Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Microwave-assisted hydrolysis of biomass over activated carbon supported polyoxometalates†
S. Tsubaki *a,
K. Oonob,
A. Ondab,
T. Uedac,
T. Mitanid and
M. Hiraokae
*a,
K. Oonob,
A. Ondab,
T. Uedac,
T. Mitanid and
M. Hiraokae
aDepartment of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, 2-12-1, Ookayama, Meguro, Tokyo, 152-8550, Japan. E-mail: shuntaro.tsubaki@gmail.com; tsubaki.s.aa@m.titech.ac.jp
bResearch Laboratory of Hydrothermal Chemistry, Faculty of Science, Kochi University, Akebono-cho 2-5-1, Kochi 780-8520, Japan
cDepartment of Marine Resource Science, Faculty of Agriculture and Marine Sciences, Kochi University, Akebono-cho 2-5-1, Kochi, Japan
dResearch Institute for Sustainable Humanosphere, Kyoto University, Gokasho, Uji, 611-0011, Japan
eUsa Marine Biological Institute, Kochi University, Inoshiri, Usa, Tosa, Kochi 781-1164, Japan
First published on 21st February 2017
Abstract
Activated carbon supported polyoxometalates (AC-POMs) were used for acceleration of hydrolysis of biomass under microwave irradiation. Microwaves exhibited a higher saccharification rate for cellobiose and green seaweed (Monostroma latissimum) over AC-POMs than conductive heating by 1.25 to 1.55-fold. The activity of the AC-POMs was positively correlated with the dielectric loss of the catalysts, suggesting that microwave susceptibility of AC-POMs is relevant to the enhancement of catalysis under microwave irradiation.
Hydrolysis of biomass is one of the key technologies for the production of bio-fuels, bio-materials, and bio-chemicals as well as biologically functional chemicals.1 Heterogeneous catalysts have been paid great attention in the green conversion of biomass since they are less hazardous than conventional mineral acids, and do not require neutralization.2 For instance, sulfonated carbons effectively hydrolyze crystalline cellulose as water-tolerant solid acids.3
Microwave irradiation is widely used for accelerating organic and inorganic reactions,4 materials processing,5 environmental treatment6 and biomass treatment.7 Microwave irradiation is capable of selectively heating microwave sensitizing materials by dielectric and conduction loss mechanisms.8 Solid catalysts such as carbon supported Pt and carbon-filled zeolite can be selectively heated in less microwave susceptive reaction media to enhance heterogeneous reactions by generating local hot spots in the vicinity of the solid catalysts.9 A combination of microwaves and a heterogeneous catalyst is, therefore, expected to be effective for enhancing biomass conversion processes.10
Polyoxometalates (POMs) are well-known for super-strong acid and oxidation activities with less corrosivity than mineral acids.11 POMs have been applied for hydrolysis of crystalline cellulose,12 delignification of lignocellulosic biomass for paper industry,13 oxidation of cellulose and hemicellulose14 and dehydration of hexose to 5-hydroxymethyl furfural.15 Previously, we have reported that homogeneous POMs facilitate hydrolysis of polysaccharides (starch and crystalline cellulose) and green seaweed (Ulva spp.) under microwaves.16 POMs exhibited higher activity than conventionally used mineral acids such as HCl and H2SO4. For greener reaction, however, heterogeneous POMs are preferred for easier separation and recyclability.
Solid POMs such as Cs+ salts and supported POMs have been used for catalyst, energy storage and sensing applications.11,17 Activated carbon (AC) is one of the useful catalyst support as well as microwave susceptors.9 Previously, we have reported that several types of ACs were effective for hydrolysis of corn starch with reduction in the reaction temperature.18 In the present study, microwave-susceptible solid acid catalysts were prepared by supporting POMs on ACs and used to study the effects of microwave generated local hot spot at the solid acid catalyst.
Microwave-susceptibility of susceptors were first compared among AC (type A, Table 1), silicon carbide (SiC) and yttria stabilized zirconia (YSZ) which are typically used microwave susceptors. These susceptors (500 mg) were suspended in water (10 mL) and microwaved by multimode microwave reactor START-D (2.45 GHz, Milestone, Inc.) equipped with PTFE high-pressure reactor and PID temperature control connected to the thermocouple thermometer. Microwave susceptibility of the supports were evaluated by the microwave energy consumption monitored by the START-D controller. ACs exhibited the best microwave susceptibility and reduced microwave energy consumption down by 39% from that of pure water (Fig. 1).
| Type | Description of ACs | Supplier |
|---|---|---|
| A | Fracture type activated carbon | Wako Pure Chemical Industries Ltd. |
| B | Bone char | |
| C | SX II | |
| D | SX PLUS | |
| E | SD | Ajinomoto Fine-Techno Co., Inc. |
| F | SHIRASAGI™ P | Osaka Gas Chemicals Co., Ltd. |
| G | SHIRASAGI™ FAC |
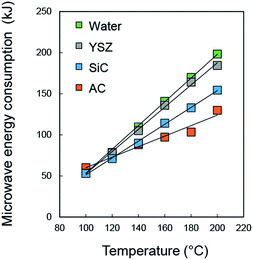 | ||
| Fig. 1 Comparison of microwave energy consumption of aqueous suspension of AC (type A), SiC and YSZ under hydrothermal condition (100–200 °C). | ||
Silicotungstate (SiW) was, then, supported on 7 kinds of ACs (Table 1) by the wet impregnation method according to Kumar et al.19 Namely, ACs were suspended in 50 mM methanol solution of SiW and shaken overnight, then, methanol was removed by evaporation. Where indicated, ACs were pretreated with 1 wt% aqueous solutions of HCl, LiCl, NaCl, CsCl, KCl, tetrabutyl ammonium bromide (TBABr), NH4Cl, and aqueous HNO3 solutions (10% and 20%) overnight to stabilize POM anions on ACs. The pretreatment solutions were thoroughly washed with water before supporting SiWs.
The aqueous suspension of POM-impregnated ACs were, then, repeatedly washed under hydrothermal condition using microwaves (START-D microwave oven, 200 °C for 10 min) to remove loosely adsorbed POMs. Subsequently, they were thoroughly washed with water and dried at 250 °C for 6 hours to obtain AC-SiW. The amount of SiW supported on ACs were also described as a function of size and BET specific surface area (Fig. 2). ACs larger than 40 μm × 20 μm (types D–G) were found effective for supporting SiW. Similarly, the BET specific surface area and micropore volume above 800 m2 g−1 and 1200 m2 g−1 were sufficient for supporting SiW, respectively.
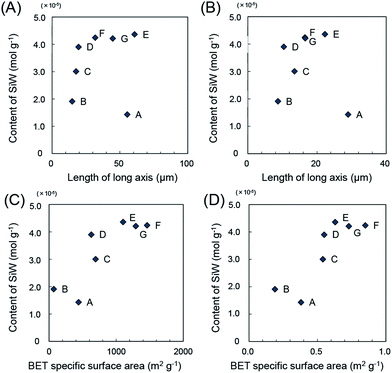 | ||
| Fig. 2 The amount of SiW supported on ACs as functions of (A and B) size of ACs and (C and D) BET nitrogen adsorption properties. Alphabets in the figures show the types of ACs as shown in Table 1. | ||
Several kinds of AC-SiW were further prepared by using types D, E, F with several pretreatments (Table 2). AC-SiWs contained 2.79 × 10−5 mol g−1 to 5.12 × 10−5 mol g−1. The amounts of acid sites of AC-POMs were, then, determined by the titration method. Namely, the AC-POMs were suspended in 10 mM NaOH and stirred for 2 hours. The AC-POMs were removed by centrifugation. The supernatant (10 mL) was titrated with 10 mM HCl. The amounts of acid sites of AC-SiWs attained 5.00 × 10−4 to 3.40 × 10−3 mol g−1 as HCl equivalent (Table 2).
| POM–pretreatment (type of AC) | Amount of SiW (mol g−1) | Amount of acid sitesa (mol g−1) |
|---|---|---|
| a HCl equivalent acid sites. | ||
| SiW–none (type D) | 3.90 × 10−5 | 5.00 × 10−4 |
| SiW–HCl (type D) | 4.52 × 10−5 | 1.82 × 10−3 |
| SiW–LiCl (type D) | 4.92 × 10−5 | 1.19 × 10−3 |
| SiW–NaCl (type D) | 4.49 × 10−5 | 1.90 × 10−3 |
| SiW–KCl (type D) | 5.12 × 10−5 | 2.13 × 10−3 |
| SiW–CsCl (type D) | 3.77 × 10−5 | 2.07 × 10−3 |
| SiW–TBABr (type D) | 4.70 × 10−5 | 2.23 × 10−3 |
| SiW–NH4Cl (type D) | 3.66 × 10−5 | 2.38 × 10−3 |
| SiW–HNO3 10% (type D) | 3.69 × 10−5 | 1.61 × 10−3 |
| SiW–HNO3 20% (type D) | 2.79 × 10−5 | 1.74 × 10−3 |
| SiW–none (type E) | 4.24 × 10−5 | 3.09 × 10−3 |
| SiW–HCl (type E) | 4.78 × 10−5 | 3.08 × 10−3 |
| SiW–none (type F) | 4.21 × 10−5 | 3.40 × 10−3 |
Cellobiose was used as model biomass substrate for testing catalysis of AC-SiWs. Cellobiose (100 mg) were suspended in pure water (10 mL) with AC-POM (100 mg) and hydrolyzed under microwaves using START-D as mentioned above. The microwave reaction was operated at 180 °C for 10 min with 8 min of heating-up time. The generation of glucose was monitored by HPLC (LC-2000 system, JASCO) equipped with a column of Shodex SC1011 (7.8 mm I.D × 300 mm, Showa Denko K.K.) with eluent of water at 1 mL min−1 of flow rate and RI detection. Saccharification rate was calculated as a follows;
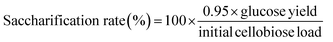 | (1) |
The amount of SiW leaching was monitored by ICP-OES. The saccharification rate attained 30.7% to 54.7% depending on the pretreatment of the catalyst (Table 3). The amounts of leached SiW were maintained below 10 μM, however, oxidation of AC by HNO3 gave higher SiW leaching than other pretreatments (Table 3). The amount of leached SiW attained 4.6% (SiW–LiCl) to 22% (SiW–HNO3 10% and SiW–HNO3 20%) of the immobilized SiW (Table S1†).
| POM–pretreatment (type of AC) | Saccharification rate (%) | Amount of leached SiW (μM) |
|---|---|---|
| SiW–none (type D) | 40.7 | 6.4 |
| SiW–HCl (type D) | 43.3 | 6.3 |
| SiW–LiCl (type D) | 39.8 | 5.0 |
| SiW–NaCl (type D) | 35.7 | 5.6 |
| SiW–KCl (type D) | 38.8 | 6.1 |
| SiW–CsCl (type D) | 34.2 | 5.0 |
| SiW–TBAB (type D) | 35.3 | 4.0 |
| SiW–NH4Cl (type D) | 37.4 | 5.2 |
| SiW–HNO3 10% (type D) | 35.1 | 12 |
| SiW–HNO3 20% (type D) | 30.7 | 16 |
| SiW–none (type E) | 46.5 | 12 |
| SiW–HCl (type E) | 54.7 | 8.0 |
| SiW–none (type F) | 44.4 | 14 |
The activities of AC-SiWs were, then, compared under microwave and conductive heating under the same thermal history using induction oven (SSN-400, Shikoku Rika. Co., Kochi, Japan).16 Microwaves gave higher saccharification rate than induction heating by 1.25- to 1.45-fold without change in the amount of leached SiW during reaction (Fig. 3).
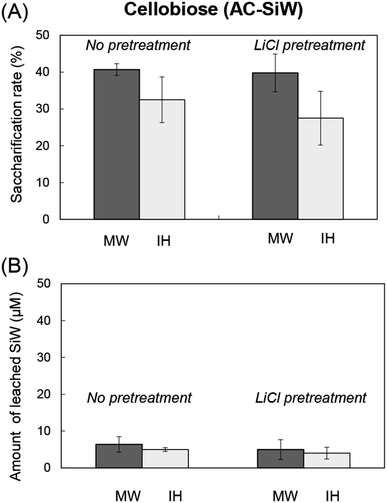 | ||
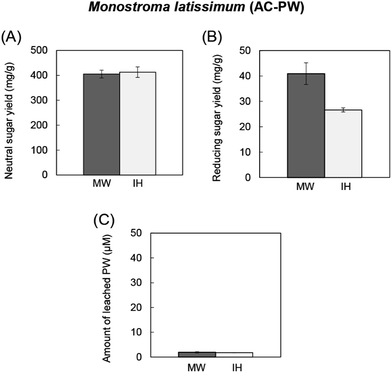
| Fig. 3 Comparison of microwave (MW) and induction heating (IH) for hydrolysis of cellobiose over AC-SiW. (A) Saccharification rate of cellobiose and (B) amount of leached SiW during reaction. | ||
In addition, the effects of AC-supported phosphotungstic acid (AC-PW) were tested for hydrolysis of green seaweed biomass (Monostroma latissimum). AC-PW was prepared by the same procedure for AC-SiW using type A activated carbon without any pretreatment. Dried and powdered M. latissimum (500 mg) and AC-PW (500 mg) were suspended in distilled water (10 mL) and microwaved at 140 °C and 10 min. The amount of generated total sugar (including both native polysaccharide and hydrolyzed sugars) were determined by the phenol sulfuric acid. The amount of reducing sugars generated after hydrolysis were determined by the DNS method.
Hydrolysis of M. latissimum over AC-PW produced 400–420 mg of total sugars by both microwave and induction heating (Fig. 4A). Microwaves, however, exhibited higher yield in reducing sugar than the induction heating by 1.55-fold (Fig. 4B) with no difference in PW leaching (Fig. 4C). The results indicated that hydrolysis of M. latissimum was enhanced under microwaves to produce lower molecular reducing sugars such as rhamnan sulfate oligosaccharides. The hydrolyzed polysaccharide (rhaman sulfate) of M. latissimum are expected for their biological effects such as anticoagulant activity and anti-herpetic effect.20
These results demonstrated that AC-POMs exhibit higher saccharification of carbohydrates under microwaves than conductive heating. Direct heating of AC-POM catalysts due to microwaves probably generated local hot spot at the solid catalyst to enhance hydrolysis of biomass. To understand the mechanism of energy transfer from microwaves to AC-POM, their complex dielectric constants in water were further examined using the coaxial probe method in the same concentration as the cellobiose hydrolysis (100 mg in 10 mL). Dielectric loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) at 2.45 GHz was calculated from the dielectric constant using the following equation;
δ) at 2.45 GHz was calculated from the dielectric constant using the following equation;
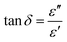 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ), acid sites as well as the amount of leached SiW (Fig. 4).
δ), acid sites as well as the amount of leached SiW (Fig. 4).
Relative permittivity, dielectric loss and dielectric loss tangent exhibited positive correlations with cellobiose saccharification indicating that complex dielectric properties of AC-POMs are one of the key parameter for catalysis under microwaves (Fig. 5A–C). Stronger microwave absorption by AC-SiWs can generate higher temperature at the catalyst. We assume that higher saccharification rate can be obtained due to the higher local hot spot generated by stronger microwave absorption of the catalyst. Another key parameter is the acidity of the AC-SiWs. The increase in the amount of acid sites also facilitated saccharification of cellobiose (Fig. 5D) without significant correlation with the amount of leached SiW (Fig. 5E).
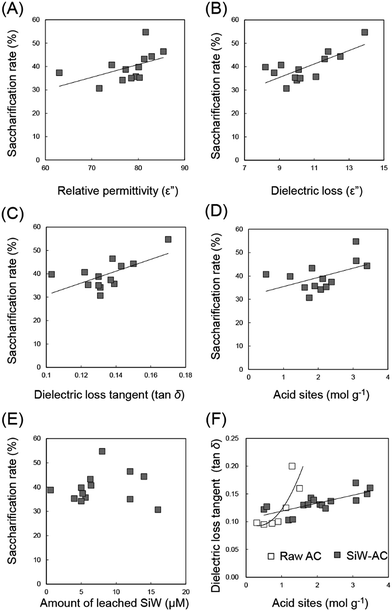 | ||
| Fig. 5 Correlations among dielectric properties of AC-SiW, saccharification rate of cellobiose, acid sites and the amount of leached POM. | ||
In addition, the dielectric loss tangent of AC-SiW positively correlated with the amount of acid sites (Fig. 5F). This could be due to enhanced dielectric loss due to ionic conduction of protons at the acid sites. The ionic conduction of electrolytes are one of the important microwave absorption mechanisms for heating materials.8 Because protons exhibit significantly higher ion conductivity than other ions by the Grotthuss mechanism,21 the ionic conduction of proton at the acid sites can improve the dielectric loss of the catalyst. The same phenomenon was also observed for raw ACs depending on the amount of acidic functional groups, such as hydroxyl and carboxyl groups, originally existing on the carbon surface (Fig. 5F). The microwave susceptibility of AC-POMs was, therefore, originated from the synergy of dielectric loss of AC support as well as ionic conduction of protons of the acid sites.
Conclusions
In this study, the effects of microwave local heating were investigated on hydrolysis of biomass using solid POMs. AC supports were used since they exhibit high microwave susceptibility. AC-POMs were, then, fabricated by the wet impregnation method. Microwaves exhibited higher saccharification rate of cellobiose over AC-SiW than induction heating by 1.25- to 1.45-fold. AC-PW also generated higher reducing sugars under microwave than induction heating by 1.55-fold. Positive correlation was observed between dielectric loss of AC-SiW and saccharification rate. The results indicated that direct and selective heating of AC-POMs facilitates hydrolysis of biomass under microwaves by generating local hot spot at the solid catalyst.Acknowledgements
This study was supported by the Adaptable and Seamless Technology Transfer Program (A-STEP) through Target-driven R&D (Exploratory Research), Japan Science and Technology Agency (AS242Z03337N), Grant-in-Aid for Young Scientists B Japan Society for the Promotion of Science (15K18814) and the Japan Prize Foundation. The dielectric measurements were conducted through Analysis and Development System for Advanced Materials (ADAM) at the Research Institute for Sustainable Humanosphere, Kyoto University.Notes and references
- A. Sun and J. Cheng, Bioresour. Technol., 2002, 83, 1 CrossRef PubMed.
- R. Rinaldi and F. Schüth, Energy Environ. Sci., 2009, 2, 610 CAS.
- M. Hara, T. Yoshida, A. Takata, J. N. Konda, S. Hayashi and L. Domen, Angew. Chem., Int. Ed., 2004, 43, 2955 CrossRef CAS PubMed; A. Onda, T. Ochi and K. Yanagisawa, Green Chem., 2008, 10, 1033 RSC.
- A. Loupy, Microwaves in organic synthesis, Wiley-VCH Verlag GmbH & Co., KGaA, Weinheim. 2006 Search PubMed.
- Y. V. Bykov, K. I. Rybakov and V. E. Semenov, J. Phys. D: Appl. Phys., 2001, 34, R55 CrossRef CAS.
- D. A. Jones, T. P. Lelyveld, S. D. Mavrofidis, S. W. Kingman and N. J. Miles, Resour., Conserv. Recycl., 2002, 34, 75 CrossRef.
- S. Tsubaki, T. Ueda and A. Onda, in Microwaves in Catalysis, Wiley-VCH Verlag GmbH & Co., KGaA, Weinheim, 2015 Search PubMed.
- C. Gabriel, S. Gabriel, E. H. Grant, B. S. J. Halstead and D. M. P. Mingos, Chem. Soc. Rev., 1998, 27, 213 RSC.
- S. Horikoshi and N. Serpone, Catal. Sci. Technol., 2014, 4, 1197 Search PubMed; D. Mochizuki, R. Sasaki, M. Maitani, M. Okamoto, E. Suzuki and Y. Wada, J. Catal., 2015, 323, 1 CrossRef CAS.
- Y. B. Huang and Y. Fu, Green Chem., 2013, 15, 1095 RSC.
- I. V. Kozhevnikov, Chem. Rev., 1998, 98, 171 CrossRef CAS PubMed.
- K. I. Shimizu, H. Furukawa, N. Kobayashi, Y. Itaya and A. Satsuma, Green Chem., 2009, 11, 1627 RSC.
- I. A. Weinstock, R. H. Attala, R. S. Reiner, M. A. Moen, K. E. Hammel, C. J. Houtman, C. L. Hill and M. K. Harrup, J. Mol. Catal. A: Chem., 1997, 116, 59 CrossRef CAS.
- B. B. Sarma and R. Neumann, Nat. Commun., 2014, 5, 4621 CAS.
- C. Fan, H. Guan, H. Zhang, J. Wang, S. Wang and X. Wang, Biomass Bioenergy., 2011, 35, 2659 CrossRef CAS.
- S. Tsubaki, K. Oono, T. Ueda, A. Onda, K. Yanagisawa, T. Mitani and J. Azuma, Bioresour. Technol., 2013, 144, 67 CrossRef CAS PubMed; S. Tsubaki, K. Oono, M. Hiraoka, T. Ueda, A. Onda and K. Yanaigsawa, Green Chem., 2014, 16, 2227 RSC.
- T. Okuhara, Chem. Rev., 2002, 102, 3641 CrossRef CAS PubMed; Y. Wang and I. A. Weinstock, Chem. Soc. Rev., 2012, 41, 7479 RSC; Y. Ji, L. Huang, J. Hu, C. Streb and Y.-F. Song, Energy Environ. Sci., 2015, 8, 776 Search PubMed.
- A. Matsumoto, S. Tsubaki, M. Sakamoto and J. Azuma, Bioresour. Technol., 2011, 102, 3985 CrossRef CAS PubMed.
- V. B. Kumar, I. N. Pulidinim and A. Gedanken, Renewable Energy, 2015, 78, 141 CrossRef CAS.
- S. L. Holdt and S. Kraan, J. Appl. Phycol., 2011, 23, 543–597 CrossRef CAS.
- S. Tsubaki, M. Hiraoka, S. Hadano, K. Okamura, T. Ueda, H. Nishimura, K. Kashimura and T. Mitani, Carbohydr. Polym., 2015, 115, 78 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra28778f |
| This journal is © The Royal Society of Chemistry 2017 |