Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Preparation, characterization, and protein-resistance of films derived from a series of α-oligo(ethylene glycol)-ω-alkenes on H–Si(111) surfaces†
Guoting Qinc,
Chi Ming Yama,
Amit Kumara,
J. Manuel Lopez-Romero *b,
Sha Lia,
Toan Huynha,
Yan Lia,
Bin Yanga,
Rafael Contreras-Caceresb and
Chengzhi Cai*a
*b,
Sha Lia,
Toan Huynha,
Yan Lia,
Bin Yanga,
Rafael Contreras-Caceresb and
Chengzhi Cai*a
aDepartment of Chemistry & Center for Materials Chemistry, University of Houston, Houston, Texas 77204-5003, USA. E-mail: cai@uh.edu; Fax: +1-713-743-2709; Tel: +1-713-743-2710
bDepartamento de Química Orgánica, Facultad de Ciencias, Universidad de Málaga, 29071 Málaga, Spain
cCollege of Optometry, University of Houston, Houston, TX 77204, USA
First published on 3rd March 2017
Abstract
A series of oligo(ethylene glycol) (OEG)-terminated monolayers were prepared by photo-activated grafting of OEG-alkenes with the general formula CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH2)m(OCH2CH2)nOCH3 (abbreviated as Cm+2EGn, m = 8, 9; n = 3–7) on hydrogen-terminated silicon (111) surfaces using different deposition conditions. The films were characterized by contact-angle goniometry, ellipsometry, X-ray photoelectron spectroscopy (XPS) and tested for protein resistance. Films prepared under a higher vacuum showed a higher thickness and exhibited better protein resistance with increasing ethylene glycol (EG) units. Remarkably, the films prepared from C10EGn were generally thicker than those from their corresponding homologues C11EGn, and displayed better resistance to protein adsorption, which were probably due to the odd–even effect from the alkyl chain. Prepared under high vacuum conditions (∼10−5 mbar), the C10EG7 films with a thickness of 40 Å adsorbed <0.8% (the detection limit of N 1s XPS) monolayer of fibrinogen in a standard assay. The films remained protein-resistant (adsorbed <3% monolayer of fibrinogen) even after 28 days in phosphate buffered saline (PBS) at 37 °C or 17 days in MC3T3-E1 cell culture with 10% fetal bovine serum at 37 °C. Therefore, the C10EG7 films prepared under high vacuum conditions represent the most protein-resistant and stable films on non-oxidized silicon substrates.
CH(CH2)m(OCH2CH2)nOCH3 (abbreviated as Cm+2EGn, m = 8, 9; n = 3–7) on hydrogen-terminated silicon (111) surfaces using different deposition conditions. The films were characterized by contact-angle goniometry, ellipsometry, X-ray photoelectron spectroscopy (XPS) and tested for protein resistance. Films prepared under a higher vacuum showed a higher thickness and exhibited better protein resistance with increasing ethylene glycol (EG) units. Remarkably, the films prepared from C10EGn were generally thicker than those from their corresponding homologues C11EGn, and displayed better resistance to protein adsorption, which were probably due to the odd–even effect from the alkyl chain. Prepared under high vacuum conditions (∼10−5 mbar), the C10EG7 films with a thickness of 40 Å adsorbed <0.8% (the detection limit of N 1s XPS) monolayer of fibrinogen in a standard assay. The films remained protein-resistant (adsorbed <3% monolayer of fibrinogen) even after 28 days in phosphate buffered saline (PBS) at 37 °C or 17 days in MC3T3-E1 cell culture with 10% fetal bovine serum at 37 °C. Therefore, the C10EG7 films prepared under high vacuum conditions represent the most protein-resistant and stable films on non-oxidized silicon substrates.
Introduction
Modification of silicon surfaces with a stable, ultrathin and biocompatible monolayer is of great interest for the development of silicon-based miniature biodevices,1 including field-effect transistors (FETs),1–5 impedance and capacitance devices,6–11 porous silicon photonic devices and probes,12–14 drug carriers,15 cantilever sensors,16–18 and implantable devices, such as silicon-based neuron interfaces.19–23 These silicon-based transducers interconvert specific biomolecular interactions/events with electrical/mechanical/optical signals of the silicon devices. One of the critical issues limiting the specificity and sensitivity of the transducers is the non-specific adsorption of proteins onto the silicon surfaces. Protein adsorption is also the first step of inflammatory and fibrotic responses leading to failure of many types of implanted devices.24,25 The conventional way to address this issue is to utilize the SiO2 layer on Si substrates and coat it with organosiloxane films terminated with poly- or oligo(ethylene glycol) (PEG or OEG).26–31 However, the protein-resistance of such siloxane monolayers is not ideal; adsorption of 3–10% monolayer of proteins was reported.31 The lower protein-resistance than the corresponding OEG-terminated alkanethiolate self-assembled monolayers (SAMs) on gold is likely due to the lower packing density and higher defect density of the siloxane films on silicon. Also, organosiloxane films are prone to hydrolysis in aqueous electrolytes, especially at basic conditions.32,33 Furthermore, for electrical transducers, the sensitivity and signal to noise ratio are limited by the relatively thick oxide dielectric layer.7,10 Although excellent protein-resistant properties have been demonstrated for zwitterions34–36 and polypeptides37 coatings, ultrathin films presenting OEG are still among the most widely used and the most protein-resistant coatings studied to date.38–57 Moreover, we recently demonstrated that the high resistance to non-specific protein adsorption of OEG films allows the selective attachment of proteins onto the nanopatterns generated on such films by local oxidation using conductive atomic force microscopy (AFM).58,59We and others have reported the photo-activated grafting of the α-OEG-ω-alkenes onto hydrogen-terminated silicon, forming OEG-terminated monolayers that are directly bound to the silicon substrate via Si–C bonds (Scheme 1).13,40,48,51,60–72 We performed this process in a simple apparatus combining a Schlenk tube with a quartz cell at a low vacuum level,68,69,73,74 using OEG-terminated alkenes C11EGn (n = 3, 6, 7 and 9). We found that C11EGn with n ≥ 6 led to films that reduced the adsorption of fibrinogen to ∼3% monolayer after immersion in fibrinogen (1 mg mL−1) for 1 h and brief washing with water.68,69,73 The OEG films remained protein-resistant after 1 week in PBS (pH 7.4) at room temperature.49 Notably, in the literature the protein-resistance of OEG-terminated films were often measured after washing the protein-treated samples with a detergent solution for a relatively long period of time.75 Such a strong washing procedure resulted in a lower value of the protein adsorption compared to the procedure employed by us.68,69,73 While the protein-resistance and stability of our previous OEG-terminated films on non-oxidized silicon were comparable to the reported OEG-terminated alkylthiolate monolayers on gold substrates,76–78 further improvement is necessary for many applications stated above, such as implantable devices and biosensors.
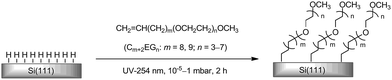 | ||
| Scheme 1 Photo-induced surface hydrosilylation of OEG-terminated alkene monolayers on silicon surfaces. | ||
The mechanism of protein resistance on OEG-terminated monolayers has been extensively studied theoretically and experimentally. It becomes clear that the packing density and the length of the OEG chains are the most important parameters. They directly affect the hydration of the OEG layer, which has been associated to the repulsion of proteins,42,51,52,57,79–84 although the detailed mechanism awaits to be fully elucidated.2a,28,53,60,78,80,85–92 In general, protein resistance increased with longer OEG chains especially at a low packing density. For alkanethiolate SAMs terminated with 3–6 EG units on Au(111) surfaces, the OEG chains adopt a helical conformation and amorphous state with a molecular cross-section of 21.3 Å2. Their packing density is 21.4 Å2 per molecule (equivalent to 4.7 molecules per nm2).45,51,78,80,81,91 The densities of previously reported alkyl terminated monolayers grown by hydrosilylation on Si are in the range of 0.45–0.55 alkyl chains per Si atom on a Si(111) surface (equivalent to 28.4–23.3 Å2 per molecule),66,71,93–95 which are lower than those of alkyl thiolate SAMs on gold due to the larger lattice parameters for Si(111). In our previous study, OEG monolayers on silicon substrate with low protein adsorption (<3% monolayer of fibrinogen)69 had a packing density of 0.37–0.39 molecules per surface Si atom.
In addition to protein-resistance, film stability can also be greatly enhanced with dense and highly ordered hydrophobic alkyl layers that serve as a barrier against the penetration of oxygen and anion that induces oxidation at the silicon interface.2a,96–98 Oligo(ethylene glycol) monolayers grafted directly onto silicon surfaces by silanization were shown to be unstable under either air or PBS solution conditions.28 This instability was attributed to the oxidation at the silicon interface underneath the alkyl chains, which was susceptible to hydrolytic cleavage.99 Lewis' group100,101 functionalized Si(111) surfaces via Si–C bonds and achieved sterically bulky alkyl monolayers in a two-step process, involving Grignard reaction of the alkyl Grignard reagent with chlorine-terminated silicon surface. Unfortunately, high-purity Grignard reagents containing OEG chains are extremely difficult to prepare. Therefore, direct hydrosilylation of alkenes onto hydrogen-terminated silicon substrates is a more practical method. However, as mentioned above, the highest density of alkyl monolayers on silicon achieved to date via hydrosilylation is not comparable to that of alkanethiolated SAMs.71 We expect that the presence of water and especially oxygen that has a high reactivity and diffusion rate, even at a low level in the system, may significantly decrease the film coverage due to the competition with the alkene for the surface active sites generated by the UV radiation.71 Moreover, the resultant oxide defects accelerate the oxidation of adjacent silicon.102 Therefore, decreasing oxygen level in the system is crucial to achieve a high packing density and low oxide defects. On the other hand, longer OEG chains generally increase protein resistance, but are expected to decrease packing density and stability of the films. In order to increase film stability, we aim to increase the packing density by optimizing the OEG length.
Herein, we report the study of monolayers derived from a series of α-OEG-ω-alkenes with the general formula of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH2)m(OCH2CH2)nOCH3 abbreviated as Cm+2EGn (m = 8, 9; n = 3–7) on H–Si(111) surfaces. The study is focused on the effect of m with an odd (m = 9) or even (m = 8) number and n from 3 to 7 on the film thickness and protein resistance prepared at low (∼1 mbar) and medium (∼0.05 mbar) vacuum conditions (Scheme 1). Contact angle goniometry, ellipsometry and XPS were used to characterize and investigate the protein resistance of OEG films. We further improved the deposition vacuum to the level of 10−5 mbar, resulting in monolayers with a thickness of 40 Å. These monolayers represent the most protein resistant and stable monolayers on non-oxidized silicon reported to date.28,75
CH(CH2)m(OCH2CH2)nOCH3 abbreviated as Cm+2EGn (m = 8, 9; n = 3–7) on H–Si(111) surfaces. The study is focused on the effect of m with an odd (m = 9) or even (m = 8) number and n from 3 to 7 on the film thickness and protein resistance prepared at low (∼1 mbar) and medium (∼0.05 mbar) vacuum conditions (Scheme 1). Contact angle goniometry, ellipsometry and XPS were used to characterize and investigate the protein resistance of OEG films. We further improved the deposition vacuum to the level of 10−5 mbar, resulting in monolayers with a thickness of 40 Å. These monolayers represent the most protein resistant and stable monolayers on non-oxidized silicon reported to date.28,75
Materials and methods
Materials
Commercial chemicals, including sulfuric acid (Sigma-Aldrich), 30% hydrogen peroxide solution (Sigma-Aldrich), 40% ammonium fluoride solution (Sigma-Aldrich), dichloromethane (Sigma-Aldrich), petroleum ether (Sigma-Aldrich), absolute ethanol (Alfa Aesar), and 10% buffer-HF (Transene) were used without purification. Fibrinogen, minimum essential medium alpha modification (αMEM) and Dulbecco's modified eagle's medium (DMEM) were purchased from Sigma Aldrich. Silicon (111) wafers were purchased from Silicon Quest Int'l. Inc. The synthesis of α-OEG-ω-alkenes with the general formula of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH2)m(OCH2CH2)nOCH3 (Cm+2EGn, m = 8, 9; n = 3–7) are described in ESI.† OEGs where prepared under N2 atmosphere and stored at 5 °C in amber vials under Ar to prevent the presence of water and O2. MC3T3-E1 fibroblast cells and osteoblast D1 cells were provided from Dr David Mooney at Harvard University.
CH(CH2)m(OCH2CH2)nOCH3 (Cm+2EGn, m = 8, 9; n = 3–7) are described in ESI.† OEGs where prepared under N2 atmosphere and stored at 5 °C in amber vials under Ar to prevent the presence of water and O2. MC3T3-E1 fibroblast cells and osteoblast D1 cells were provided from Dr David Mooney at Harvard University.
Preparation of H–Si(111) substrates
Single side polished silicon (111) wafer (boron-doped, P-type, 1–10 Ω cm resistivity, miscut angle of ±0.5°) was cut into pieces of 1 × 1 cm2, cleaned with Piranha solution (concentrated H2SO4/30% H2O2, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) for 10 min at ∼80 °C to remove organic contaminates. Caution: Piranha solutions react violently with organic materials and should be handled with extreme care. The freshly cleaned sample was immersed in an Ar-saturated, 40% NH4F solution (prepared by gently bubbling the solution with argon for 10 minutes prior to use) for 20 min followed by rinse with argon-saturated Millipore water and dried with a stream of argon.
1 v/v) for 10 min at ∼80 °C to remove organic contaminates. Caution: Piranha solutions react violently with organic materials and should be handled with extreme care. The freshly cleaned sample was immersed in an Ar-saturated, 40% NH4F solution (prepared by gently bubbling the solution with argon for 10 minutes prior to use) for 20 min followed by rinse with argon-saturated Millipore water and dried with a stream of argon.
Photo-activated grafting
Contact-angle goniometry
Water drops were dispersed onto the film surfaces using a micro-Electrapette 25 (Matrix Technologies). Advancing and receding contact angles (θa/r) were measured using a goniometer (Rame-Hart, model 100). The pipette tip was kept in contact with the drop during the measurements. At least four drops of probe liquid were measured for each sample, and the mean values were reproducible within ±1°.Ellipsometry
An ellipsometer (Rudolph Research, Auto EL III), operated with a 632.8 nm He–Ne laser at an incident angle of 70°, was employed for thickness measurement. A refractive index of 1.45 was assumed for the prepared films. At least four measurements were taken for each sample, and the mean values were reproducible within ±1 Å.X-ray photoelectron spectroscopy (XPS)
A PHI 5700 X-ray photoelectron spectrometer, equipped with a monochromatic Al Kα X-ray source (hν = 1486.7 eV) at a take-off angle (TOA) of 45° from the film surface was employed for XPS measurement. High-resolution XPS spectra were obtained by applying a window pass energy of 23.5 eV and the following numbers of scans: Si 2p, 5 scans; C 1s, 20 scans; O 1s, 10 scans; N 1s, 20–40 scans. The binding energy scales were referenced to the Si 2p peak at 99.0 eV. XPS spectra were curve fitted and the intensities measured as peak areas were calculated using Phi Multipak V5.0A from Physical Electronics.Protein resistance
The fibrinogen solution was prepared by dissolving 1 mg fibrinogen (Sigma) in 1 mL of PBS (consisting of 0.01 M phosphate and 0.14 M NaCl, pH 7.4, Sigma) in a vial. The test samples were immersed in the fibrinogen solution at 20–25 °C for 1 h. The samples were then washed under a gentle flow of Millipore water for about 15 seconds, dried with a flow of Ar, and subjected to contact angle, ellipsometric and XPS measurements.In addition to the fibrinogen adsorption experiment above, the protein-resistance of the OEG monolayers on silicon was also evaluated in culture of αMEM for MC3T3-E1 cells and DMEM for D1 cells. In these experiments, the test samples were incubated in cell culture media with or without live cells over a specific period of time. It should be noted that the culture media in all cases contained 10% fetal bovine serum (FBS) that consists of a wide variety of proteins necessary for the cultured cells to survive, grow and divide.
Long-term stability of C10EG7 films prepared under high vacuum
The stability of the C10EG7 films on silicon substrates was evaluated by incubating the samples in PBS at 37 °C for 28 days followed by the fibrinogen adsorption experiment, or in MC3T3 cell culture in αMEM with 10% fetal bovine serum at 37 °C for 17 days. The amount of adsorbed proteins was measured by the increase of ellipsometric thickness. The optimized films C10EG7 films were also measured by N 1s XPS using fibrinogen monolayer adsorbed on H–Si(111) as a standard (see below).Amount of protein adsorbed on the C10EG7 films
In addition to ellipsometric measurement, the amount of proteins adsorbed onto the C10EG7 films prepared under high vacuum was also quantified by N 1s XPS and expressed as percentage of a fibrinogen monolayer (% ML) calculated using eqn (1):| Adsorbed protein (% ML) = 100 × N(OEG)/N(H–Si) | (1) |
Results and discussion
C10EGn films on Si(111) prepared under low vacuum
We carried out the photo-induced surface hydrosilylation of H–Si(111) substrates with a series of OEG-alkenes (C10EGn) using the setup described elsewhere.69 Table 1 summarizes the advancing and receding contact angles of water (θa/r) and ellipsometric thicknesses (Te) for the C10EGn films on Si(111) surfaces upon exposure to UV-254 nm irradiation under ∼1 mbar for 2 h. The advancing water contact angle θa and ellipsometric thickness Te of the C10EGn films on Si(111) surfaces decreased from 59 to 51° and increased from 21 to 28 Å, respectively, with an increase of EG units from three to seven. These contact angle values are consistent with those reported for other OEG-terminated alkene films prepared under similar conditions on Si(111) surfaces.68,69 The low hysteresis (Δθ = 2–3°) for the films indicates smooth and homogeneous surfaces. As shown in Table 2, XPS data for the C10EGn films on Si(111) surfaces show two C 1s peaks and one O 1s peak. For the carbon peaks, the one at higher binding energy (∼287 eV) is assigned to the carbon atoms that are adjacent to an oxygen atom, and the one at lower binding energy (∼285 eV) is assigned to the rest of the carbon atoms.43,69,80,105 The ratios of the integrated areas of the deconvoluted C 1s signals from these two types of carbon atoms, (CC–C/CC–O), are close to the stoichiometric ratio. The peak at 533 eV is assigned to the oxygen atoms of the EG units.| Film | Low vacuum (∼1 mbar) | Medium vacuum (∼0.05 mbar) | ||||||
|---|---|---|---|---|---|---|---|---|
| Before treatment with fibrinogen | After treatment with fibrinogen | Before treatment with fibrinogen | After treatment with fibrinogen | |||||
| θa/r,a deg | Te,b °C | θa/r,a deg | T′e,b °C | θa/r,a deg | Te,b °C | θa/r,a deg | T′e,b °C | |
| a Standard deviation of measurements were ±1°.b Standard deviation of measurements were ±1 Å. | ||||||||
| C10EG3 | 59/56 | 21 | 66/49 | 35 | ||||
| C10EG4 | 56/54 | 22 | 63/41 | 31 | 55/52 | 30 | 56/53 | 32 |
| C10EG5 | 55/53 | 25 | 59/47 | 29 | 52/50 | 33 | 52/50 | 35 |
| C10EG6 | 53/51 | 27 | 55/52 | 29 | 52/49 | 34 | 53/50 | 35 |
| C10EG7 | 51/49 | 28 | 52/49 | 29 | 50/47 | 36 | 50/47 | 36 |
| Film | XPS | |||
|---|---|---|---|---|
| C 1s | O 1s | [CC–C]/[CC–O] | ||
| Expected | Measured | |||
| C10EG3 | 284.8, 286.7 | 533.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 0.9 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 0.9 |
| C10EG4 | 284.9, 286.6 | 532.9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
| C10EG5 | 284.8, 286.7 | 532.9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
| C10EG6 | 284.8, 286.7 | 532.9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
| C10EG7 | 284.8, 286.6 | 533.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 1.8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9 1.9 |
Based on the data above, calculated packing densities of the films derived from C10EGn (n = 3, 6) were similar to those we previously reported.69 These data are summarized in Table S1 in the ESI.† As expected, the packing density, represented as Am (unit surface area per grafted molecule) and Nchains/Nsurf,Si (number of hydrocarbon chains per surface silicon atom), decreases with increasing OEG monolayer length grafted on the silicon substrate. It should be noted, however, that the measured ellipsometric thickness may represent an overestimate due to the adsorption of water to the OEG-terminated films. Indeed, recent studies106–108 indicated the presence of tightly bound water on top of organic thin films, even those presenting hydrophobic alkynyl groups, which could not be removed by ultrahigh vacuum.
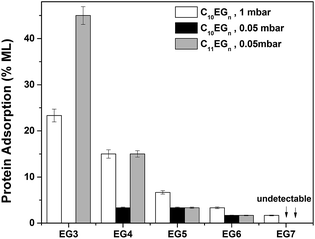
After immersing the C10EGn-coated Si(111) substrates with a fibrinogen solution for 1 h, both Te and θa/r indicate that the films of C10EG3, C10EG4, and C10EG5 prepared on Si(111) surfaces readily adsorbed the protein, as shown by an increase of both of Te (14, 9, and 4 Å, respectively) and θa (7, 7, and 4°, respectively), while very low protein adsorption was found on the C10EG6- and C10EG7-coated surfaces, as indicated by the nearly unchanged θa/r and Te values (Tables 1 and S1†). For comparison, a freshly prepared H–Si(111) substrate was also subjected to protein treatment for 1 h. As expected, fibrinogen readily adsorbed on the hydrophobic H–Si(111) surface, resulting in a film with Te = ∼60 Å and θa/r = ∼80°/<20°, corresponding to a monolayer of fibrinogen.69 Thus, by ellipsometric measurement, the degree of protein adsorption on the C10EGn-coated surfaces decreased from ∼23% to ∼2% monolayer of fibrinogen with an increase of EG units from three to seven (Fig. 2). Therefore, with at least six EG units, the OEG-terminated films derived from the C10EGn series on Si(111) surfaces and prepared at low vacuum conditions (∼1 mbar) effectively resisted the protein adsorption, which was comparable to those films derived from OEG-terminated thiolates on Au(111) surfaces that also displayed increasing protein resistance with longer EG chain length.49,53,57,78
C10EGn films on Si(111) prepared at medium vacuum conditions
In order to reduce the presence of water and specially oxygen which compete with the alkene for the surface active sites generated by UV, we prepared the C10EGn films on Si(111) surfaces under an improved vacuum (∼0.05 mbar, termed as medium vacuum). Under such a vacuum, the C10EG3 films could not be prepared since the adsorbate C10EG3 readily evaporated. Table 1 summaries θa/r and Te of the C10EGn films on Si(111) surfaces upon exposure to 254 nm UV irradiation under ∼0.05 mbar for 2 h. θa and Te of the C10EGn films on Si(111) surfaces decreased from 55 to 50° and increased from 30 to 36 Å, respectively, with an increase of n from 4 to 7. There was a significant increase of Te by ∼8 Å for the C10EGn films with the same number of EG units on Si(111) surfaces as the pressure of the system was reduced from ∼1 to ∼0.05 mbar. A low hysteresis (Δθ = 2–3°) was still observed for the films.After treatment of the C10EGn-coated Si(111) substrates with a fibrinogen solution for 1 h, only a low level of protein adsorption on the EG3-, EG4-, EG5- and EG6-terminated films was observed, as indicated by the small changes of θa/r (≤2°) and Te (≤2 Å) values (Tables 1, S1† and Fig. 2). The increase of 2 Å in thickness represents an adsorption of ∼3% monolayer of fibrinogen (60 Å thick for a monolayer). No protein adsorption on the C10EG7 films could be detected by water contact angle and ellipsometry.
Notably, the thicknesses of the C10EGn films are almost equal to the theoretical molecular length of the compounds, assuming the molecules adopt a zig-zag ‘all-trans’ conformation.80 However, the maximum packing density of long alkyl chains grafted on H–Si(111) by surface hydrosilylation is only ∼0.55 alkyl chains/surface Si atom,66 corresponding to a density of 23.3 Å2 per molecule. Hence, in our case it is highly unlikely that the packing density reaches the theoretical value of 19.1 Å2 per molecule when adopting a zig-zag conformation.80 Furthermore, the improved protein resistance (Fig. 2) indicates that the OEG chains most likely are in an amorphous state. Also, XPS did not show the presence of silicon oxide underneath the film. Therefore, the high thickness can be attributed to the growth of a partial second layer due to the presence of radicals on the film. There might be three likely pathways to generate radicals from monolayers:109 (1) direct photodissociation of C–H or C–C bonds via vacuum ultraviolet (VUV); (2) photoelectron mediated dissociation of monolayer; (3) reactive oxyspecies generated by UV light, such as singlet oxygen and hydroxyl radicals. The UV induced generation of reactive oxy species is possible109,110 although the oxygen level in our vacuum system was 1.5 × 104 times lower than that in air. Also, the radicals could be generated via the cleavage of the C–H bonds on the CH3 headgroup of the monolayers by the very minor amount of 185 nm UV light, since the CC–C/CC–O ratios match those of the molecules (Tables 2 and 5).
We then investigated the thickness of C10EG7 films prepared from hydrosilylation at different exposure time under UV. The film thickness was monitored by ellipsometry, and was also validated by angle-resolved XPS (Fig. 3). It can be seen that the film thickness rapidly increased under UV light before it slowed down at around 2 h, during which the formation of the first layer was probably the dominating factor of the thickness growth. After that, the growth of a second layer may account for the increasing thickness, although it was much slower than that of the first layer. Interestingly, the surface hydrophilicity and homogeneity seem to be constant regardless of the UV exposure time, as can bee seen from the water contact angle data (Table 3).
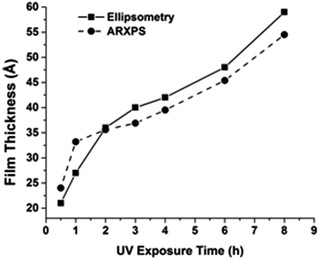 | ||
| Fig. 3 Thickness of C10EG7 films prepared under medium vacuum condition at different UV exposure time. One sample was prepared at each time point. | ||
C11EGn films on Si(111) prepared at medium vacuum conditions
OEG-terminated films derived from C11EGn (n = 3, 6, 7, 9) on Si(111) under low vacuum conditions were described in our previously reports.68,69 The films with three EG units adsorbed 30–60% monolayer of fibrinogen, while the films with more than six EG units reduced the adsorption of fibrinogen to ∼3% monolayer. We expected that both the packing density and the protein resistance of the C11EGn films on Si(111) surfaces would be improved under higher vacuum conditions (∼0.05 mbar). Table 4 summaries θa/θr and Te for the C11EGn films on Si(111) surfaces upon exposure to UV-254 nm irradiation under ∼0.05 mbar for 2 h. The advancing water contact angle θa and ellipsometric thickness Te of the C11EGn films decreased from 61 to 50° and increased from 25 to 34 Å, respectively, with the increase of OEG chain length (n) from 3 to 7. Indeed, there was a ∼5 Å increase of Te for the C11EGn films with the same number of EG units on Si(111) surfaces as the pressure of the system was reduced from ∼1 to ∼0.05 mbar. A low hysteresis (Δθ = 2–3°) was still observed for the films. The XPS data for the C11EGn films on Si(111) surfaces (Table 5) show two C 1s peaks at ∼285 and ∼287 eV, respectively, and one O 1s peak at ∼533 eV, similar to those for the C10EGn films described in the previous section. The (CC–C/CC–O) ratios are also in good agreement with the expected ratios.| Film | Before protein adsorption | After protein adsorption | ||
|---|---|---|---|---|
| θa/r,a deg | Te,b Å | θa/r,a deg | Te,b Å | |
| a Standard deviation of measurements were ±1°.b Standard deviation of measurements were ±1 Å. | ||||
| C11EG3 | 61/59 | 25 | 75/<20 | 52 |
| C11EG4 | 57/55 | 28 | 63/47 | 37 |
| C11EG5 | 52/49 | 30 | 53/50 | 32 |
| C11EG6 | 51/48 | 32 | 52/49 | 33 |
| C11EG7 | 50/47 | 34 | 50/47 | 34 |
| Film | XPS | |||
|---|---|---|---|---|
| C 1s | O 1s | [CC–C]/[CC–O] | ||
| Expected | Measured | |||
| C11EG3 | 284.7, 286.5 | 533.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 0.7 |
| C11EG4 | 284.8, 286.6 | 533.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
| C11EG5 | 284.8, 286.6 | 533.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
| C11EG6 | 284.8, 286.6 | 533.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
| C11EG7 | 284.6, 286.4 | 533.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 1.7 |
After treatment of the C11EGn-coated Si(111) substrates with a fibrinogen solution for 1 h, both Te and θa/r indicate that the films of C11EG3 and C11EG4 prepared on Si(111) surfaces readily adsorbed the protein, as shown by both an increase of θa (14 and 6°, respectively) and Te (27 and 9 Å, respectively). The estimated fibrinogen adsorption based on the ellipsometric data is presented in Fig. 2 and Table S1,† showing a decrease of fibrinogen adsorption from ∼45% to ∼3% monolayer with increasing EG units from three to six, and the fibrinogen adsorption on the C11EG7 films was not detectable.
C10EGn vs. C11EGn films – thickness and packing
Since the methylene chain of the OEG films is ca. 1–2 Å per unit (–CH2–), the C11EGn films are expected to be 1–2 Å thicker than the C10EGn films with the same number of EG units. However, Te of the C11EGn films was smaller than that of the C10EGn films by ∼2 Å (Fig. 4). As shown above, for the C10EGn films, an increase of one EG unit led to an increase of 1–3 Å of the film thickness. However, an increase of one carbon atom in the alkyl chain with the same EG units showed, surprisingly, a decrease of ∼2 Å. Additionally, the calculated packing densities decreased with the increasing number of EG units from 3 to 7 for both C10EGn and C11EGn films as shown in Table S1.† To rationalize this intriguing result, we note that there is probably an ‘Odd–Even’ effect from the alkyl chain as discussed below using a simplified packing model. The ‘Odd–Even’ effect often affects the packing density and wettability of SAMs, such as n-carboxylic acids and alkylthiolates, on various substrates, such as graphite, Au(111), and Ag(111).111,112 However, to our knowledge, no study has been reported on the possible odd–even effect on OEG-terminated alkyl monolayers on silicon. Confirming this effect for our systems requires systematic study of the OEG-terminated films with a series of alkyl chain length, which is out of the scope for the current study.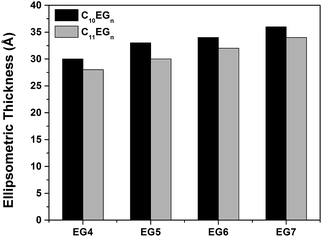 | ||
| Fig. 4 Ellipsometric thicknesses (Å) of C10EGn and C11EGn films on Si(111) prepared at medium vacuum conditions. | ||
To account for the above observed higher packing density of the C10EGn films over the corresponding C11EGn films, we use an idealized packing model shown in Fig. 5. The lone-pair electrons of the bottom oxygen atoms (O–C(11)) in the C11EGn films, which has an odd number of carbon atoms in the alkyl chain, interact with the H2C(11) moieties in the adjacent alkyl chains. In comparison, the bottom O atoms (O–C(10)) in the C10EGn films, which has an even number of carbon atoms in the alkyl chain, interact with the H2C(11) moieties in the adjacent ethylene oxide chains that are more electronegative than the alkyl chains in the above case. Therefore, the bottom oxygen atoms have a more favorable interaction with the methylene moieties in the C10EGn films than the C11EGn films, leading to higher packing density in the former. To a lesser extent, the top oxygen atoms may prefer to interact with the adjacent ethylene oxide moieties in the C10EGn films than with the methyl groups in the C11EGn films.
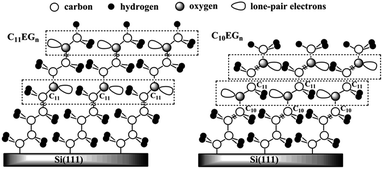 | ||
| Fig. 5 Schematic representations of C11EGn (odd-numbered) and C10EGn (even-numbered) films on Si(111). | ||
C10EG7 films grown with improved apparatus under high vacuum conditions
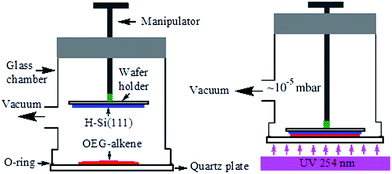
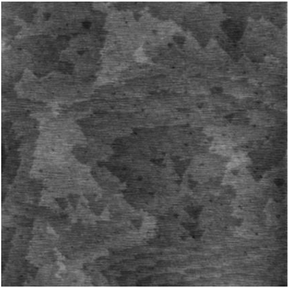
Despite of the simplicity of our previous apparatus for photo-activated grafting, it has several limitations. First, due to the close proximity of the H–Si(111) substrate to the alkene droplet placed in the small quartz cell,68,69 the Si–H surface is easily contaminated during the degassing process by the volatile reactive impurities such as H2O and O2. Second, the size (<1 cm2) of the silicon sample was limited by the size of the available quartz cells. Third, the manipulation of the sample was difficult to master, and flipping of the samples often occurred. To address these drawbacks, we designed the second-generation apparatus for photo-activated grafting of thin films (Fig. 1). The new apparatus has the following improvements. First, during the degassing step, the H–Si substrate surface is located above the outlet to the vacuum and is far away from the alkene droplet, thus reducing the possibility of contamination. Second, only the quartz window needs to be cleaned before each use. Finally, the apparatus is easy to use, and can handle larger samples (up to 2 × 2 cm2). Most importantly, the vacuum was improved from 0.05 mbar to 10−5 mbar. On the basis of the above results, we expected that the improvement of the vacuum conditions would greatly increase the packing density of the OEG-terminated films and thus enhance the protein-resistance and long-term stability of the films. The above study identified C10EG7 as the best adsorbate, also because we observed substantial evaporation of EGn-alkenes with n < 6 during deposition under such a high vacuum condition, resulting in poor quality film. Therefore, we focused on the study of the C10EG7 films prepared using the new apparatus under high vacuum (10−5 mbar).The resultant C10EG7 films on silicon (111) were characterized by contact angle goniometry, ellipsometry, and AFM. As shown in Fig. 6, the AFM contact mode image of a C10EG7 film revealed the underlying atomic steps of the silicon substrate, indicating that the film was ultraflat. The advancing and receding water contact angles were 51°/49° with a low hysteresis Δθ = 2°, also indicating a homogeneous surface. The ellipsometric thickness of the film was increased from 36 Å to 40 ± 1 Å, reaches the molecular length of C10EG7 (38.7 Å). This ellipsometric thickness was in agreement with the thickness of 39.5 Å measured by variable angle XPS (see ESI, Fig. S2†). These results indicated that the molecules on the surfaces were densely packed.
Protein resistance and stability of the C10EG7 films prepared under high vacuum conditions
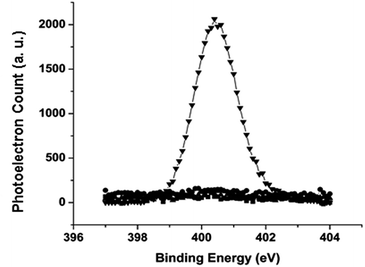
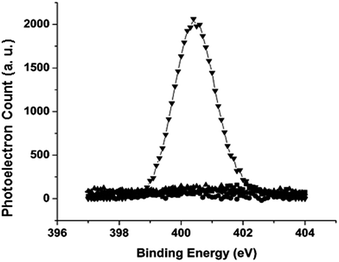
The stability of the films on silicon substrates was evaluated by incubation of the samples in PBS or cell culture media at 37 °C for the specified period of time, followed by the fibrinogen adsorption experiment. The amount of the adsorbed proteins was determined by the N 1s signal from XPS using eqn (1). As shown by the N 1s region of the XPS spectrum in Fig. 7, freshly prepared C10EG7 films did not adsorb protein to the detection limit of N 1s XPS (0.8% monolayer of fibrinogen). Even after storage for 14 d under ambient conditions or 28 d in PBS (pH 7.4) at 37 °C, the C10EG7 coated silicon surfaces remained protein resistant, with no N 1s signal was detected after the fibrinogen adsorption experiment. Furthermore, the protein-resistance and stability of the C10EG7 films on silicon was tested with cell culture media (αMEM for MC3T3-E1 cells and DMEM for D1 cells) and cell cultures with 10% fetal bovine serum (FBS), which contained a rich variety of proteins. As shown in Fig. 8, the amount of protein adsorbed onto the C10EG7 surfaces were 2.0% ± 0.8% ML after 12 d in αMEM with 10% FBS, 2.8% ± 0.8% ML after 17 d in MC3T3-E1 cell culture, 1.4% ± 0.8% ML after 5 d in DMEM with 10% FBS, and 1.2% ± 0.8% ML after 7 d in D1 cell culture.Conclusion
Protein-resistant films derived from a series of α-oligo(ethylene glycol)-ω-alkenes (Cm+2EGn, m = 8, 9, n = 3–7) with odd and even numbers of methylene chains (m) and various numbers of EG units (n) were prepared on H–Si(111) surfaces by photo-induced hydrosilylation under low (∼1 mbar) and medium (∼0.05 mbar) vacuum conditions. All films generally exhibited a low hysteresis of water contact angles, indicating a high homogeneity. Both the film thickness and resistance to protein adsorption were improved when the films were prepared at improved vacuum conditions. Under the same vacuum conditions, the even-numbered C10EGn films exhibit both higher thickness and better resistance to protein adsorption than the odd-numbered C11EGn films with the equal number of EG units. Furthermore, the films derived from C11EGn with n ≥ 5 and C10EGn with n ≥ 4 reduced the adsorption of fibrinogen to <3% monolayer as they were prepared at medium vacuum conditions. The optimal film C10EG7 on Si(111) prepared at a high vacuum (10−5 mbar) showed no protein adsorption, and good stability in various media including cell culture media.Acknowledgements
This work was supported by the National Science Foundation grants DMR-0706627, DMR-1207583 and DMR-1508722, the Welch Foundation grant E-1498, University of Houston (GEAR and TcSAM Special Funding), the Spanish Research Project CTQ13-48418P and CTQ16-76311, and the Marie Curie COFUND program “U-mobility” co-financed by University of Málaga and the European Community's Seventh Framework Programme under Grant Agreement No. 246550.Notes and references
-
(a) A. Andres-Arroyo, B. Gupta, F. Wang, J. J. Gooding and P. J. Reece, Nano Lett., 2016, 16, 1903–1910 CrossRef CAS PubMed
; (b) X. Cheng, E. Hinde, D. M. Owen, S. B. Lowe, P. J. Reece, K. Gaus and J. J. Gooding, Adv. Mater., 2015, 27, 6144–6150 CrossRef CAS PubMed
.
-
(a) V. R. Gonçales, Y. Wu, B. Gupta, S. G. Parker, Y. Yang, S. Ciampi, R. Tilley and J. J. Gooding, J. Phys. Chem. C, 2016, 120, 15941–15948 CrossRef
; (b) Q. N. Minh, S. P. Pujari, B. Wang, Z. Wang, H. Haick, H. Zuilhof and C. J. M. van Rijn, Appl. Surf. Sci., 2016, 387, 1202–1210 CrossRef
; (c) S. P. Pujari, E. van Andel, O. Yaffe, D. Cahen, T. Weidner, C. J. M. van Rijn and H. Zuilhof, Langmuir, 2013, 29, 570–580 CrossRef CAS PubMed
.
- Y. Paska, T. Stelzner, S. Christiansen and H. Haick, ACS Nano, 2011, 5, 5620–5626 CrossRef CAS PubMed
.
- K. Smaali, D. Guerin, V. Passi, L. Ordronneau, A. Carella, T. Melin, E. Dubois, D. Vuillaume, J. P. Simonato and S. Lenfant, J. Phys. Chem. C, 2016, 120, 11180–11191 CAS
.
- M. W. Shinwari, M. J. Deen and D. Landheer, Microelectron. Reliab., 2007, 47, 2025–2057 CrossRef
.
-
(a) S. Ciampi, M. H. Choudhury, S. A. B. A. Ahmad, N. Darwish, A. Le Brun and J. J. Gooding, Electrochim. Acta, 2015, 186, 216–222 CrossRef CAS
; (b) B. Fabre, Y. Li, L. Scheres, S. P. Pujari and H. Zuilhof, Angew. Chem., Int. Ed., 2013, 52, 12024–12027 CrossRef CAS PubMed
.
- M. Birkholz, A. Mai, C. Wenger, C. Meliani and R. Scholz, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2016, 8, 355–377 CrossRef CAS PubMed
.
- S. Kumar, V. Vandana, C. M. S. Rauthan, V. K. Kaul, S. N. Singh and P. K. Singh, IEEE J. Photovolt., 2014, 4, 380–386 CrossRef
.
- G. Panzarasa, G. Soliveri and V. Pifferi, J. Mater. Chem. C, 2016, 4, 340–347 RSC
.
- H. Abiri, M. Abdolahad, M. Gharooni, S. A. Hosseini, M. Janmaleki, S. Azimi, M. Hosseini and S. Mohajerzadeh, Biosens. Bioelectron., 2015, 68, 577–585 CrossRef CAS PubMed
.
- M. N. Masood, S. Chen, E. T. Carlen and A. van den Berg, All-(111) Surface Silicon Nanowires: Selective Functionalization for Biosensing Applications, ACS Appl. Mater. Interfaces, 2010, 2, 3422–3428 CAS
.
- B. Gupta, K. Mai, S. B. Lowe, D. Wakefield, N. D. Girolamo, K. Gaus, P. J. Reece and J. J. Gooding, Anal. Chem., 2015, 87, 9946–9953 CrossRef CAS PubMed
.
-
(a) B. Guan, A. Magenau, S. Ciampi, K. Gaus, P. J. Reece and J. J. Gooding, Bioconjugate Chem., 2014, 25, 1282–1289 CrossRef CAS PubMed
; (b) Y. Zhu, A. H. Soeriyadi, S. G. Parker, P. J. Reece and J. J. Gooding, J. Phys. Chem. B, 2014, 2, 3582–3588 CAS
.
- B. Guan, A. Magenau, K. A. Kilian, S. Ciampi, K. Gaus, P. J. Reece and J. J. Gooding, Faraday Discuss., 2011, 149, 301–317 RSC
.
- D. L. Sonin, D. V. Korolev, V. N. Postnov, E. B. Naumysheva, E. I. Pochkaeva, M. L. Vasyutina and M. M. Galagudza, Drug Delivery, 2016, 23, 1747–1756 CrossRef CAS PubMed
.
- R. E. Fernandez, V. Hareesh, E. Bhattacharya and A. Chadha, Biosens. Bioelectron., 2009, 24, 1276–1280 CrossRef CAS PubMed
.
- J. Bowen and D. Cheneler, Nanoscale, 2016, 8, 4245–4521 RSC
.
- M. del Rey, R. A. da Silva, D. Meneses, D. F. S. Petri, J. Tamayo, M. Calleja and P. M. Kosaka, Sens. Actuators, B, 2014, 204, 602–610 CrossRef CAS
.
- C. L. Kolarcik, S. D. Luebben, S. A. Sapp, J. Hanner, N. Snyder, T. D. Y. Kozai, E. Chang, J. A. Nabity, S. T. Nabity, C. F. Lagenaur and X. T. Cui, Soft Matter, 2015, 11, 4847–4861 RSC
.
- L. R. Hochberg, M. D. Serruya, G. M. Friehs, J. A. Mukand, M. Saleh, A. H. Caplan, A. Branner, D. Chen, R. D. Penn and J. P. Donoghue, Nature, 2006, 442, 164–171 CrossRef CAS PubMed
.
- S. Kamath, D. Bhattacharyya, C. Padukudru, R. B. Timmons and L. P. Tang, J. Biomed. Mater. Res., Part A, 2008, 86, 617–626 CrossRef PubMed
.
- J. Yakovleva, R. Davidsson, A. Lobanova, M. Bengtsson, S. Eremin, T. Laurell and J. Emneus, Anal. Chem., 2002, 74, 2994–3004 CrossRef CAS PubMed
.
- M. J. Sweetman, C. J. Shearer, J. G. Shapter and N. H. Voelcker, Langmuir, 2011, 27, 9497–9503 CrossRef CAS PubMed
.
- J. P. Seymour and D. R. Kipke, Neural probe design for reduced tissue encapsulation in CNS, Biomaterials, 2007, 28, 3594–3607 CrossRef CAS PubMed
.
- M. Huebner, M. Ben Haddada, C. Methivier, R. Niessner, D. Knopp and S. Boujday, Biosens. Bioelectron., 2015, 67, 334–341 CrossRef CAS PubMed
.
- R. Murthy, B. M. Bailey, C. Valentin-Rodriguez, A. Ivanisevic and M. A. Grunlan, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 4108–4119 CrossRef CAS
.
- A. S. Anderson, A. M. Dattelbaum, G. A. Montano, D. N. Price, J. G. Schmidt, J. S. Martinez, W. K. Grace, K. M. Grace and B. I. Swanson, Langmuir, 2008, 24, 2240–2247 CrossRef CAS PubMed
.
- F. Cecchet, B. De Meersman, S. Demoustier-Champagne, B. Nysten and A. M. Jonas, Langmuir, 2006, 22, 1173–1181 CrossRef CAS PubMed
.
- N. Shirahata and A. Hozumi, Ultrathin poly(ethylene glycol) monolayers formed by chemical vapor deposition on silicon substrates, J. Nanosci. Nanotechnol., 2006, 6, 1695–1700 CrossRef CAS PubMed
.
- S. J. Sofia, V. Premnath and E. W. Merrill, Macromolecules, 1998, 31, 5059–5070 CrossRef CAS PubMed
.
- R. Contreras-Caceres, C. M. Santos, S. H. Li, A. Kumar, Z. L. Zhu, S. S. Kolar, M. A. Casado-Rodriguez, Y. K. Huang, A. McDermott, J. M. López-Romero and C. Cai, J. Colloid Interface Sci., 2015, 458, 112–118 CrossRef CAS PubMed
.
- M. CalistriYeh, E. J. Kramer, R. Sharma, W. Zhao, M. H. Rafailovich, J. Sokolov and J. D. Brock, Langmuir, 1996, 12, 2747–2755 CrossRef CAS
.
- T. Tsukagoshi, Y. Kondo and N. Yoshino, Colloids Surf., B, 2007, 54, 82–87 CrossRef CAS PubMed
.
-
(a) S. C. Lange, E. van Andel, M. M. J. Smulders and H. Zuilhof, Langmuir, 2016, 32, 10199–10205 CrossRef CAS PubMed
; (b) Z. Wang and H. Zuilhof, Langmuir, 2016, 32, 6310–6318 CrossRef CAS PubMed
.
- A. T. Nguyen, J. Baggerman, J. M. J. Paulusse, H. Zuilhof and C. J. M. van Rijn, Langmuir, 2012, 28, 604–610 CrossRef CAS PubMed
.
- A. T. Nguyen, J. Baggerman, J. M. J. Paulusse, C. J. M. van Rijn and H. Zuilhof, Langmuir, 2011, 27, 2587–2594 CrossRef CAS PubMed
.
- K. H. A. Lau, C. L. Ren, S. H. Park, I. Szleifer and P. B. Messersmith, Langmuir, 2012, 28, 2288–2298 CrossRef CAS PubMed
.
- L. Cao, S. Sukavaneshvar, B. D. Ratner and T. A. Horbett, J. Biomed. Mater. Res., Part A, 2006, 79, 788–803 CrossRef PubMed
.
- J. R. Capadona, D. M. Collard and A. J. Garcia, Langmuir, 2003, 19, 1847–1852 CrossRef CAS
.
- C. Fairman, J. Z. Ginges, S. B. Lowe and J. J. Gooding, ChemPhysChem, 2013, 14, 2183–2189 CrossRef CAS PubMed
.
- S. S. Li, D. Y. Yang, H. Y. Tu, H. T. Deng, D. Du and A. D. Zhang, J. Colloid Interface Sci., 2013, 402, 284–290 CrossRef CAS PubMed
.
- S. Herrwerth, W. Eck, S. Reinhardt and M. Grunze, J. Am. Chem. Soc., 2003, 125, 9359–9366 CrossRef CAS PubMed
.
- S. Herrwerth, T. Rosendahl, C. Feng, J. Fick, W. Eck, M. Himmelhaus, R. Dahint and M. Grunze, Langmuir, 2003, 19, 1880–1887 CrossRef CAS
.
- M. Rosso, A. T. Nguyen, E. de Jong, J. Baggerman, J. M. J. Paulusse, M. Giesbers, R. G. Fokkink, W. Norde, K. Schroën, C. J. M. van Rijn and H. Zuilhof, ACS Appl. Mater. Interfaces, 2011, 3, 697–704 CAS
.
- L. Y. Li, S. F. Chen, J. Zheng, B. D. Ratner and S. Y. Jiang, J. Phys. Chem. B, 2005, 109, 2934–2941 CrossRef CAS PubMed
.
- X. F. Hu and C. B. Gorman, Acta Biomater., 2014, 10, 3497–3504 CrossRef CAS PubMed
.
- M. J. Felipe, P. Dutta, R. Pernites, R. Ponnapati and R. C. Advincula, Polymer, 2012, 53, 427–437 CrossRef CAS
.
- B. S. Flavel, M. Jasieniak, L. Velleman, S. Ciampi, E. Luais, J. R. Peterson, H. J. Griesser, J. G. Shapter and J. J. Gooding, Langmuir, 2013, 29, 8355–8562 CrossRef CAS PubMed
.
- D. E. Heath, A. R. M. Sharif, C. P. Ng, M. G. Rhoads, L. G. Griffith, P. T. Hammond and M. B. Chan-Park, Lab Chip, 2015, 15, 2073–2089 RSC
.
- C. M. Yam, M. Deluge, D. Tang, A. Kumar and C. Z. Cai, J. Colloid Interface Sci., 2006, 296, 118–130 CrossRef CAS PubMed
.
- J. Zheng, L. Y. Li, S. F. Chen and S. Y. Jiang, Langmuir, 2004, 20, 8931–8938 CrossRef CAS PubMed
.
- J. Zheng, L. Y. Li, H. K. Tsao, Y. J. Sheng, S. F. Chen and S. Y. Jiang, Biophys. J., 2005, 89, 158–166 CrossRef CAS PubMed
.
- K. S. Lee, I. In and S. Y. Park, Appl. Surf. Sci., 2014, 313, 532–536 CrossRef CAS
.
- M. Zwahlen, S. Herrwerth, W. Eck, M. Grunze and G. Hahner, Langmuir, 2003, 19, 9305–9310 CrossRef CAS
.
- Y. C. Chiag, Y. Chang, W. Y. Chen and R. C. Ruaan, Langmuir, 2012, 28, 1399–1407 CrossRef CAS PubMed
.
- M. Rosso, A. T. Nguyen, E. de Jong, J. Baggerman, J. M. J. Paulusse, M. Giesbers, R. G. Fokkink, W. Norde, K. Schroën, C. J. M. v. Rijn and H. Zuilhof, ACS Appl. Mater. Interfaces, 2011, 3, 697–704 CAS
.
- T. Hayashi, Y. Tanaka, Y. Koide, M. Tanaka and M. Hara, Phys. Chem. Chem. Phys., 2012, 14, 10196–10206 RSC
.
- G. T. Qin and C. Z. Cai, Nanotechnology, 2009, 20, 355306 CrossRef PubMed
.
- G. T. Qin, J. H. Gu, K. Liu, Z. D. Xiao, C. M. Yam and C. Z. Cai, Langmuir, 2011, 27, 6987–6994 CrossRef CAS PubMed
.
- M. Sanchez-Molina, J. M. Lopez-Romero, J. Hierrezuelo-Leon, M. Martin-Rufian, M. Valpuesta and R. Contreras-Caceres, Asian J. Org. Chem., 2016, 5, 550–559 CrossRef CAS
.
- A. Lucena-Serrano, C. Lucena-Serrano, R. Contreras-Caceres, A. Diaz, M. Valpuesta, C. Cai and J. M. Lopez-Romero, Appl. Surf. Sci., 2016, 360, 419–428 CrossRef CAS
.
- R. J. Flamers, Annu. Rev. Anal. Chem., 2008, 1, 707–736 CrossRef PubMed
.
- K. A. Kilian, T. Bocking, K. Gaus, M. Gal and J. J. Gooding, Biomaterials, 2007, 28, 3055–3062 CrossRef CAS PubMed
.
- K. A. Kilian, T. Bocking, S. Ilyas, K. Gaus, W. Jessup, M. Gal and J. J. Gooding, Adv. Funct. Mater., 2007, 17, 2884–2890 CrossRef CAS
.
- T. L. Lasseter, B. H. Clare, N. L. Abbott and R. J. Hamers, J. Am. Chem. Soc., 2004, 126, 10220–10221 CrossRef CAS PubMed
.
- G. T. Qin, C. M. Santos, W. Zhang, Y. Li, A. Kumar, U. J. Erasquin, K. Liu, P. Muradov, B. W. Trautner and C. Cai, J. Am. Chem. Soc., 2010, 132, 16435–16441 Search PubMed
.
- M. Stutzmann, J. A. Garrido, M. Eickhoff and M. S. Brandt, Phys. Status Solidi, 2006, 203, 3424–3437 CrossRef CAS
.
- C. M. Yam, J. H. Gu, S. Li and C. Z. Cai, Comparison of resistance to protein adsorption and stability of thin films derived from alpha-hepta-(ethylene glycol) methyl omega-undecenyl ether on H–Si(111) and H–Si(100) surfaces, J. Colloid Interface Sci., 2005, 285, 711–718 CrossRef CAS PubMed
.
- C. M. Yam, J. M. Lopez-Romero, J. H. Gu and C. Z. Cai, Chem. Commun., 2004, 2510–2511 RSC
.
- J. M. Buriak, Chem. Rev., 2002, 102, 1271–1308 CrossRef CAS PubMed
.
- R. L. Cicero, M. R. Linford and C. E. D. Chidsey, Langmuir, 2000, 16, 5688–5695 CrossRef CAS
.
- Q. Y. Sun, L. de Smet, B. van Lagen, M. Giesbers, P. C. Thune, J. van Engelenburg, F. A. de Wolf, H. Zuilhof and E. J. R. Sudholter, J. Am. Chem. Soc., 2005, 127, 2514–2523 CrossRef CAS PubMed
.
- J. H. Gu, C. M. Yam, S. Li and C. Z. Cai, J. Am. Chem. Soc., 2004, 126, 8098–8099 CrossRef CAS PubMed
.
- G. T. Qin, R. Zhang, B. Makarenko, A. Kumar, W. Rabalais, J. M. L. Romero, R. Rico and C. Z. Cai, Chem. Commun., 2010, 46, 3289–3291 RSC
.
- E. Perez, K. Lahlil, C. Rougeau, A. Moraillon, J.-N. Chazalviel, F. Ozanam and A. C. Gouget-Laemmel, Langmuir, 2012, 28, 14654–14664 CrossRef CAS PubMed
.
- N. T. Flynn, T. N. T. Tran, M. J. Cima and R. Langer, Langmuir, 2003, 19, 10909–10915 CrossRef CAS
.
- K. Jans, K. Bonroy, R. De Palma, G. Reekmans, H. Jans, W. Laureyn, M. Smet, G. Borghs and G. Maes, Langmuir, 2008, 24, 3949–3954 CrossRef CAS PubMed
.
- L. Y. Li, S. F. Chen and S. Y. Jiang, J. Biomater. Sci., Polym. Ed., 2007, 18, 1415–1427 CrossRef CAS PubMed
.
- Y. He, Y. Chang, J. C. Hower, J. Zheng, S. Chen and S. Jiang, Phys. Chem. Chem. Phys., 2008, 10, 5539–5544 RSC
.
- T. Hayashi, Y. Tanaka, Y. Koide, M. Tanakac and M. Hara, Phys. Chem. Chem. Phys., 2012, 14, 10196–10206 RSC
.
- A. E. Ismail, G. S. Grest and M. J. Stevens, Structure and dynamics of water near the interface with oligo(ethylene oxide) self-assembled monolayers, Langmuir, 2007, 23, 8508–8514 CrossRef CAS PubMed
.
- C. Fairman, J. Z. Ginges, S. B. Lowe and J. J. Gooding, ChemPhysChem, 2013, 14, 2183–2189 CrossRef CAS PubMed
.
- M. W. A. Skoda, R. M. J. Jacobs, J. Willis and F. Schreiber, Langmuir, 2007, 23, 970–974 CrossRef CAS PubMed
.
- S. Chen, L. Li, C. Zhao and J. Zheng, Polymer, 2010, 51, 5283–5293 CrossRef CAS
.
- G. M. Liu, Y. J. Chen, G. Z. Zhang and S. H. Yang, Phys. Chem. Chem. Phys., 2007, 9, 6073–6082 RSC
.
- L. K. Ista and G. P. Lopez, Langmuir, 2012, 28, 12844–12850 CrossRef CAS PubMed
.
- T. Satomi, Y. Nagasaki, H. Kobayashi, H. Otsuka and K. Kataoka, Langmuir, 2007, 23, 6698–6703 CrossRef CAS PubMed
.
- M. J. Shuster, A. Vaish, M. L. Gilbert, M. Martinez-Rivera, R. M. Nezarati, P. S. Weiss and A. M. Andrews, J. Phys. Chem. C, 2011, 115, 24778–24787 CAS
.
- L. D. Unsworth, H. Sheardown and J. L. Brash, Langmuir, 2005, 21, 1036–1041 CrossRef CAS PubMed
.
- L. D. Unsworth, H. Sheardown and J. L. Brash, Langmuir, 2008, 24, 1924–1929 CrossRef CAS PubMed
.
- D. J. Vanderah, H. L. La, J. Naff, V. Silin and K. A. Rubinson, J. Am. Chem. Soc., 2004, 126, 13639–13641 CrossRef CAS PubMed
.
- N. Bonnet, D. O'Hagan and G. Hahner, Phys. Chem. Chem. Phys., 2010, 12, 4367–4374 RSC
.
- X. Y. Zhu, Y. Jun, D. R. Staarup, R. C. Major, S. Danielson, V. Boiadjiev, W. L. Gladfelter, B. C. Bunker and A. Guo, Langmuir, 2001, 17, 7798–7803 CrossRef CAS
.
- A. B. Sieval, B. van den Hout, H. Zuilhof and E. J. R. Sudholter, Langmuir, 2000, 16, 2987–2990 CrossRef CAS
.
- A. B. Sieval, B. van den Hout, H. Zuilhof and E. J. R. Sudholter, Langmuir, 2001, 17, 2172–2181 CrossRef CAS
.
- P. Gorostiza, C. H. de Villeneuve, Q. Y. Sun, F. Sanz, X. Wallart, R. Boukherroub and P. Allongue, J. Phys. Chem. B, 2006, 110, 5576–5585 CrossRef CAS PubMed
.
- M. F. Juarez, F. A. Soria, E. M. Patrito and P. Paredes-Olivera, J. Phys. Chem. C, 2008, 112, 14867–14877 CAS
.
- L. J. Webb, D. J. Michalak, J. S. Biteen, B. S. Brunschwig, A. S. Y. Chan, D. W. Knapp, H. M. Meyer, E. J. Nemanick, M. C. Traub and N. S. Lewis, J. Phys. Chem. B, 2006, 110, 23450–23459 CrossRef CAS PubMed
.
- C. M. Dekeyser, C. C. Buron, K. Mc Evoy, C. C. Dupont-Gillain, J. Marchand-Brynaert, A. M. Jonas and P. G. Rouxhet, J. Colloid Interface Sci., 2008, 324, 118–126 CrossRef CAS PubMed
.
- E. J. Nemanick, P. T. Hurley, B. S. Brunschwig and N. S. Lewis, J. Phys. Chem. B, 2006, 110, 14800–14808 CrossRef CAS PubMed
.
- L. E. O'Leary, E. Johansson, B. S. Brunschwig and N. S. Lewis, J. Phys. Chem. B, 2010, 114, 14298–14302 CrossRef PubMed
.
- C. Hogan, L. Caramella and G. Onida, Phys. Status Solidi B, 2012, 249, 1132–1139 CrossRef CAS
.
- C. M. Yam, J. Gu, S. Li and C. Cai, J. Colloid Interface Sci., 2005, 285, 711–718 CrossRef CAS PubMed
.
- A. Ulman, An Introduction to Ultrathin Organic Films: From Langmuir–Blodgett to Self-Assembly, Academic Press, Boston, 1991 Search PubMed
.
- D. Briggs and M. P. Seah, Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy, Wiley, New York, 1984 Search PubMed
.
- M. James, T. A. Darwish, S. Ciampi, S. O. Sylvester, Z. Zhang, A. Ng, J. J. Gooding and T. L. Hanley, Soft Matter, 2011, 7, 5309–5318 RSC
.
- C. Wang, H. Lu, Z. Wang, P. Xiu, B. Zhou, G. Zuo, R. Wan, J. Hu and H. Fang, Phys. Rev. Lett., 2009, 103, 137801 CrossRef PubMed
.
- C. Wang, B. Zhou, P. Xiu and H. Fang, J. Phys. Chem. C, 2011, 115, 3018–3024 CAS
.
- T. Ye, E. A. McArthur and E. Borgue, J. Phys. Chem. B, 2005, 109, 9927–9938 CrossRef CAS PubMed
.
- T. Ye, D. Wynn, R. Dudek and E. Borguet, Langmuir, 2001, 17, 4497–4500 CrossRef CAS
.
- F. Tao and S. L. Bernasek, Chem. Rev., 2007, 107, 1408–1453 CrossRef CAS PubMed
.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103–1169 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Procedure of the synthesis of α-oligo(ethylene glycol)-ω-alkenes, calculation of thickness from ARXPS, and protein adsorption from ellipsometry. See DOI: 10.1039/c6ra28497c |
| This journal is © The Royal Society of Chemistry 2017 |