Open Access Article
Open Access ArticleModular synthesis of propargylamine modified cyclodextrins by a gold(III)-catalyzed three-component coupling reaction†
Tsz-Wai Hui‡
ab,
Jian-Fang Cui‡ab and
Man-Kin Wong *ab
*ab
aThe Hong Kong Polytechnic University Shenzhen Research Institute, Shenzhen, PR China
bState Key Laboratory of Chirosciences, Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, PR China. E-mail: mankin.wong@polyu.edu.hk; Fax: +852 2364 9932
First published on 3rd March 2017
Abstract
An efficient modular approach for the synthesis of propargylamine modified β-cyclodextrins has been developed. Using mono-(6-benzylamino-6-deoxy)-β-cyclodextrins, formaldehyde, and alkynes, mono-(6-(benzylpropargyl)amino-6-deoxy)-β-cyclodextrins have been synthesized through a three-component coupling reaction catalyzed by gold(III) salt in water at 40 °C.
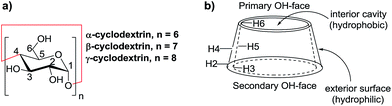
Cyclodextrins (CDs) are naturally occurring cyclic oligosaccharides consisting of α-1,4-D-glucopyranose units. The commonly used cyclodextrins with six, seven, and eight glucopyranose units are referred to as α-, β-, and γ-cyclodextrins, respectively (Fig. 1a). Cyclodextrin is an amphipathic molecule bearing a hydrophilic exterior surface and a hydrophobic interior cavity (Fig. 1b). Owing to the hydrophilic surface, cyclodextrins are water-soluble. The hydrophobic cavity of cyclodextrins allows “host–guest” inclusion complex formation with a wide variety of guest molecules through hydrophobic interaction. Therefore, the use of cyclodextrins and their modified derivatives as versatile supramolecular hosts has been widely employed in supramolecular catalysis, enzymatic mimics, analytical chemistry, and the pharmaceutical and food industry.1–9
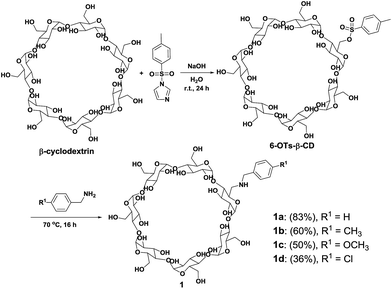
In general, modified cyclodextrins exhibit distinctive properties compared with native cyclodextrins. Modification of cyclodextrins provides novel derivatives with unique structures and functions to support the development of different research fields.1,4–9 In the past decades, various methods for cyclodextrin modification have been developed to fine-tune their physicochemical properties (solubility, inclusion complexation properties, etc.). In particularly, 6-OTs monosubstituted cyclodextrins have been employed as orthogonal handle for conjugation with different functional molecules (Scheme 1a).10–12 Through transformation of the OTs group of the mono[6-O-(p-toluenesulfonyl)]-cyclodextrin (6-OTs-CD), amine and alkyne functionalities could be easily introduced into cyclodextrins. Further synthetic elaborations through amidation and click reaction with carboxylic acids/halides and azides containing molecules offer an easy access to multifunctional molecules. Moreover, alkyne containing cyclodextrins function as versatile building blocks for the synthesis of multifunctional cyclodextrins through click reaction, 1,3-dipolar cycloaddition, and Sonogashira cross-coupling reaction.13,14 However, cyclodextrin modification is still a challenging task in synthetic chemistry due to the multiple hydroxyl groups present at the 2-, 3-, and 6-positions competing with reactants, leading to complex product formation and difficult product purification. Thus, there remains a significant interest to develop new approaches for the synthesis of multifunctional cyclodextrins.
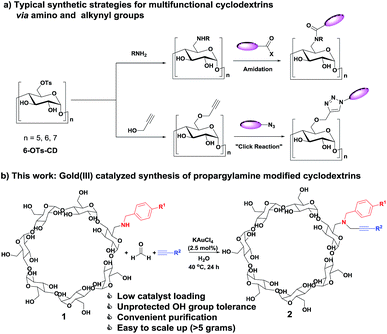 | ||
| Scheme 1 (a) Typical synthetic strategies for multifunctional cyclodextrins via amino and alkynyl groups; (b) gold(III) catalyzed synthesis of propargylamine modified cyclodextrins. | ||
Over the years, we have been developing gold catalysis for organic synthesis,15–18 and bioconjugation of oligosaccharides, peptides and proteins.19–21 Efficient methods for the synthesis of multifunctional biomolecules via Morita–Baylis–Hillman reaction and gold catalyzed/mediated reactions have also been developed in our group.19–22 Particularly, a modular approach for single-site incorporation of two independent functionalities (amines and alkynes) into aldehyde-containing oligosaccharides by using a one-pot gold-mediated three component coupling reaction has been developed.17,19 As cyclodextrins are cyclic oligosaccharides, it is envisaged that multifunctional propargylamine modified cyclodextrins could be synthesized from a gold-catalyzed three component coupling reaction23–25 of amine containing cyclodextrins, aldehydes, and alkynes.
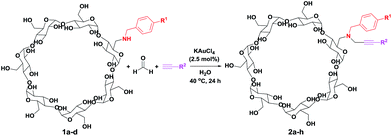
Here we first present an efficient method for the synthesis of a new class of propargylamine modified β-cyclodextrins through a modular approach of gold(III)-catalyzed three component coupling reaction of amine containing cyclodextrins, formaldehyde, and alkynes in water at 40 °C (Scheme 1b). Using mono-(6-benzylamino-6-deoxy)-β-cyclodextrins (1, 6-BA-β-CD), formaldehyde, and various alkynes, mono-(6-(benzylpropargyl)amino-6-deoxy)-β-cyclodextrins (2, 6-BPA-β-CD) have been achieved in moderate to good yields with excellent purity through convenient precipitation in acetone and recrystallization in water successively. Notably, the reaction proceeded with high chemoselectivity for formaldehyde and remarkable functional group compatibility for the unprotected hydroxyl groups of mono-(6-benzylamino-6-deoxy)-β-cyclodextrins 1.
One key challenge for selective modification of cyclodextrins is the competitive reactions of the hydroxyl groups at the 2-, 3- and 6-positions. Highly reactive reagents tend to non-selectively react with the hydroxyl groups of any positions, while less reactive reagents allow selective modification of the hydroxyl groups at the 6-position.1 Typically, mono-, and multi-(6-tosyl)cyclodextrins are the most commonly used precursors for the synthesis of a wide variety of 6-position modified cyclodextrins. Therefore, we set out to synthesize mono-(6-benzylamino-6-deoxy)-β-cyclodextrins (1, 6-BA-β-CD)26 from 6-OTs-β-CD as the amine component for the gold(III)-catalyzed three component coupling reaction. In this regard, 6-OTs-β-CD was synthesized via the reaction of β-cyclodextrin and p-toluenesulfonyl imidazole in alkaline aqueous solution according to literature procedure (Scheme 2).27 Starting from 6-OTs-β-CD, 6-BA-β-CD 1 was synthesized accordingly.28 Upon treatment with benzylamine as solvent and reactant at 70 °C for 16 h, 6-OTs-β-CD was converted to 6-BA-β-CD 1a with 83% yield. Then, various substituted benzylamines were used to synthesize 6-BA-β-CDs (1b–1d) under the same reaction conditions with moderate to good yields (36–83%, Scheme 2).
6-BA-β-CD (1a) was used as the amine component coupling with phenylacetylene and various aldehydes, as well as different solvents and transition-metal catalysts were used to study the reaction conditions (Table 1). Using 1a (0.1 mmol), benzaldehyde (1.0 mmol), and phenylacetylene (1.0 mmol) with KAuCl4 (2.5 mol%) as catalyst in water at 40 °C for 24 h gave no desired three component coupling product (Entry 1). Benzaldehydes bearing electron-donating group (4-OMe) and electron-deficient group (4-NO2) on the phenyl ring also did not afford the desired product (Entries 2 and 3). To our delight, when formaldehyde was used as the aldehyde component under the same reaction conditions, the expected product 2a was isolated in 50% yield (Entry 4). Yet, only a trace amount or no product was obtained when ACN, THF and toluene were used instead of H2O as solvent (Entries 5–7). AuCl3 and AuCl gave comparable yield in 46% and 41%, respectively (Entries 8 and 9). A trace amount of product was obtained by using HAuCl4 as the catalyst (Entry 10). Other gold(III) complexes [(C^N)AuCl2] and [(C^N)2AuBF4] (HC^N = 2-phenylpyridine or 2-phenylquinoline) were also employed as the catalyst for the reaction, but only a trace amount of coupling product was detected by ESI-MS analysis (Entries 11 and 12). Note that 34% yield of product was obtained when Salen-gold(III) complex [(Salen)AuBF4] was used as catalyst (Entry 13). Then, the catalytic effect of various transition-metal catalysts, including Cu(OAc)2, Cu(OTf)2, AgOTf, NiBr2, Zn(OAc)2 and RuCl3, for this reaction was examined (Entries 14–19). The results showed that only RuCl3 catalyzed this reaction to give product in 25% yield. Furthermore, aliphatic aldehydes, such as acetaldehyde, butyraldehyde and heptaldehyde, did not give the corresponding products with combination of amine 1a and phenylacetylene (Entries 20–22). These results indicated that this three component coupling reaction proceeded with high chemoselectivity for formaldehyde and the use of simple gold salt KAuCl4 as the catalyst and water as the solvent are essential. Moreover, a primary amine modified cyclodextrin 1aa was synthesized and employed as amine component coupling with phenylacetylene and various aromatic and aliphatic aldehydes. Yet, for all of tested aldehydes, no desired three component coupling products were obtained (see ESI, Table S1†).
| Entry | R | Catalyst | Solvent | Yield of 2b (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), aldehyde (1.0 mmol), phenylacetylene (1.0 mmol), 40 °C, 24 h.b Isolated yield.c Detected by ESI-MS analysis. | ||||
| 1 | Ph | KAuCl4 | H2O | 0 |
| 2 | 4-OMeC6H4 | KAuCl4 | H2O | 0 |
| 3 | 4-NO2C6H4 | KAuCl4 | H2O | 0 |
| 4 | H | KAuCl4 | H2O | 50 |
| 5 | H | KAuCl4 | ACN | Tracec |
| 6 | H | KAuCl4 | THF | Tracec |
| 7 | H | KAuCl4 | Toluene | 0 |
| 8 | H | AuCl3 | H2O | 46 |
| 9 | H | AuCl | H2O | 41 |
| 10 | H | HAuCl4 | H2O | Tracec |
| 11 | H | (C^N)AuCl2 | H2O | Tracec |
| 12 | H | (C^N)2AuBF4 | H2O | Tracec |
| 13 | H | (Salen)AuBF4 | H2O | 34 |
| 14 | H | Cu(OAc)2 | H2O | 0 |
| 15 | H | Cu(OTf)2 | H2O | 0 |
| 16 | H | AgOTf | H2O | 0 |
| 17 | H | NiBr2 | H2O | 0 |
| 18 | H | Zn(OAc)2 | H2O | 0 |
| 19 | H | RuCl3 | H2O | 25 |
| 20 | CH3 | KAuCl4 | H2O | 0 |
| 21 | n-C3H7 | KAuCl4 | H2O | 0 |
| 22 | n-C6H13 | KAuCl4 | H2O | 0 |
 |
||||
To examine the substrate scope of this three component coupling reaction, we extended our studies to various combinations of 6-BA-β-CD 1 and alkynes. As depicted in Table 2, this synthetic strategy works well for a wide range of substrates with moderate to good yields (up to 67%). Coupling of 1a with formaldehyde and phenylacetylene led to propargylamine modified β-cyclodextrin 2a in 50% yield (Entry 1). Coupling product 2a was characterized by NMR spectroscopic techniques (including 1H, 13C, COSY, ROESY, HMQC and HMBC experiments) and ESI-MS spectrometry. Using mono-(6-benzylamino-6-deoxy)-β-cyclodextrins 1b–1d with electron-donating group (Me, OMe) and electron-deficient group (Cl) on the phenyl ring of the benzyl moieties also proceeded smoothly, and the corresponding coupling products 2b–2d were obtained in good yields (42–51%, Entries 2–4). Using 1a, various aromatic and aliphatic alkynes also reacted with formaldehyde to give 2e–2h in 26–67% yield (Entries 5–8). Notably, the reactive functional groups (hydroxyl, cyclohexenyl) on the alkynes remain intact after the coupling reactions (2f and 2h, Entry 6 and Entry 8).
| Entry | R1 | R2 | Product | Yieldb (%) |
|---|---|---|---|---|
| a The reaction was conducted with β-CD amine 1 (0.8 mmol), formaldehyde (8.0 mmol), alkyne (8.0 mmol), KAuCl4 (2.5 mol%) in water (10 mL) at 40 °C for 24 h.b Isolated yield.c β-CD amine 1 (0.1 mmol), formaldehyde (1.0 mmol), alkyne (1.0 mmol). | ||||
| 1 | H |  |
2a | 50c |
| 2 | CH3 |  |
2b | 51 |
| 3 | OCH3 | 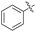 |
2c | 42 |
| 4 | Cl | 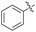 |
2d | 49 |
| 5 | H | 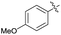 |
2e | 50 |
| 6 | H | 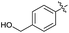 |
2f | 67 |
| 7 | H | 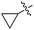 |
2g | 52 |
| 8 | H | 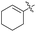 |
2h | 26 |
In conclusion, a new method for convenient preparation of propargylamine modified β-cyclodextrins has been established through a gold(III)-catalyzed three component coupling reaction. This modular synthetic strategy would be extended to conversion of cyclodextrins bearing secondary amine moieties to propargylamine modified cyclodextrin derivatives. Given the synthetic utility of propargylamines, it is envisioned that these propargylamine modified β-cyclodextrins could serve as versatile synthetic building blocks for the development of new cyclodextrin derivatives as hosts for applications on supramolecular catalysis and drug delivery.
Acknowledgements
The authors gratefully acknowledged the financial support by the National Natural Science Foundation of China (21272198), The Hong Kong Polytechnic University (4-BCA4) and State Key Laboratory of Chirosciences.Notes and references
- A. R. Khan, P. Forgo, K. J. Stine and V. T. D'Souza, Chem. Rev., 1998, 98, 1977–1996 CrossRef CAS PubMed.
- F. Bellia, D. La Mendola, C. Pedone, E. Rizzarelli, M. Saviano and G. Vecchio, Chem. Soc. Rev., 2009, 38, 2756–2781 RSC.
- A. Martinez, C. Ortiz Mellet and J. M. Garcia Fernandez, Chem. Soc. Rev., 2013, 42, 4746–4773 RSC.
- J. Tang and W. Tang, in Modified Cyclodextrins for Chiral Separation, ed. W. Tang, S.-C. Ng and D. Sun, Springer Berlin Heidelberg, Berlin, Heidelberg, 2013, pp. 1–25 Search PubMed.
- A. García, D. Leonardi, M. O. Salazar and M. C. Lamas, PLoS One, 2014, 9, e88234 Search PubMed.
- H. Bricout, F. Hapiot, A. Ponchel, S. Tilloy and E. Monflier, Sustainability, 2009, 1, 924–945 CrossRef CAS.
- Y. Miao, F. Djedaïni-Pilard and V. Bonnet, Beilstein J. Org. Chem., 2014, 10, 2654–2657 CrossRef PubMed.
- M. Fukudome, K. Yoshikawa, K. Koga, D.-Q. Yuan and K. Fujita, Chem. Commun., 2007, 3157–3159 RSC.
- W.-K. Chan, W.-Y. Yu, C.-M. Che and M.-K. Wong, J. Org. Chem., 2003, 68, 6576–6582 CrossRef CAS PubMed.
- H. Yamamura, Y. Sugiyama, K. Murata, T. Yokoi, R. Kurata, A. Miyagawa, K. Sakamoto, K. Komagoe, T. Inoue and T. Katsu, Chem. Commun., 2014, 50, 5444–5446 RSC.
- V. Oliveri and G. Vecchio, Chem.–Asian J., 2016, 11, 1648–1657 CrossRef CAS PubMed.
- Y.-C. Lin, P.-I. Wang and S.-W. Kuo, Soft Matter, 2012, 8, 9676–9684 RSC.
- F. G. Calvo-Flores, J. Isac-García, F. Hernández-Mateo, F. Pérez-Balderas, J. A. Calvo-Asín, E. Sanchéz-Vaquero and F. Santoyo-González, Org. Lett., 2000, 2, 2499–2502 CrossRef CAS PubMed.
- F. Ortega-Caballero, J. J. Giménez-Martínez and A. Vargas-Berenguel, Org. Lett., 2003, 5, 2389–2392 CrossRef CAS PubMed.
- V. K.-Y. Lo, K. K.-Y. Kung, M.-K. Wong and C.-M. Che, J. Organomet. Chem., 2009, 694, 583–591 CrossRef CAS.
- V. K.-Y. Lo, Y. Liu, M.-K. Wong and C.-M. Che, Org. Lett., 2006, 8, 1529–1532 CrossRef CAS PubMed.
- H.-M. Ko, K. K.-Y. Kung, J.-F. Cui and M.-K. Wong, Chem. Commun., 2013, 49, 8869–8871 RSC.
- K. K.-Y. Kung, V. K.-Y. Lo, H.-M. Ko, G.-L. Li, P.-Y. Chan, K.-C. Leung, Z. Zhou, M.-Z. Wang, C.-M. Che and M.-K. Wong, Adv. Synth. Catal., 2013, 355, 2055–2070 CrossRef CAS.
- K. K.-Y. Kung, G.-L. Li, L. Zou, H.-C. Chong, Y.-C. Leung, K.-H. Wong, V. K.-Y. Lo, C.-M. Che and M.-K. Wong, Org. Biomol. Chem., 2012, 10, 925–930 CAS.
- K. K.-Y. Kung, H.-M. Ko, J.-F. Cui, H.-C. Chong, Y.-C. Leung and M.-K. Wong, Chem. Commun., 2014, 50, 11899–11902 RSC.
- A. O.-Y. Chan, J. L.-L. Tsai, V. K.-Y. Lo, G.-L. Li, M.-K. Wong and C.-M. Che, Chem. Commun., 2013, 49, 1428–1430 RSC.
- G.-L. Li, K. K.-Y. Kung, L. Zou, H.-C. Chong, Y.-C. Leung, K.-H. Wong and M.-K. Wong, Chem. Commun., 2012, 48, 3527–3529 RSC.
- C. Wei and C.-J. Li, J. Am. Chem. Soc., 2003, 125, 9584–9585 CrossRef CAS PubMed.
- C.-J. Li, Acc. Chem. Res., 2010, 43, 581–590 CrossRef CAS PubMed.
- N. Uhlig and C.-J. Li, Chem. Sci., 2011, 2, 1241–1249 RSC.
- C. Wang, F. Chen, X.-W. He, S.-Z. Kang, C.-C. You and Y. Liu, Analyst, 2001, 126, 1716–1720 RSC.
- R. C. Petter, J. S. Salek, C. T. Sikorski, G. Kumaravel and F. T. Lin, J. Am. Chem. Soc., 1990, 112, 3860–3868 CrossRef CAS.
- Y. Liu, C.-C. You, S.-Z. Kang, C. Wang, F. Chen and X.-W. He, Eur. J. Org. Chem., 2002, 2002, 607–613 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra00249a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |