4, 141.4, 137.0, 136.0, 135.8, 134.6, 132.7, 131.0, 130.7, 130.1, 129.9, 129.2, 129.0, 128.8, 128.4, 128.0, 127.4, 125.8, 124.8, 44.1, 43.2, 40.0, 32.1, 21.0; HRMS (ESI) m/z calcd for C33H27O2NCl [M]+ 504.1735; found 504.1738.
4-(3-Acetyl-6-chloro-4-phenylquinolin-2-yl)-3-(4-methoxyphenyl)-1-phenylbutan-1-one (5g). Yield 70%; brown solid; mp 205–207 °C; 1H NMR (500 MHz, CDCl3): δ 7.99 (d, J = 9 Hz, 1H), 7.87 (d, J = 7.5 Hz, 2H), 7.63 (dd, J = 9 Hz, 9 Hz, 1H), 7.58–7.51 (m, 6H), 7.42–7.39 (m, 3H), 7.24 (d, J = 8.5 Hz, 2H), 6.81 (d, J = 8.5 Hz, 2H), 4.24–4.18 (m, 1H), 3.76 (s, 3H), 3.64 (dd, J = 17 Hz, 17 Hz, 1H), 3.42–3.31 (m, 3H), 1.89 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.4, 155.4, 145.3, 143.5, 141.2, 137.1, 136.2, 135.2, 134.8, 133.1, 132.6, 131.0, 130.4, 130.1, 130.0, 129.3, 129.0, 128.6, 128.1, 127.9, 127.1, 125.6, 124.3, 55.5, 44.2, 43.5, 40.1, 32.4; HRMS (ESI) m/z calcd for C33H28O2N [M + H] 534.1830; found 534.1833.
4-(3-Acetyl-6-chloro-4-phenylquinolin-2-yl)-3-(4-nitrophenyl)-1-phenylbutan-1-one (5h). Yield 72%; brown solid; mp 212–214 °C; 1H NMR (500 MHz, CDCl3): δ 7.87 (d, J = 9 Hz, 1H), 7.78–7.76 (m, 2H), 7.54 (dd, J = 9 Hz, 9 Hz, 1H), 7.49–7.44 (m, 5H), 7.33–7.29 (m, 3H), 7.20–7.18 (m, 3H), 7.15–7.13 (m, 2H), 4.20–4.14 (m, 1H), 3.54 (dd, J = 17 Hz, J = 17 Hz, 1H), 3.33–3.28 (m, 1H), 3.21 (d, J = 7 Hz, 2H), 1.81 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.2, 198.2, 155.1, 142.9, 136.8, 135.6, 134.4, 133.0, 132.1, 131.2, 130.4, 130.3, 130.0, 129.9, 129.4, 129.2, 129.1, 129.0, 128.9, 128.6, 128.5, 128.1, 128.0, 126.1, 125.9, 124.9, 44.0, 42.7, 39.7, 32.1; HRMS (ESI) m/z calcd for C33H25ClO4N2 [M + H] 548.1502; found 548.1506.
3-(3-Acetyl-6-chloro-4-phenylquinolin-2-yl)-2-(4-chlorophenyl)-1-phenylpropan-1-one (5i). Yield 67%; brown solid; mp 220–222 °C; 1H NMR (500 MHz, CDCl3): δ 7.98 (d, J = 9 Hz, 1H), 7.86 (d, J = 7.5 Hz, 1H), 7.65–7.63 (m, 1H), 7.63–7.51 (m, 5H), 7.42–7.37 (m, 3H), 7.37–7.27 (m, 4H), 7.23 (d, J = 8.5 Hz, 2H), 4.27–4.23 (m, 1H), 3.64 (dd, J = 17 Hz, 17 Hz, 1H), 3.42–3.37 (m, 1H), 3.31–3.30 (m, 2H), 1.90 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.6, 198.3, 155.1, 142.8, 136.7, 135.6, 133.0, 132.2, 130.0, 129.9, 129.8, 129.5, 129.1, 129.0, 128.9, 128.6, 128.5, 128.0, 125.9, 124.9, 43.9, 42.6, 39.7, 32.1; HRMS (ESI) m/z calcd for C32H23Cl2NO2 [M + H] 524.4359; found 524.4360.
4-(3-Acetyl-6-nitro-4-phenylquinolin-2-yl)-1,3-diphenylbutan-1-one (5j). Yield 72%; light brown solid; mp 128–130 °C; 1H NMR (500 MHz, CDCl3): δ 8.14 (d, J = 9 Hz, 2H), 8.03 (d, J = 8 Hz, 1H), 7.90–7.88 (m, 2H), 7.74–7.71 (m, 1H), 7.65–7.63 (m, 1H), 7.56–7.53 (m, 6H), 7.49–7.46 (m, 1H), 7.42 (t, J = 8 Hz, 2H), 7.38–7.33 (m, 2H), 4.05–4.44 (m, 1H), 3.69 (dd, J = 17.5 Hz, 17.5 Hz, 1H), 3.54–3.48 (m, 1H), 3.34–3.31 (m, 2H), 1.92 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.8, 197.4, 153.9, 152.6, 147.4, 146.5, 144.6, 136.6, 135.1, 134.7, 133.2, 130.3, 130.1, 129.1, 128.9, 128.8, 128.7, 128.6, 128.0, 127.0, 126.2, 125.0, 123.7, 43.7, 42.4, 40.1, 32.3; HRMS (ESI) m/z calcd for C33H26N2O4 [M + H] 514.5706; found 514.5708.
General experimental procedure for the synthesis of 1-(4-phenyl-2-(2-phenyl-3-(quinolin-2-yl)propyl)quinolin-3-yl)ethan-1-one (8a). 2-Aminobenzophenone (0.507 mmol, 100 mg), acetylacetone (0.507 mmol, 50.7 mg), Ca(OTf)2 (0.05 mmol), nBuNPF6 (0.05 mmol) were heated at 120 °C under solvent-free conditions for 4–5 h. After completion of the reaction, 2-methylquinoline (0.507 mmol, 72.6 mg) and benzaldehyde (0.507 mmol, 53.8 mg) were added to the reaction mixture. Heating of the reaction was continued for another 7–8 h at 120 °C. After completion, the reaction mass was diluted with water and extracted into ethyl acetate thrice. The solvent was evaporated under reduced pressure. The crude obtained was purified with column chromatography to obtain pure 8a (mg, 78% yield) as yellow solid mp 88–90 °C; 1H NMR (500 MHz, CDCl3): δ 8.53 (s, 2H), 8.47–8.44 (m, 2H), 8.15 (d, J = 9.5 Hz, 2H), 8.10 (d, J = 8.5 Hz, 2H), 7.63–7.57 (m, 8H), 7.32 (d, J = 4.5 Hz, 4H), 4.72 (t, J = 7 Hz, 1H), 3.59–3.54 (m, 2H), 3.43–3.39 (m, 2H), 1.94 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 204.4, 158.3, 152.1, 149.2, 146.6, 146.1, 145.8, 136.1, 133.5, 130.9, 1230.0, 129.9, 129.8, 129.4, 129.3, 128.9, 128.4, 126.6, 124.3, 124.2, 123.8, 123.7, 123.6, 123.1, 71.7, 42.6, 42.2, 32.1; HRMS (ESI) m/z calcd for C35H28O2N2 [M + H]+ 492.2201; found 492.2204.
Ethyl 4-phenyl-2-(2-phenyl-3-(quinolin-2-yl)propyl)quinoline-3-carboxylate (8b). Yield 70%; brown solid; mp 134–136 °C; 1H NMR (500 MHz, CDCl3): δ 7.96 (d, J = 8.5 Hz, 2H), 7.88 (d, J = 8 Hz, 2H), 7.74 (d, J = 8.5 Hz, 2H), 7.60 (m, 6H), 7.42–7.40 (m, 2H), 7.37–7.35 (m, 2H), 7.33–7.29 (m, 3H), 7.20–7.19 (m, 5H), 7.16–7.14 (m, 1H), 7.10 (t, J = 7.5 Hz, 3H), 7.05–7.01 (m, 3H), 4.15–4.12 (m, 1H), 3.92–3.84 (m, 2H), 3.45–3.42 (m, 4H), 0.78 (t, J = 7 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 168.4, 161.0, 156.0, 147.7, 147.5, 146.2, 144.4, 135.8, 135.5, 130.0, 129.5, 129.3, 129.1, 129.0, 128.8, 128.3, 128.2, 128.1, 128.0, 127.8, 127.5, 127.3, 126.5, 126.4, 126.3, 126.1, 125.5, 125.1, 122.1, 61.3, 45.2, 45.1, 43.4, 13.5; HRMS (ESI) m/z calcd for C36H30O2N2 [M + H]+ 522.2307; found 522.2309.
1-(6-Chloro-4-phenyl-2-(2-phenyl-3-(quinolin-2-yl)propyl)quinolin-3-yl)ethan-1-one (8c). Yield 72%; light brown solid; mp 132–134 °C; 1H NMR (500 MHz, CDCl3): δ 7.98 (s, 1H), 7.82 (d, J = 8.5 Hz, 2H), 7.65 (d, J = 7.5 Hz, 3H), 7.63–7.62 (m, 1H), 7.51–7.48 (m, 2H), 7.45 (d, J = 2 Hz, 2H), 7.28–7.23 (m, 3H), 7.21–7.20 (m, 2H), 7.20–7.18 (m, 2H), 7.16–7.14 (m, 1H), 4.29–4.23 (m, 1H), 3.55–3.50 (m, 1H), 3.42–3.38 (m, 3H), 1.79 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.6, 160.8, 155.8, 147.8, 147.6, 145.6, 144.4, 142.9, 136.2, 135.6, 135.5, 134.5, 132.4, 130.8, 130.7, 130.1, 129.9, 129.8, 129.4, 129.3, 129.2, 129.1, 128.0, 128.8, 128.7, 128.6, 128.3, 127.8, 127.5, 127.4, 126.6, 126.5, 126.3, 125.7, 125.6, 124.7, 122.2, 122.0, 45.2, 45.0, 43.3, 32.1; HRMS (ESI) m/z calcd for C35H27ClN2O [M + H] 527.0544; found 527.0548.
Ethyl 6-nitro-4-phenyl-2-(2-phenyl-3-(quinolin-2-yl)propyl)quinoline-3-carboxylate (8d). Yield 78%; yellow solid; mp 133–134 °C; 1H NMR (500 MHz, CDCl3): δ 7.95–7.94 (m, 1H), 7.77 (d, J = 8.5 Hz, 1H), 7.61–7.58 (m, 1H), 7.54 (d, J = 8.5 Hz, 1H), 7.50–7.47 (m, 3H), 7.43 (d, J = 2.5 Hz, 1H), 7.37–7.35 (m, 1H), 7.32–7.30 (m, 1H), 7.27–7.19 (m, 1H), 7.16–7.10 (m, 2H), 4.30–4.24 (m, 1H), 4.07–3.98 (m, 2H), 3.58–3.48 (m, 2H), 3.46–3.38 (m, 1H), 0.91 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 168.0, 162.1, 156.4, 148.1, 148.0, 145.8, 145.3, 145.2, 144.8, 144.4, 143.6, 135.5, 135.3, 135.2, 135.1, 134.8, 130.8, 130.7, 129.3, 129.2, 128.7, 128.6, 128.5, 128.4, 128.3, 128.0, 127.9, 127.8, 127.7, 126.7, 126.5, 126.3, 125.8, 125.0, 122.3, 61.4, 45.8, 45.6, 45.2, 44.6, 43.4, 43.3, 42.8, 13.5; HRMS (ESI) m/z calcd for C33H28O2N [M + H]+ 567.2228; found 568.2231.
1-(6-Chloro-2-(2-(4-nitrophenyl)-3-(quinolin-2-yl)propyl)-4-phenylquinolin-3-yl)ethan-1-one (8e). Yield 76%; brown solid; mp 128–130 °C; 1H NMR (500 MHz, CDCl3): δ 8.06 (d, J = 8.5 Hz, 2H), 7.97–7.93 (m, 2H), 7.89 (d, J = 8.5 Hz, 1H), 7.69–7.61 (m, 3H), 7.51–7.45 (m, 7H), 7.23 (d, J = 6 Hz, 1H), 7.18–7.17 (m, 1H), 7.14 (d, J = 8.5 Hz, 1H), 4.52–4.46 (m, 1H), 3.57–3.53 (m, 3H), 3.42–3.37 (m, 1H), 1.89 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.4, 159.6, 154.5, 152.4, 147.6, 146.4, 145.6, 143.3, 136.0, 135.3, 134.3, 132.8, 131.1, 130.6, 130.0, 129.9, 129.5, 129.3, 129.1, 129.0, 128.9, 128.8, 128.6, 127.5, 126.5, 125.9, 125.7, 124.8, 123.6, 123.5, 121.9, 44.8, 44.4, 42.3, 32.2; HRMS (ESI) m/z calcd for C35H26ClN3O3 [M + H]+ 572.0520; found 572.0526.
Ethyl 2-(3-(3-(ethoxycarbonyl)quinolin-2-yl)-2-phenylpropyl)-4-phenylquinoline-3-carboxylate (8f). Yield 71%; yellow solid; mp 124–126 °C; 1H NMR (500 MHz, CDCl3): δ 8.17 (d, J = 7.5 Hz, 1H), 8.06 (d, J = 8.5 Hz, 1H), 7.66 (t, J = 10 Hz, 2H), 7.49–7.46 (m, 7H), 7.38 (d, J = 7 Hz, 3H), 7.25–7.20 (m, 6H), 7.11 (t, J = 7 Hz, 1H), 4.43 (t, J = 7 Hz, 1H), 4.05–3.97 (m, 4H), 3.66–3.56 (m, 4H), 0.89 (t, J = 7 Hz, 6H); 13C NMR (125 MHz, CDCl3): δ 168.3, 156.4, 147.3, 146.2, 144.9, 135.9, 133.3, 130.1, 129.9, 129.5, 129.4, 129.0, 128.4, 128.3, 128.1, 128.0, 127.9, 127.5, 126.4, 126.3, 126.0, 125.0, 61.2, 43.9, 42.8, 29.7, 13.5; HRMS (ESI) m/z calcd for C33H28O2N [M + H]+ 671.2074; found 671.2080.
General experimental procedure for the synthesis of 2-(2-(3-acetyl-4-phenylquinolin-2-yl)-1-phenylethyl)malononitrile (10a). 2-Aminobenzophenone (0.507 mmol, 100 mg), acetylacetone (0.65 mmol, 50.7 mg), Ca(OTf)2 (0.05 mmol), nBuNPF6 (0.05 mmol) were heated at 120 °C under solvent-free conditions for 4–5 h. After the completion of the reaction, malononitrile (0.507 mmol, 33.5 mg) and benzaldehyde (0.507 mmol, 53.8 mg) were added to the reaction mixture along with water as solvent. The reaction was refluxed for another 5–6 h at 120 °C. After completion, the reaction mass was filtered and washed with cold ethanol. The solid obtained was dried (no further purification was required) as 10a, white solid, (92% yield); mp 201–202 °C; 1H NMR (500 MHz, CDCl3): δ 8.33 (d, J = 8 Hz, 2H), 8.13 (d, J = 8.5 Hz, 1H), 7.84 (t, J = 7 Hz, 1H), 7.76–7.72 (m, 2H), 7.57 (s, 2H), 7.43 (s, 1H), 7.33 (d, J = 5.5 Hz, 1H), 5.29 (d, J = 4 Hz, 1H), 4.46–4.44 (m, 1H), 3.64–3.59 (m, 1H), 3.45 (dd, J = 17 Hz, 16.5 Hz, 1H), 1.94 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.3, 151.4, 148.2, 147.1, 145.7, 144.2, 134.7, 134.2, 131.0, 130.0, 129.9, 129.4, 129.2, 129.1, 128.9, 127.8, 126.5, 125.4, 124.3, 112.0, 111.5, 43.6, 37.1, 32.0, 27.9; HRMS (ESI) m/z calcd for C28H21ON3 [M + H]+ 415.1684; found 415.1690.
2-(2-(3-Acetyl-4-phenylquinolin-2-yl)-1-phenylethyl)malononitrile (10a). Yield 92%; light brown solid; mp 131–133 °C 1H NMR (500 MHz, CDCl3): δ 8.11 (d, J = 8.5 Hz, 1H), 7.88 (d, J = 7.5 Hz, 2H), 7.76–7.73 (m, 1H), 7.65–7.61 (m, 2H), 7.53–7.52 (m, 4H), 7.50–7.44 (m, 2H), 7.41–7.38 (m, 2H), 7.28–7.25 (m, 3H), 7.16 (t, J = 7 Hz, 2H), 4.32–4.26 (m, 1H), 3.67 (dd, J = 16.5 Hz, 17 Hz, 1H), 3.47–3.42 (m, 1H). 3.38–3.30 (m, 2H), 2.03 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.8, 198.7, 155.1, 147.4, 144.6, 137.2, 135.3, 135.2, 135.1, 134.8, 130.0, 128.9, 128.7, 128.4, 128.3, 128.0, 127.6, 126.6, 126.5, 126.1, 126.0, 125.1, 125.0; HRMS (ESI) m/z calcd for C28H21ON3 [M + H]+ 415.1648; found 415.1688.
2-(2-(3-Acetyl-4-phenylquinolin-2-yl)-1-(p-tolyl)ethyl)malononitrile (10b). Yield 94%; solid; mp 135–136 °C; 1H NMR (500 MHz, CDCl3): δ 8.17 (d, J = 7 Hz, 1H), 7.83 (s, 1H), 7.72 (d, J = 6.5 Hz, 1H), 7.56 (s, 4H), 7.42 (s, 4H), 7.27 (m, 2H), 5.27 (s, 1H), 4.26 (s, 1H), 3.63–3.57 (m, 1H), 3.44–3.41 (m, 1H), 2.40 (s, 3H), 1.93 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.3, 152.4, 147.2, 145.1, 138.7, 134.8, 134.6, 134.3, 130.7, 130.1, 129.9, 129.8, 129.3, 129.2, 129.0, 128.8, 128.0, 127.5, 126.3, 125.3, 112.7, 112.1, 43.5, 37.6, 32.0, 28.5, 21.2; HRMS (ESI) m/z calcd for C33H28O2N [M + H] 430.1914; found 430.1920.
2-(2-(3-Acetyl-4-phenylquinolin-2-yl)-1-(4-methoxyphenyl)ethyl)malononitrile (10c). Yield 96%; white solid; mp 162–164 °C; 1H NMR (500 MHz, CDCl3): δ 13C NMR (125 MHz, CDCl3) δ 8.15 (d, J = 8 Hz, 1H), 7.92 (d, J = 8 Hz, 1H), 7.81 (t, J = 7 Hz, 1H), 7.71–7.66 (m, 2H), 7.55 (s, 1H), 7.45–7.43 (m, 3H), 7.03 (d, J = 8 Hz, 1H), 6.97 (d, J = 7.5 Hz, 1H), 5.21 (d, J = 4 Hz, 1H), 4.24–4.23 (m, 1H), 3.93 (s, 3H), 3.60–3.55 (m, 1H), 3.42–3.39 (m, 1H), 1.93 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.2, 164.8, 159.9, 158.8, 152.4, 147.2, 145.1, 133.4, 129.3, 127.4, 126.3, 125.3, 124.0, 115.1, 114.5, 55.3, 43.3, 37.7, 32.0, 28.7. HRMS (ESI) m/z calcd for C29H23N3O2 [M + H] 445.5119; found 445.5124.
2-(2-(3-Acetyl-4-phenylquinolin-2-yl)-1-(4-nitrophenyl) ethyl)malononitrile (10d). Yield 93%; white solid; mp 143–145 °C; 1H NMR (500 MHz, CDCl3): δ 8.33 (d, J = 8 Hz, 2H), 8.13 (d, J = 8 Hz, 1H), 7.83 (t, J = 6 Hz, 1H), 7.76–7.72 (m, 3H), 7.57 (s, 4H), 7.43 (s, 1H), 7.33 (d, J = 4.5 Hz, 1H), 5.28 (d, J = 4 Hz, 1H), 4.45 (t, J = 5 Hz, 1H), 3.64–3.59 (m, 1H), 3.48–3.44 (m, 1H), 1.94 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.3, 151.4, 148.2, 147.1, 145.7, 144.2, 134.7, 134.2, 131.0, 130.0, 129.9, 129.4, 129.1, 128.9, 127.7, 126.4, 125.4, 124.3, 112.0, 111.5, 43.7, 37.1, 32.0, 27.9; HRMS (ESI) m/z calcd for C33H28O2N [M + H]+ 461.1608; found 461.1614.
2-(2-(3-Acetyl-4-phenylquinolin-2-yl)-1-(4-(benzyloxy)phenyl)ethyl)malononitrile (10e). Yield 93%; light brown solid; mp 143–145 °C; 1H NMR (500 MHz, CDCl3): δ 8.64 (d, J = 2.5 Hz, 1H), 8.57 (d, J = 7 Hz, 1H), 8.29 (d, J = 9.5 Hz, 1H), 7.64–7.60 (m, 3H), 7.45–7.34 (m, 10 H), 7.04 (d, J = 8.5 Hz, 2H), 5.09 (s, 2H), 4.96 (d, J = 5 Hz, 1H), 4.29–4.26 (m, 1H), 3.64–3.59 (m, 1H), 3.49–3.45 (m, 1H), 1.94 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 204.1, 159.2, 156.6, 149.1, 146.9, 146.2, 136.6, 136.0, 133.4, 133.3, 131.1, 130.2, 129.9, 129.8, 129.5, 129.4, 129.3, 129.1, 128.6, 128.1, 127.5, 124.7, 124.1, 123.3, 115.5, 112.3, 111.9, 70.1, 43.1, 38.2, 31.8, 28.9; HRMS (ESI) m/z calcd for C33H28O2N [M + H] 522.2176; found 522.2184.
2-(2-(3-Acetyl-6-chloro-4-phenylquinolin-2-yl)-1-(4-nitrophenyl)ethyl)malononitrile (10f). Yield 95%; light brown solid; mp 131–133 °C 1H NMR (500 MHz, CDCl3): δ 8.32 (d, J = 8.5 Hz, 2H), 8.07 (d, J = 9 Hz, 1H), 7.77–7.72 (m, 3H), 7.67 (d, J = 2 Hz, 1H), 7.60–7.55 (m, 3H), 7.40–7.39 (m, 1H), 7.30 (d, J = 6.5 Hz, 1H), 4.45–4.41 (m, 1H), 3.63–3.57 (m, 1H), 3.46–3.41 (m, 1H), 1.94 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 204.9, 151.8, 148.2, 145.5, 144.8, 144.1, 134.9, 134.0, 133.9, 131.9, 130.7, 129.9, 129.8, 129.8, 129.4, 129.4, 129.3, 129.2, 128.9, 126.4, 125.2, 124.4, 111.9, 111.4, 43.5, 37.1, 31.9, 28.0; HRMS (ESI) m/z calcd for C28H19O3N4Cl [M + H] 494.1145; found 494.1150.
2-(2-(3-Acetyl-6-chloro-4-phenylquinolin-2-yl)-1-(4-methoxyphenyl)ethyl)malononitrile (10g). Yield 92%; light brown solid; mp 188–190 °C; 1H NMR (500 MHz, CDCl3): δ 8.16 (d, J = 8.5 Hz, 1H), 7.93 (d, J = 8.5 Hz, 3H), 7.70 (t, J = 8 Hz, 1H), 7.56 (s, 3H), 7.44 (t, J = 8.5 Hz, 2H), 7.04 (d, J = 8.5 Hz, 3H), 5.21 (d, J = 4.5 Hz, 1H), 4.25 (t, J = 5 Hz, 1H), 3.85 (s, 3H), 3.61–3.56 (m, 1H), 3.44–3.40 (m, 1H), 1.94 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.2, 164.8, 159.9, 158.8, 152.4, 133.4, 130.6, 130.1, 129.9, 129.3, 127.4, 126.3, 124.1, 115.1, 114.5, 55.8, 43.3, 37.7, 32.0, 28.6; HRMS (ESI) m/z calcd for C29H22O2N3Cl [M + H]+ 479.1400; found 479.1404.
2-(2-(3-Acetyl-6-chloro-4-phenylquinolin-2-yl)-1-(4-methoxyphenyl)ethyl)malononitrile (10h). Yield 94%; white solid; mp 130–132 °C; 1H NMR (500 MHz, CDCl3): δ 8.33 (d, J = 8.5 Hz, 2H), 8.13 (d, J = 8.5 Hz, 1H), 7.85–7.82 (m, 1H), 7.76–7.73 (m, 3H), 7.74 (t, J = 8.5 Hz, 4H), 7.59–7.53 (m, 1H), 7.43–7.32 (m, 1H), 5.29 (d, J = 5 Hz, 1H), 4.47–4.43 (m, 1H), 3.64–3.59 (m, 1H), 3.47–3.43 (m, 1H), 2.46 (s, 3H), 1.94 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.4, 164.8, 151.4, 148.2, 147.1, 145.7, 144.2, 134.7, 134.2, 131.0, 130.0, 129.9, 129.4, 129.1, 128.9, 127.8, 126.5, 125.4, 124.4, 112.0, 111.5, 43.6, 37.1, 32.0, 27.9; HRMS (ESI) m/z calcd for C29H22N3ClO [M + H]+ 463.9573; found 463.9576.
2-(2-(3-Acetyl-6-nitro-4-phenylquinolin-2-yl)-1-phenylethyl)malononitrile (10i). Yield 92%; white solid; mp 135–137 °C; 1H NMR (500 MHz, CDCl3): δ 8.33 (d, J = 8.5 Hz, 2H), 8.13 (d, J = 8.5 Hz, 1H), 7.85–7.82 (m, 1H), 7.76–7.73 (m, 3H), 7.59–7.53 (m, 4H), 7.43–7.42 (m, 1H), 7.33–7.32 (m, 1H), 5.29 (d, J = 5 Hz, 1H), 4.47–4.43 (m, 1H), 3.64–3.59 (m, 1H), 3.47–3.43 (m, 1H), 1.94 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.3, 151.4, 148.2, 147.1, 145.7, 144.2, 134.7, 134.2, 131.0, 130.0, 129.9, 129.4, 129.2, 129.1, 128.9, 127.8, 126.5, 125.4, 124.4, 112.0, 111.5, 43.6, 37.1, 32.0, 27.9; HRMS (ESI) m/z calcd for C33H28O2N [M + H]+ 461.1608; found 461.1614.
General experimental procedure for the synthesis of 2-(2-(3-acetyl-4-phenylquinolin-2-yl)-1-(2-oxoindolin-3-yl)ethyl)malononitrile (12a). 2-Aminobenzophenone (0.50 mmol, 100 mg), acetylacetone (0.65 mmol, 50.7 mg), Ca(OTf)2 (0.05 mmol), nBuNPF6 (0.05 mmol) were heated at 120 °C under solvent free conditions for 4–5 h. After completion of the reaction, malononitrile (0.507 mmol, 33.5 mg) and isatin (0.507 mmol, 84.7 mg) were added to the reaction mixture along with water as solvent. The reaction was refluxed for another 5–6 h at 120 °C. After completion, the reaction mass was filtered and washed with cold ethanol. The solid obtained was dried and characterised without any further purification (12a). Yield 91%; light blue solid; mp 186–188 °C; 1H NMR (500 MHz, CDCl3): δ 8.19 (s, 1H), 7.93 (d, J = 8.5 Hz, 1H), 7.34 (t, J = 7 Hz, 1H), 7.65 (d, J = 8 Hz, 1H), 7.53–7.47 (m, 5H), 7.36–7.33 (m, 3H), 7.09–7.04 (m, 2H), 5.23 (s, 1H), 3.72 (q, J = 16.5 Hz, 2H), 1.85 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.2, 175.5, 150.2, 146.7, 145.3, 141.1, 134.9, 130.6, 130.4, 130.1, 129.9, 129.3, 129.0, 128.9, 128.8, 127.5, 126.7, 126.3, 125.2, 124.3, 123.4, 110.6, 110.2, 51.6, 38.2, 32.1, 30.2; HRMS (ESI) m/z calcd for C29H20O2N4 [M + H] 456.1586; found 470.1590.
2-(3-((3-Acetyl-4-phenylquinolin-2-yl)methyl)-1-methyl-2-oxoindolin-3-yl)malononitrile (12b). Yield 92%; pink solid; mp 215–217 °C; 1H NMR (500 MHz, CDCl3): δ 7.81 (d, J = 8 Hz, 1H), 7.73–7.70 (m, 1H), 7.64 (d, J = 7.5 Hz, 1H), 7.52–7.48 (m, 5H), 7.41 (d, J = 7.5 Hz, 1H), 7.33–7.31 (m, 2H), 7.09 (t, J = 7.5 Hz, 1H), 7.02 (d, J = 7.5 Hz, 1H), 5.12 (s, 1H), 3.72 (dd, J = 16.5 Hz, 16.5 Hz, 2H), 3.14 (s, 3H), 2.12 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.2, 174.1, 150.2, 146.6, 145.1, 144.4, 137.7, 134.9, 134.1, 130.5, 130.4, 130.1, 130.0, 129.2, 128.9, 128.8, 127.4, 126.7, 126.3, 126.1, 125.2, 123.9, 123.8, 123.4, 111.1, 110.2, 109.6, 108.9, 51.2, 38.3, 32.1, 30.4, 26.8; HRMS (ESI) m/z calcd for C30H22O2N4 [M + H] 470.1742; found 470.1745.
2-(3-((3-Acetyl-4-phenylquinolin-2-yl)methyl)-5-methyl-2-oxoindolin-3-yl)malononitrile (12c). Yield 92%; pink solid; mp 189–191 °C; 1H NMR (500 MHz, CDCl3): δ 8.49 (s, 1H), 7.94 (d, J = 8 Hz, 1H), 7.72 (t, J = 7.5 Hz, 1H), 7.64 (d, J = 8 Hz, 1H), 7.53–7.47 (m, 4H), 7.31–7.26 (m, 3H), 7.13 (d, J = 8 Hz, 1H), 6.94 (d, J = 7.5 Hz, 1H), 5.15 (s, 1H), 3.75–3.65 (m, 2H), 2.29 (s, 3H), 1.84 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.2, 175.6, 150.2, 146.7, 145.2, 138.7, 135.0, 134.2, 133.7, 133.1, 130.8, 130.6, 130.2, 130.1, 130.0, 129.2, 129.0, 128.9, 128.9, 128.8, 128.8, 128.7, 127.4, 127.1, 126.7, 126.6, 126.3, 126.2, 125.2, 124.8, 118.8, 111.3, 111.1, 110.7, 110.3, 51.7, 38.3, 32.1, 30.3, 21.2; HRMS (ESI) m/z calcd for C30H22O2N4 [M + H] 470.1742; found 470.1745.
2-(3-((3-Acetyl-6-chloro-4-phenylquinolin-2-yl)methyl)-1-methyl-2-oxoindolin-3-yl)malononitrile (12d). Yield 91%; blue solid; mp 125–127 °C; 1H NMR (500 MHz, CDCl3): δ 7.81 (d, J = 8.5 Hz, 1H), 7.72 (t, J = 7 Hz, 1H), 7.64 (d, J = 8.5 Hz, 1H), 7.52–7.40 (m, 4H), 7.34–7.30 (m, 3H), 7.10 (t, J = 7.5 Hz, 1H), 7.02 (d, J = 7.5 Hz, 1H), 5.11 (s, 1H), 3.79–3.64 (m, 2H), 3.41 (s, 3H), 1.86 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 205.1, 174.1, 150.2, 146.6, 145.1, 144.4, 134.9, 134.2, 130.5, 130.4, 130.1, 130.0, 129.2, 128.9, 128.8, 127.4, 126.3, 126.1, 125.2, 123.8, 123.3, 111.1, 110.1, 108.9, 51.2, 38.3, 32.1, 30.4, 26.8; HRMS (ESI) m/z calcd for C30H21O2N4Cl [M + H] 504.1725; found 504.1732.
Acknowledgements
SY acknowledge DST-India, for their financial support (SB/FT/CS-149/2012). GS thanks Central University of Rajasthan for a Fellowship.
Notes and references
-
(a) V. R. Solomon and H. Lee, Curr. Med. Chem., 2011, 18, 1488 CrossRef CAS PubMed;
(b) S. Kumar, S. Bawa and H. Gupta, Mini-Rev. Med. Chem., 2009, 9, 1648 CrossRef CAS PubMed.
-
(a) K. Kaur, M. Jain, R. P. Reddy and R. Jain, Eur. J. Med. Chem., 2010, 45, 3245 CrossRef CAS PubMed;
(b) B. N. Acharya, D. Thavaselvam and M. B. Kaushik, Med. Chem. Res., 2008, 17, 487 CrossRef;
(c) K. Chibale, J. R. Moss, M. Blackie, D. Schalkwyk and P. J. Smith, Tetrahedron Lett., 2000, 41, 6231 CrossRef CAS.
-
(a) A. H. Abadi, G. H. Hegazy and A. A. E. Zaher, Bioorg. Med. Chem., 2005, 13, 5759 CrossRef CAS PubMed;
(b) A. Baba, N. Kawamura, H. Makino, Y. Ohta, S. Taketomi and T. Sohda, J. Med. Chem., 1996, 39, 5176 CrossRef CAS PubMed;
(c) Y. Chen, Y. Zhao, C. Lu, C. Tzeng and J. P. Wang, Bioorg. Med. Chem., 2006, 14, 4373 CrossRef CAS PubMed.
- S. Eswaran, A. V. Adhikari, I. H. Chowdhury, N. K. Pal and K. D. Thomas, Eur. J. Med. Chem., 2010, 45, 3374 CrossRef CAS PubMed.
- M. I. F. Bachiller, C. Perez, G. C. G. Munoz, S. Conde, M. G. Lopez, M. Villarroya, A. G. Garcia and M. I. R. Franco, J. Med. Chem., 2010, 53, 4927 CrossRef PubMed.
-
(a) R. C. Bernotas, R. R. Singhaus, D. H. Kaufman, J. Ullrich, H. Fletcher, E. Quinet, P. Nambi, R. Unwalla, A. Wilhelmsson, A. G. Nilsson, M. Farnegardh and J. Wrobel, Bioorg. Med. Chem., 2009, 17, 1663 CrossRef CAS PubMed;
(b) Y. Bi, P. Stoy, L. Adam, B. He, J. Krupinski, D. Normandin, R. Pongrac, L. Seliger, A. Watson and J. E. Macor, Bioorg. Med. Chem. Lett., 2004, 14, 1577 CrossRef CAS PubMed.
-
(a) J. P. Michael, Nat. Prod. Rep., 2002, 19, 742 RSC;
(b) M. N. Bayle, C. Bénard, F. Zouhiri, J.-F. Mouscadet, H. Leh, C.-M. Thomas, G. Mbemba and D. D. Angelo, Bioorg. Med. Chem. Lett., 2005, 15, 4019 CrossRef PubMed;
(c) C. Benard, F. Zouhiri, M. N. Bayle, M. Canet, D. Desmaele, H. Leh, J.-F. Mouscadet, G. Mbemba, C.-M. Thomas, S. Bonnenfant, M. L. Bret and J. Angelo, Bioorg. Med. Chem., 2004, 14, 2473 CrossRef CAS PubMed;
(d) A. Mousnier, H. Leh, J.-F. Mouscadet and C. Dargemont, Mol. Pharmacol., 2004, 66, 783 CrossRef CAS PubMed.
-
(a) B. Qian, S. Guo, J. Shao, Q. Zhu, L. Yang, C. Xia and H. Huang, J. Am. Chem. Soc., 2010, 132, 3650 CrossRef CAS PubMed;
(b) B. Qian, S. Guo, C. Xia and H. Huang, Adv. Synth. Catal., 2010, 352, 3195 CrossRef CAS;
(c) M. Rueping and N. Tolstoluzhsky, Org. Lett., 2011, 13, 1095 CrossRef CAS PubMed;
(d) B. Qian, P. Xie, Y. Xie and H. Huang, Org. Lett., 2011, 13, 2580 CrossRef CAS PubMed;
(e) Y. Yan, K. Xu, Y. Fang and Z. Wang, J. Org. Chem., 2011, 76, 6849 CrossRef CAS PubMed;
(f) G. Wang, S. Li, Q. Wu and S. D. Yang, Org. Chem. Front., 2015, 2, 569 RSC;
(g) N. S. Kumar, L. C. Rao, N. Jagdeeshbabu, V. D. Kumar, U. S. N. Murthy and H. M. Meshram, Synlett, 2015, 26, 1808 CrossRef CAS;
(h) H. Dong, L. Xu, S.-S. Li, L. Wang, C.-L. Shao and J. Xiao, ACS Comb. Sci., 2016, 18, 604 CrossRef CAS PubMed;
(i) N. N. Rao and H. M. Meshram, Tetrahedron Lett., 2013, 54, 5087 CrossRef;
(j) S. Yaragorla, G. Singh and R. Dada, Tetrahedron Lett., 2015, 56, 5924 CrossRef CAS;
(k) S. Yaragorla, G. Singh and R. Dada, Tetrahedron Lett., 2016, 57, 591 CrossRef CAS;
(l) S. Yaragorla, R. Dada and G. Singh, Synlett, 2016, 27, 912 CrossRef CAS;
(m) H. Komai, T. Yoshino, S. Matsunaga and M. Kanai, Org. Lett., 2011, 13, 1706 CrossRef CAS PubMed;
(n) Z. Jamal, Y.-C. Teo and L.-K. Wong, Eur. J. Org. Chem., 2014, 7343 CrossRef CAS.
-
(a) P. Friedländer, Ber. Dtsch. Chem. Ges., 1882, 15, 2572 CrossRef;
(b) J. M. Contelles, E. P. Mayoral, A. Samadi, M. D. C. Carreiras and E. Soriano, Chem. Rev., 2009, 109, 2652 CrossRef PubMed;
(c) M. Barbero, S. Bazzi, Cadamuro and S. Dughera, Tetrahedron Lett., 2010, 51, 2342 CrossRef CAS;
(d) B. V. S. Reddy, A. Venkateswarlu, G. N. Reddy and Y. V. R. Rami Reddy, Tetrahedron Lett., 2013, 54, 5767 CrossRef CAS;
(e) J. S. Yadav, P. P. Rao, D. Sreenu, R. S. Rao, V. N. Kumar, K. Nagaiah and A. R. Prasad, Tetrahedron Lett., 2005, 46, 7249 CrossRef CAS.
- M. Dabiri, P. Salehi, M. Baghbanzadeh and M. S. Nikcheh, Tetrahedron Lett., 2008, 49, 5366 CrossRef CAS.
- D. Kumar, A. Kumar, M. M. Qadri, M. I. Ansari, A. Gautam and A. K. Chakraborti, RSC Adv., 2015, 5, 2920 RSC.
-
(a) A. Dömling and I. Ugi, Angew. Chem., 2000, 39, 3168 CrossRef;
(b) H. R. Benjamin, Z. Serge, R. Vishal and K. Y. Andrei, Chem. Rev., 2014, 114, 8323 CrossRef PubMed.
-
(a) R. Dada, G. Singh, A. Pareek, S. Kausar and S. Yaragorla, Tetrahedron Lett., 2016, 57, 3739 CrossRef CAS;
(b) S. Yaragorla, A. Pareek, R. Dada, A. I. Almansour and N. Arumugam, Tetrahedron Lett., 2016, 57, 5841 CrossRef CAS;
(c) S. Yaragorla, A. Pareek, R. Dada and G. Singh, RSC Adv., 2016, 6, 28865 RSC;
(d) A. Pareek, R. Dada, M. Rana, A. K. Sharma and S. Yaragorla, RSC Adv., 2016, 6, 89732 RSC;
(e) G. Singh, R. Dada and S. Yaragorla, Tetrahedron Lett., 2016, 57, 4424 CrossRef CAS;
(f) S. Yaragorla, A. Pareek and R. Dada, Tetrahedron Lett., 2015, 56, 4770 CrossRef CAS.
- Though the other conditions found to be reasonably satisfactory for Friedlander synthesis, only entry 4, Table 1 was found to be the best condition for the next step (conjugate addition).
- See ESI† for more details about optimization of reaction conditions and copies of spectra.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra28642a |
|
| This journal is © The Royal Society of Chemistry 2017 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *ab
*ab