Open Access Article
Open Access ArticleUpconversion luminescence of Sm2+ ions
Xiaohui Liua,
Tuerxun Aidilibikeab,
Junjie Guoa,
Yangyang Lia,
Weihua Dia and
Weiping Qin *a
*a
aState Key Laboratory on Integrated Optoelectronics, College of Electronic Science & Engineering, Jilin University, Changchun, Jilin 130012, China. E-mail: wpqin@jlu.edu.cn
bYili Normal University, Electronic and Information Engineering, Yining, Xinjiang 835000, China
First published on 3rd March 2017
Abstract
Herein, we report the phenomenon of upconversion luminescence from Sm2+ ions, which demonstrates that changeable valence lanthanides can serve as ions for optical frequency transformation. Upon excitation with a 980 nm diode laser, the doped Sm2+ ions in the hybrid material BaFCl0.5Br0.5:1%Sm2+–CaF2:1%Yb3+ emit red upconversion fluorescence peaks at 631 nm, 644 nm, 665 nm, 689 nm, 704 nm, and 729 nm from the 5D0,1 → 7F0,1,2 transitions. By transient dynamic analysis, we attributed the excitation of Sm2+ ions to the cooperation energy transfer process, in which two excited Yb3+ ions simultaneously transfer their energy to one Sm2+ ion.
Introduction
Upconversion luminescence (UCL) refers to a nonlinear optical process in which the sequential absorption of two or more photons leads to the emission of light at a shorter wavelength than the excitation wavelength (anti-Stokes type emission). Since the 1960s, optical frequency upconversion (UC) has been developed as a luminescence technology. The most important evolution of UC focuses on visible (VIS) UCL including red, green, and blue.1–6 Owing to their excellent spectroscopic properties, lanthanide (Ln)-doped UCL materials have gained significant attention in the fields of 3D displays, light-emitting devices, biomarkers, bioassays, solid lasers, light-emitting diodes (LEDs), NIR photocatalysis, and temperature sensors.7–16 In general, trivalent lanthanide ions, such as Tm3+, Er3+, Tb3+, and Ho3+, usually act as activators in UC materials, and Yb3+ ions with a large absorption cross-section in the NIR region17,18 act as popular sensitizers to increase optical absorption.19,20 In the past few decades, researchers used trivalent lanthanides with stable valence states in various UCL materials, and to date, almost no reports have been found on UCL from a divalent lanthanide ion or a changeable valence lanthanide, except for the study reporting UCL in Tm2+ materials in 2006 by Hans U. Güdel et al.21 However, changeable valence lanthanides may produce more abundant wavelengths beyond those from trivalent lanthanides.As a popular changeable valence lanthanide ion, Sm2+ has been heavily studied in the past few decades due to its red downconversion (DC) luminescence as well as its ability in optical storage.22–25 Sm2+ has a similar electronic structure to that of Eu3+ and emits mainly bright red fluorescence in a downconversion way. Since the persistent spectral hole burning (PSHB) based on Sm2+ ions was reported, Sm2+-doped fluoride halide mixed crystals have been exhibiting fascinating potential application in ultrahigh density optical data storage.26–28 However, Sm2+ ion has never been considered as an UCL ion working in the regime of NIR excitation because it cannot be excited by a NIR photon or sensitized by an excited Yb3+ ion. In principle, two-photon absorption can possibly lead to the UCL of Sm2+ ions. However, to date, this has not been achieved because of the low efficiency (η = ∼10−13) of two-photon absorption.29 Compared with two-photon absorption, the cooperative energy transfer (CET) from a Yb3+-dimer to another lanthanide ion is more efficient (η = ∼10−6).30 CET is the physical process in which two identical excited ions simultaneously exhibit transitions and transfer energy to another ion. Cooperative transition, which is closely associated with clustered lanthanide ions in solid hosts, involves cooperative luminescence (CL), cooperative absorption (CA), and CET. Clusters of lanthanide ions, such as Yb3+-dimers, can easily form in the alkaline-earth fluoride crystal AF2 (A = Ca, Sr, and Ba), depending on the type of lanthanide ion and its doping concentrations.31,32 In ref. 30, the Yb3+ emission is a featureless band peaking at 497 nm, whereas a much more complex band with at least five individual peaks centered at around 520 nm was observed in the materials mentioned in this study. Calcium fluoride consists of a simple cubic lattice of fluorine ions in which every other body center position is occupied by a Ca2+ ion. Yb3+ ions occupy the Ca2+ sites when they are introduced into the CaF2 lattice. Cooperative sensitization based on Yb3+-dimers can excite Sm2+ ions by bridging the energy gap between the ground state and excited state of Sm2+ ions, yet it has not been performed to date.
The main reason for this should be that it is a real challenge to prepare a material simultaneously containing Yb3+ and Sm2+ ions using the conventional solid state reduction reaction. Samarium is a lanthanide that usually takes on a trivalent state, and an intense reduction reaction is needed to change it from the trivalent state to the divalent state. However, trivalent ytterbium has a similar reduction-oxidation potential as that of trivalent samarium; thus, it will also be reduced to the bivalent state under the same conditions for the reduction of trivalent samarium ions. Therefore, a reasonable synthetic route should be considered for the coexistence of Sm2+ ions and Yb3+ ions in a material.
Herein, we synthesized a hybrid material containing the Yb3+-dimer and Sm2+ ions via a stepwise synthetic reaction and report the first experimental observation of UC emissions from Sm2+ ions upon 980 nm excitation.
Experimental
Yb3+-doped CaF2 and Sm2+-doped BaFCl0.5Br0.5 were prepared via high temperature solid state reactions in argon and reducing atmospheres, respectively. Stoichiometric amounts of the raw materials CaF2 and YbF3 (1 mol%) were thoroughly mixed by grinding and heated to 1200 °C for 2 h in an Ar atmosphere. Sm3+-doped BaFCl0.5Br0.5 was prepared in the same way but in a H2 reducing atmosphere. A suitable melting temperature is the key to ensure a close enough distance between Yb3+ and Sm2+ ions and achieve efficient energy transfer from the Yb3+-dimers to Sm2+ ions. To make this a reality, the prepared powders BaFCl0.5Br0.5:1%Sm2+ and CaF2:1%Yb3+ were mixed in the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and annealed at 900 °C in an Ar atmosphere for 10 min to obtain the final hybrid material BaFCl0.5Br0.5:1%Sm2+–CaF2:1%Yb3+.
1 and annealed at 900 °C in an Ar atmosphere for 10 min to obtain the final hybrid material BaFCl0.5Br0.5:1%Sm2+–CaF2:1%Yb3+.
Characterization
A Rigaku RU-200b X-ray powder diffractometer (XRD) was used to analyze the crystal structures using nickel-filtered Cu-Kα radiation (λ = 0.15405 nm) in the range of 10° ≤ 2θ ≤ 70°. The accelerating voltage was 40 kV, and the emission current was 200 mA. Absorption spectra in the UV to NIR region were obtained using a Shimadzu UV3600 spectrophotometer in the range of 200–1100 nm. Excitation and emission spectra were obtained using a Hitachi F-4500 spectrometer equipped with a Hamamatsu R928 photomultiplier (PMT) and a 2 W 980 nm continuous wave diode laser as the excitation source. Luminescence spectra were obtained using a one-meter monochromator (SPEX 1000M; HORIBA Jobin Yvon Inc., Edison, NJ, USA) equipped with an 1800 line mm−1 grating. The excitation light source was a power adjustable continuous wave laser diode (978 nm, 10 W; BWT Beijing Ltd, Beijing, China). Spectral measurements at low temperature were performed using a helium-cycled cryostat (ARS-2HW; Advanced Research Systems, Macungie, PA, USA). A digital oscilloscope (DPO4104B, bandwidth 1 GHz, sampling rate 5 GSs−1; Tektronix, Shanghai, China), a power-adjustable continuous wave laser diode (CW978 nm, 10 W), and a chopper were used to obtain the decay curves.Results and discussion
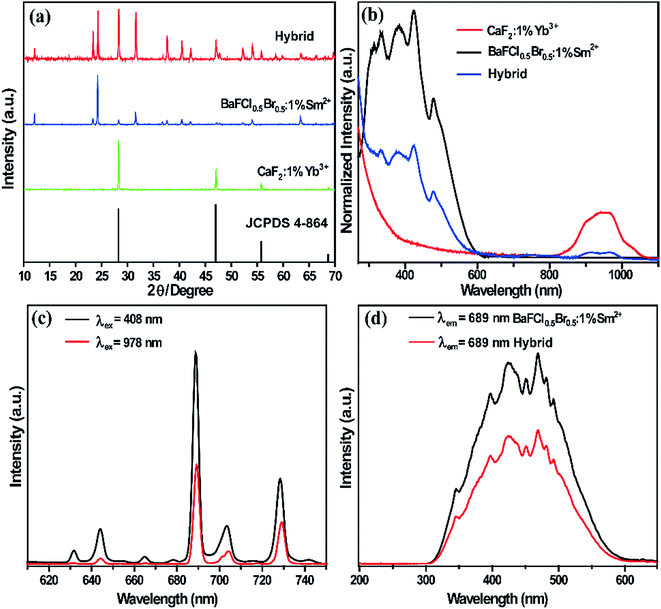
The composition and phase purity of the products were first examined by XRD. It is well known that CaF2 has a cubic crystal structure and its lattice parameter values are a = 5.463 nm, b = 5.463 nm, and c = 5.463 nm. The blue line in Fig. 1a shows the XRD patterns of the as-prepared CaF2:1%Yb3+. All the patterns can be indexed to the pure cubic phase of CaF2 (JCPDS 4-864) and no impurity peaks were observed. There is no standard card corresponding to the sample BaFCl0.5Br0.5:1%Sm2+ since it is a fluoride halide-mixed crystal (Fig. 1a, red line). According to the XRD patterns of the products, neither impurity peaks nor a second phase can be detected at the current mixing level. This clearly implies that the doping of Yb3+ and Sm2+ ions does not cause any significant change in the host structure.The absorption spectra of obtained powder samples, with the characteristic bands of Sm2+ ions and Yb3+ ions in the UV-vis and the near-infrared (NIR) regions, respectively, reveal the existence of both Sm2+ and Yb3+ ions, as shown in Fig. 1b. The absorption band at around 950 nm is attributed to the electronic transition from the 2F7/2 ground state to the 2F5/2 excited states of Yb3+ ions. For the samples doped with Sm2+ ions, an obvious broad absorption band with four peaks (black and blue curves) appears in the UV-vis region, which are assigned to the 4f5d bands of Sm2+ ions. The emission spectrum of BaFCl0.5Br0.5:1%Sm2+–CaF2:1%Yb3+ was obtained under 408 nm excitation, which is shown as the black line in Fig. 1c. The emissions consist of several sharp peaks in the region from 600 to 750 nm, which are assigned to the 5D0 → 7Fj (j = 0, 1, and 2) and 5D1 → 7Fj (j = 0, 1, and 2) transitions of Sm2+ ions. It is well-known that the 689 nm emission from the 5D0 → 7F0 transition of Sm2+ ions is efficient and well isolated. To understand the origin of the emissions, the excitation spectra of the 4f5d bands were obtained by monitoring the transition of 5D0 → 7F0 (689 nm), as shown in Fig. 1d. It can be seen that there are four main peaks in the 4f5d band. The half diffraction peak of the emission 5D0 → 7F0 is located at about 345 nm. The UC emissions of BaFCl0.5Br0.5:1%Sm2+–CaF2:1%Yb3+ was obtained under 978 nm excitation, which is shown as the red line in Fig. 1c. The emission curve is almost the same as that in the DC spectrum of BaFCl0.5Br0.5:1%Sm2+ without Yb3+ ions, indicating that two different ways of excitation can induce the emissions of Sm2+ ions. Therefore, it is reasonable to attribute the UC emission peaks at 631 nm, 644 nm, 665 nm, 689 nm, 704 nm, and 729 nm to the 5Di (i = 0, 1) → 7Fj (j = 0, 1, 2) transitions of the Sm2+ ion.
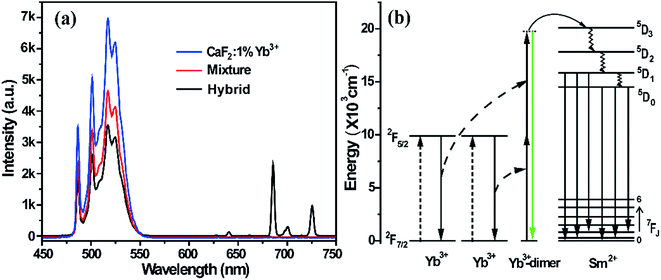
Under the pumping of an NIR laser, a pair of Yb3+ ions (Yb3+-dimer) were both exited from the 2F7/2 to 2F5/2 level, and then depopulated from the excited state 2F5/2 simultaneously to emit a visible photon, which is called cooperative luminescence. Fig. 2a shows the photoluminescence spectrum of the mixture, hybrid, and CaF2:1%Yb3+ under 980 nm excitation. A broad band ranging from 470 to 570 nm is observed, which originates from the cooperative luminescence of the Yb3+-dimers. It was found that this broad band is composed of a few sharp emission peaks connected with each other. It is well-known that the 2F5/2 to 2F7/2 levels of Yb3+ ions split into three and four Stark components in a tetragonal crystal field, respectively.33 Therefore, a random combination of two Stark components in the cooperative luminescence of a Yb3+-dimer forms emissions located at different energies but close to each other.34 These approaching emission peaks are connected to form a broad band.
As shown in Fig. 2a and 1d, there is obvious spectral overlap between the emission of the Yb3+-dimers and the excitation of Sm2+ ions. Radiation reabsorption may happen when the excitation and emission spectra overlap. However, there is no UCL in the physical mixture of BaFCl0.5Br0.5:1%Sm2+ and CaF2:1%Yb3+, which indicates that the mechanism of radiation reabsorption does not play a main role in the process of UCL of Sm2+ ions. In contrast, the emissions of Yb3+-dimers are still very strong in the hybrid material, and Yb3+-dimers have a partial radiative transition accompanied with CL, thus demonstrating that only some of the energy of the Yb3+-dimers transfers to Sm2+ ions and realizes the UCL of Sm2+ ions. Fig. 2b schematically describes the possible UC processes in the energy level diagrams of the cooperation energy transfer from Yb3+ to Sm2+ ions. The large energy gap between the ground state and the excited state in Sm2+ ions requires the cooperation sensitization of Yb3+-dimers because there is no intermediate level in Sm2+ that can be resonant with a 980 nm photon or an excited Yb3+ ion. Under the NIR laser excitation, an Yb3+-dimer simultaneously absorbs two NIR photons and then transfers their energy to a Sm2+ ion through a cooperative sensitization process to promote it to the state of 5D3. The lower energy levels 5D0,1 can be populated through a series of non-radiative relaxations from the higher 5D3 state.
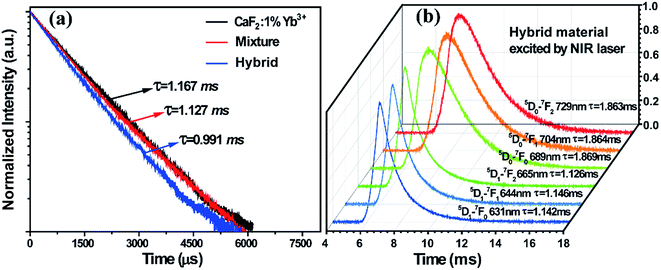
To further confirm the CET from the Yb3+-dimers to Sm2+ ions, the excited state dynamics was investigated under 978 nm excitation by monitoring the emission of Yb3+-dimers at 500 nm, as shown in Fig. 3a. The decay time of the Yb3+-dimers decreases in the hybrid material, indicating that CET occurred between the Yb3+-dimers and Sm2+ ions. It can be seen that these decay curves have no rising edge, which means that the CL of the Yb3+-dimers originates from the direct excitation of 978 nm and no energy transfer process exists. However, the UCL of Sm2+ ions shows a rising edge, as shown in Fig. 3b, which indicates that the energy that stimulates Sm2+ ions originates from the energy transfer of the Yb3+-dimers. The decay times were measured at around 1.13 ms and 1.86 ms for the 5D1 → 7F0,1,2 and 5D0 → 7F0,1,2 transitions of Sm2+ ions, respectively. The decay parts of these curves can be fitted well into a single-exponential function as I = I0 exp(−t/τ) (I0 is the initial emission intensity at t = 0, and τ is the lifetime), demonstrating the populating processes as discussed above.
To fully analyse the nature of the UC processes, the dependence of the integral luminescence intensities on the pumping laser power (at 978 nm) was examined. For an unsaturated UC process, the integrated UCL intensity If is proportional to Pn, where, P denotes the pumping power, and the exponent n represents the number of photons involved in populating the upper emitting state in the UC process. Fig. 4 shows the typical double-logarithmic plots of UCL intensities versus the pump power densities. The values of the photon number n are listed in Table 1. It can be seen from the table that these transitions are of two-photon UC processes. This result corresponds to the analysis of the UC population and emission processes in the schematic energy level diagram of the Sm2+ ion and Yb3+-dimer.
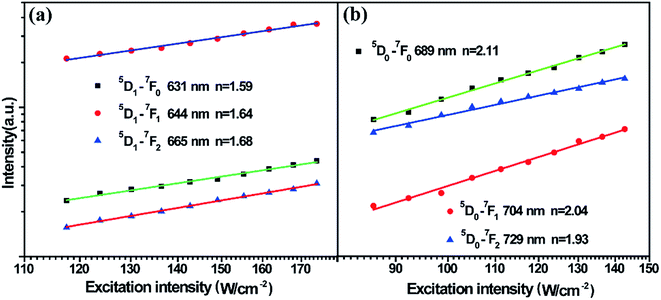 | ||
| Fig. 4 (a) Excitation power dependence of the UCL of 5D1→ 7Fj (j = 0, 1, 2) in Sm2+ ions. (b) Excitation power dependence of UCL of 5D0 → 7Fj (j = 0, 1, 2) in Sm2+ ions. | ||
| Emitting level | 5D1 → 7F0 | 5D1 → 7F1 | 5D1 → 7F2 | 5D0 → 7F0 | 5D0 → 7F1 | 5D0 → 7F2 |
| Number of photons | 1.59 | 1.64 | 1.68 | 2.11 | 2.04 | 1.93 |
Conclusion
In summary, the hybrid phosphor BaFCl0.5Br0.5:1%Sm2+–CaF2:1%Yb3+ was prepared via a stepwise high temperature reaction to achieve a material containing both Yb3+ and Sm2+ ions. The UCL of the 5Di (i = 0, 1) → 7Fj (j = 0, 1, 2) transitions from Sm2+ was observed under NIR excitation. The analysis results indicate that the population of Sm2+ excited states originates from the cooperative energy transfer of Yb3+-dimers. Based on our results, it is actually possible to achieve the upconversion luminescence of changeable valence lanthanides under NIR excitation, which moreover, opens up a new field for the upconversion of rare earth ions.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (Grants 11474132 and 11274139).Notes and references
- L. Johnson and H. Guggenheim, Appl. Phys. Lett., 1973, 23, 96–98 CrossRef CAS.
- A. Silversmith, W. Lenth and R. Macfarlane, Appl. Phys. Lett., 1987, 51, 1977–1979 CrossRef CAS.
- R. Macfarlane, F. Tong, A. Silversmith and W. Lenth, Appl. Phys. Lett., 1988, 52, 1300–1302 CrossRef CAS.
- T. Danger, J. Koetke, R. Brede, E. Heumann, G. Huber and B. Chai, J. Appl. Phys., 1994, 76, 1413–1422 CrossRef CAS.
- M.-F. Joubert, Opt. Mater., 1999, 11, 181–203 CrossRef CAS.
- M. Pollnau, D. Gamelin, S. Lüthi, H. Güdel and M. Hehlen, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 3337 CrossRef CAS.
- W. Miniscalco, L. Andrews, B. Thompson, R. Quimby, L. Vacha and M. Drexhage, Electron. Lett., 1988, 24, 28–29 CrossRef.
- J. Allain, M. Monerie and H. Poignant, Electron. Lett., 1991, 27, 1156–1157 CrossRef CAS.
- J. Allain, M. Monerie and H. Poignant, Electron. Lett., 1992, 28, 111–113 CrossRef.
- H. Többen, Electron. Lett., 1992, 28, 1361–1362 CrossRef.
- W. Park, M. Jung and D. Yoon, Sens. Actuators, B, 2007, 126, 324–327 CrossRef CAS.
- L. Yi, X. He, L. Zhou, F. Gong, R. Wang and J. Sun, J. Lumin., 2010, 130, 1113–1117 CrossRef CAS.
- J. Alonso, J. Ferrer, A. Salinas-Castillo, R. Mallavia and S. F. de Ávila, Solid-State Electron., 2010, 54, 1269–1272 CrossRef CAS.
- W. Qin, D. Zhang, D. Zhao, L. Wang and K. Zheng, Chem. Commun., 2010, 46, 2304–2306 RSC.
- L. Wei, S. Doughan, Y. Han, M. V. DaCosta, U. J. Krull and D. Ho, Sensors, 2014, 14, 16829–16855 CrossRef PubMed.
- Y. Tang, W. Di, X. Zhai, R. Yang and W. Qin, ACS Catal., 2013, 3, 405–412 CrossRef CAS.
- J. Nees, S. Biswas, F. Droun, J. Faure, M. Nantel, G. A. Mourou, A. Nishimura, H. Takuma, J. Itatani and J.-C. Chanteloup, IEEE J. Sel. Top. Quantum Electron., 1998, 4, 376–384 CrossRef CAS.
- A. Diening, P.-A. Möbert and G. Huber, J. Appl. Phys., 1998, 84, 5900–5904 CrossRef CAS.
- D. Dosev, I. Kennedy, M. Godlewski, I. Gryczynski, K. Tomsia and E. Goldys, Appl. Phys. Lett., 2006, 88, 1906 CrossRef.
- S. Das, A. A. Reddy and G. V. Prakash, Chem. Phys. Lett., 2011, 504, 206–210 CrossRef CAS.
- E. Beurer, J. Grimm, P. Gerner and H. U. Güdel, J. Am. Chem. Soc., 2006, 128(10), 3110–3111 CrossRef CAS PubMed.
- W. Kaiser, C. Garrett and D. Wood, Phys. Rev., 1961, 123, 766 CrossRef CAS.
- J. O'Connor and H. Bostick, J. Appl. Phys., 1962, 33, 1868–1870 CrossRef.
- G. H. Dieke and R. Sarup, J. Chem. Phys., 1962, 36, 371–377 CrossRef CAS.
- D. Wood and W. Kaiser, Phys. Rev., 1962, 126, 2079 CrossRef CAS.
- R. Jaaniso and H. Bill, Europhys. Lett., 1991, 16, 569 CrossRef CAS.
- K. Holliday, C. Wei, M. Croci and U. P. Wild, J. Lumin., 1992, 53, 227–230 CrossRef CAS.
- J. Zhang, S. Huang, W. Qin, D. Gao and J. Yu, J. Lumin., 1992, 53, 275–278 CrossRef CAS.
- E. Bayer and G. Schaack, Phys. Status Solidi B, 1970, 41, 827–835 CrossRef CAS.
- T. Kushida, J. Phys. Soc. Jpn., 1973, 34, 1327–1333 CrossRef CAS.
- E. Nakazawa and S. Shionoya, Phys. Rev. Lett., 1970, 25, 1710 CrossRef CAS.
- V. Petit, P. Camy, J.-L. Doualan, X. Portier and R. Moncorgé, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 085131 CrossRef.
- M. J. Weber and R. W. Bierig, Phys. Rev. B: Condens. Matter Mater. Phys., 1964, 134, A1492 CAS.
- W. P. Qin, Z. Y. Liu, C. N. Sin, C. F. Wu, G. S. Qin, Z. Chen and K. Z. Zheng, Light: Sci. Appl., 2014, 3, e193 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |