Open Access Article
Open Access ArticleSoft colloidal lithography†
M. Weilerab and
C. Pacholski *ac
*ac
aMax Planck Institute for Intelligent Systems, Department of New Materials and Biosystems, Heisenbergstr. 3, 70569 Stuttgart, Germany. E-mail: cpachols@uni-potsdam.de
bUniversity of Heidelberg, Biophysical Chemistry, Im Neuenheimer Feld 253, 69120 Heidelberg, Germany
cUniversity of Potsdam, Institute of Chemistry, Am Mühlenberg 3, OT Golm, 14476 Potsdam, Germany
First published on 10th February 2017
Abstract
The exceptional properties of hydrogel colloids are exploited for the formation of tailor-made 2D colloidal crystals. The presented strategy facilitates the self-assembly of hydrogel colloids into highly ordered arrays in which the lattice constant and the diameter of the employed colloids can be independently adjusted by solely chemical means providing easy access to complex nanostructures.
Colloidal lithography is a well-established technique for fabricating nanostructured surfaces (reviewed e.g. in1–3). The appeal of this bottom-up approach is based on its simplicity, time- as well as cost-efficiency and its applicability to structuring large surface areas. In general it exploits the self-assembly of hard spheres such as silica4 or polystyrene5 nanoparticles into ordered two-dimensional arrays. Different methods were utilized for assembling and depositing the colloids onto surfaces such as the Langmuir–Blodgett approach6,7 pioneered by e.g. Möhwald,8 dip coating,9 spin coating,10,11 and lift-off from interfaces.12,13 Most often hexagonally close-packed colloidal monolayers were prepared but also other arrangements including loosely packed hexagonally ordered arrays14 and square arrays15,16 were realized by carefully choosing appropriate deposition parameters including ionic strength, pH, and concentration of the colloidal solution. In order to generate loosely packed hexagonally ordered arrays of hard spheres very often previously deposited close-packed arrangements were reworked with reactive ion etching17 or thermal treatment.18 The latter provides more elaborated nanostructures. Also the degree of order of the resulting array depends strongly on the fabrication process and at least two requirements have to be fulfilled in order to obtain colloidal arrays composed of hard spheres with a minimal amount of defects: highly monodisperse spheres and a clean environment (if possible free of dust).
An elegant way to promote the formation of defect-free colloidal crystals is the utilization of soft spheres for their production. Their softness and compressibility facilitate the dissipation of defect energies over long distances. Thereby the formation of dislocations in the crystal lattices by point defects, resulting from e.g. contamination of the surface with dust particles, is prevented.19 For example, by assembling stimuli-responsive hydrogel microgels composed of poly(N-isopropylacrylamide) (polyNIPAM) at an air/liquid interface and applying mechanical shear 2D colloidal crystals with exceptional long-range order on large areas were obtained.20 The assembly of hard spheres into hexagonally ordered, close-packed monolayers at air/liquid interfaces has already been extensively studied.21–24 Also the Langmuir–Blodgett technique was successfully transferred to arranging polyNIPAM nanospheres into highly ordered lattices.25 Moreover, this method allowed a very precise control of the polyNIPAM interparticle distance. However, the diameter of the hydrogel colloids in this publication was controlled by reactive ion etching even though it has already be shown for single polyNIPAM microspheres adsorbed on glass substrates that their diameter can be changed by immersion and drying in certain solvents.26
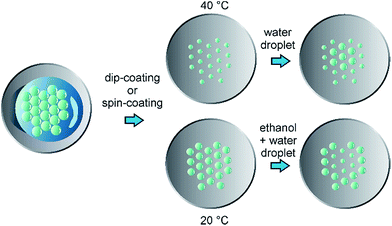
In this work, a new technique referred to as soft colloidal lithography (SCL) is presented which allows for preparing 2D colloidal crystals with independent control over lattice constants and colloid diameters by taking advantage of the remarkable properties of polyNIPAM colloids. Solely chemical stimuli supply the necessary external preconditions in order to vary both parameters in the resulting highly ordered, loosely packed colloidal array. Hence, SCL paves the road to the simple, fast, and cost-efficient fabrication of tailor-made 2D colloidal crystals which can be utilized to obtain highly desired photonic metamaterials.
The process of SCL is schematically shown in Fig. 1. Briefly, polyNIPAM microgels were wet-chemically synthesized according to Pelton et al.27 and adapted from Kim et al.28 (details of the synthesis can be found in the ESI†). Two different types of polyNIPAM microspheres were thereby obtained which were investigated throughout this work: on the one hand polyNIPAM colloids with a diameter of ∼1 μm and on the other hand anionic polyNIPAM colloids with a diameter ∼2 μm. To guarantee the formation of highly ordered 2D colloidal crystals, polyNIPAM microspheres were pre-assembled at an air/liquid interface by addition of ethanol to the colloidal dispersion. The resulting hexagonally ordered loosely packed array of polyNIPAM colloids was mechanically annealed and afterwards transferred onto cleaned glass cover slips using dip coating or spin coating.20
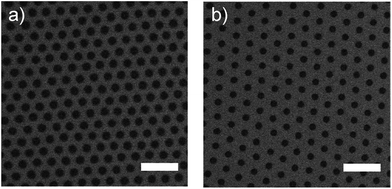
In Fig. 2 images of hexagonally arranged polyNIPAM arrays on glass substrates are displayed which were obtained by scanning electron microscopy (SEM) detecting backscattered electrons.
Both arrays were prepared using polyNIPAM colloids with a diameter of ∼2 μm which were pre-assembled on a glass substrate and transferred onto a water surface at a defined temperature.
Then the polyNIPAM monolayer was deposited onto glass substrates via dip coating. During this process the temperature of the water on which the pre-assembled polyNIPAM colloids floated was changed from 20 °C (Fig. 2a) to 50 °C (Fig. 2b). Details of the procedure are given in the ESI (Fig. S2†).
The influence of only this one parameter on the resulting 2D colloidal crystal is striking. The resulting colloidal arrays are characterized by their high degree of order, low variation in their periodicity, and their significant change in the diameter of the polyNIPAM colloids.
To get quantitative results for the geometrical arrangement of these arrays, the periodicity of the lattice and its degree of order was extracted from several SEM images according to a published method.29,30 Briefly, the position of each polyNIPAM colloid in the array was determined using an algorithm implemented in the software ImageJ which served as basis for subsequent calculation of the radial distribution functions. All prepared colloidal arrays showed a very high degree of order (see Fig. S1a and b†). The lattice constant of the polyNIPAM colloids was determined to be 1.2 μm ± 0.1 μm whereas the anionic polyNIPAM colloids assembled into an array with a higher periodicity of 2.1 μm ± 0.1 μm. The diameters of the polyNIPAM colloids on the surface were analyzed using the routine of ImageJ. If a deposition temperature of 20 °C was used, the diameter of the deposited colloid types was determined to be 0.72 μm ± 0.02 μm and 1.15 μm ± 0.09 μm for polyNIPAM and anionic polyNIPAM colloids, respectively. By changing the deposition temperature to 50 °C a reduction in the diameter of both types of polyNIPAM colloids was achieved: 0.61 μm ± 0.04 μm for polyNIPAM colloids and 0.83 μm ± 0.13 μm for anionic polyNIPAM spheres. Similar results were obtained using spin coating which are presented in the ESI (Fig. S3 and S4†). The large difference in the diameters of the polyNIPAM colloids at different temperatures is based on the stimuli-responsive properties of polyNIPAM which can react to small differences in its environment, including temperature, pH, and ionic strength of the dispersion, with an abrupt volume change (reviewed e.g. in31). This exceptional response of polyNIPAM is based on altering the balance between hydrophilic and hydrophobic interactions in the polymeric network. Also polyNIPAM colloids adsorbed to a glass surface change their volume (reviewed e.g. in32). In Fig. S6† AFM images of anionic polyNIPAM microspheres deposited at different temperatures onto glass surfaces are displayed. The cross-section demonstrates that not only the diameter of the anionic polyNIPAM colloids (‘footprint’) but also their height varies considerably: 82 nm ± 3 nm for arrays fabricated at low temperatures and 160 nm ± 7 nm for arrays prepared at high temperatures, for example. These results are in accordance with published values, which were obtained for similar experiments on the volume changes of polyNIPAM colloids on glass surfaces which were induced by other stimuli.33
However, the lattice constants of the obtained polyNIPAM arrays are not significantly influenced by the deposition temperature (Fig. S5†). This behaviour is intuitively unexpected but has already been reported for the assembly of large polyNIPAM colloids at oil/water interfaces. Here, polyNIPAM colloids change their shape and develop a flattened morphology based on their amphiphilic properties. Depending on the softness (cross-linking density) of the polyNIPAM colloids their peripheral shells can overlap to a different extent leading to a constant interparticle distance in the formed hexagonal array.34,35
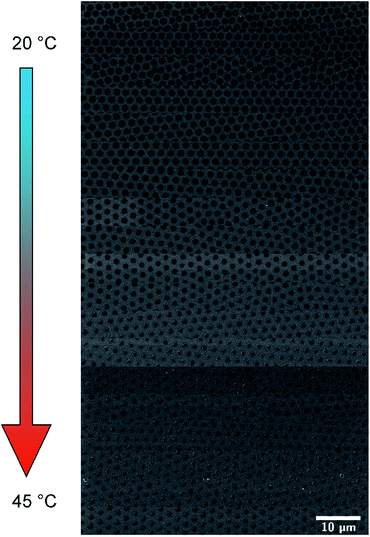
Moreover, the diameters of polyNIPAM colloids on glass substrate surfaces were gradually changed. For this purpose a pre-assembled colloidal polyNIPAM monolayer was deposited onto the surface of a temperature-controlled water bath. Then the polyNIPAM monolayer was transferred onto a glass substrate using dip coating. During the dip coating process the temperature of the water bath was gradually changed. In Fig. 3 SEM images of highly ordered polyNIPAM arrays at different positions of the glass substrate, which correspond to increasing water bath temperatures, are displayed.
Variations in the colloidal diameter can be easily noticed. Quantitative results for the polyNIPAM microsphere diameters and the lattice constants are shown in Fig. 4a and b, respectively. Whereas the diameter of the deposited polyNIPAM colloids changes gradually, the variation in the interparticle distance is not significant. By trend the lattice constant seems to increase with increasing temperature. This observation could be attributed to a change in the density of the polyNIPAM microsphere array during dip coating. Zhang et al. and others already demonstrated that the density of a colloidal polyNIPAM monolayer determines the lattice constant of the 2D colloidal crystal.7,36
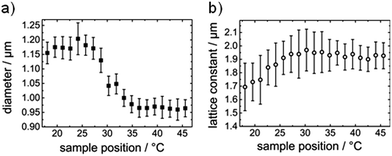 | ||
| Fig. 4 Diameter of polyNIPAM colloids (a) and lattice constants (b) of prepared loosely packed colloidal arrays as a function of deposition temperature during dip coating. | ||
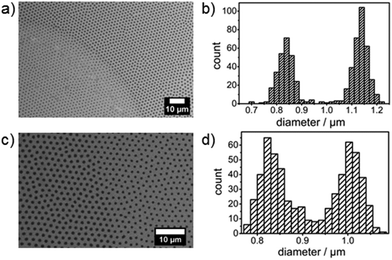
The diameter of polyNIPAM colloids absorbed on a glass surface can also be changed locally providing a chemical route to more complex structures. Fig. 5a shows a SEM image of a loosely packed polyNIPAM microsphere array which has been prepared at 20 °C and subsequently treated locally with a mixture of ethanol/bidest. Water containing 20 vol% ethanol, i.e. placing a droplet at RT onto the substrate surface followed by drying at RT. On the right hand side of the image the diameter of the polyNIPAM microspheres is significantly larger than on the left hand side. A histogram of the polyNIPAM diameters present on the substrate surface is displayed in Fig. 5b and demonstrates the bimodal distribution. The process works also vice versa. PolyNIPAM colloids which have been deposited at 40 °C onto the substrate surface have a diameter of ∼0.8 μm which can be changed to ∼1 μm by water treatment at RT (Fig. 5c and d).
The lattice constant of the colloidal array is not influenced by locally applying water to the substrate surface and drying the sample at RT. Moreover, the swelling/collapse of polyNIPAM colloids deposited on glass substrates is reversible in accordance with published results on the thermoinduced behaviour of single polyNIPAM colloids adsorbed on surfaces.33
Conclusions
To summarize, the first example of independent control of interparticle distance and particle diameter in highly ordered 2D colloidal crystals using solely chemical methods is demonstrated. Highly ordered hydrogel microsphere arrays were obtained by self-assembly at an air/liquid interface and the diameter of the colloids was controlled by the deposition protocol. By changing the temperature during the transfer of the hydrogel microgel monolayer onto the substrate surface the diameter of the colloids was successfully tuned gradually as well as over the whole substrate surface. Furthermore, by placing small droplets of a ethanol/water mixture onto a 2D colloidal crystal made of hydrogel microspheres, their diameter was locally changed suggesting a promising application of the presented method to the fabrication of photonic metamaterials using e.g. metal-assisted etching (MAE) of silicon.Acknowledgements
M. Weiler and C. Pacholski acknowledge financial support from the German Federal Ministry of Education and Research (BMBF, project PhoNa, contract no. 03IS2101E) and the Max Planck Society.Notes and references
- P. Colson, C. Henrist and R. Cloots, J. Nanomater., 2013, 948510 Search PubMed.
- J. Y. Park, Korean J. Chem. Eng., 2014, 31, 541–547 CrossRef CAS.
- N. Vogel, C. K. Weiss and K. Landfester, Soft Matter, 2012, 8, 4044–4061 RSC.
- W. Wang, B. H. Gu, L. Y. Liang and W. Hamilton, J. Phys. Chem. B, 2003, 107, 3400–3404 CrossRef CAS.
- S. M. Yang, S. G. Jang, D. G. Choi, S. Kim and H. K. Yu, Small, 2006, 2, 458–475 CrossRef CAS PubMed.
- K. U. Fulda and B. Tieke, Adv. Mater., 1994, 6, 288–290 CrossRef CAS.
- B. van Duffel, R. H. A. Ras, F. C. De Schryver and R. A. Schoonheydt, J. Mater. Chem., 2001, 11, 3333–3336 RSC.
- R. M. Kenn, C. Bohm, A. M. Bibo, I. R. Peterson, H. Mohwald, J. Alsnielsen and K. Kjaer, J. Phys. Chem., 1991, 95, 2092–2097 CrossRef CAS.
- A. S. Dimitrov and K. Nagayama, Langmuir, 1996, 12, 1303–1311 CrossRef CAS.
- P. Jiang and M. J. McFarland, J. Am. Chem. Soc., 2004, 126, 13778–13786 CrossRef CAS PubMed.
- H. W. Deckman and J. H. Dunsmuir, J. Vac. Sci. Technol., B: Microelectron. Process. Phenom., 1983, 1, 1109–1112 CAS.
- M. Kondo, K. Shinozaki, L. Bergstrom and N. Mizutani, Langmuir, 1995, 11, 394–397 CrossRef CAS.
- H. H. Wickman and J. N. Korley, Nature, 1998, 393, 445–447 CrossRef CAS.
- L. Isa, K. Kumar, M. Muller, J. Grolig, M. Textor and E. Reimhult, ACS Nano, 2010, 4, 5665–5670 CrossRef CAS PubMed.
- C. D. Dushkin, G. S. Lazarov, S. N. Kotsev, H. Yoshimura and K. Nagayama, Colloid Polym. Sci., 1999, 277, 914–930 CAS.
- H. Cong and W. X. Cao, Langmuir, 2003, 19, 8177–8181 CrossRef CAS.
- C. Haginoya, M. Ishibashi and K. Koike, Appl. Phys. Lett., 1997, 71, 2934–2936 CrossRef CAS.
- Z. C. Zhou, Q. F. Yan, Q. Li and X. S. Zhao, Langmuir, 2007, 23, 1473–1477 CrossRef CAS PubMed.
- A. S. Iyer and L. A. Lyon, Angew. Chem., Int. Ed., 2009, 48, 4562–4566 CrossRef CAS PubMed.
- S. B. Quint and C. Pacholski, Soft Matter, 2011, 7, 3735–3738 RSC.
- C. Geng, L. Zheng, J. Yu, Q. Yan, T. Wei, X. Wang and D. Shen, J. Mater. Chem., 2012, 22, 22678–22685 RSC.
- M. Kondo, K. Shinozaki, L. Bergstroem and N. Mizutani, Langmuir, 1995, 11, 394–397 CrossRef CAS.
- J. Yu, C. Geng, L. Zheng, Z. H. Ma, T. Y. Tan, X. Q. Wang, Q. F. Yan and D. Z. Shen, Langmuir, 2012, 28, 12681–12689 CrossRef CAS PubMed.
- J. Yu, Q. F. Yan and D. Z. Shen, ACS Appl. Mater. Interfaces, 2010, 2, 1922–1926 CAS.
- M. Rey, R. Elnathan, R. Ditcovski, K. Geisel, M. Zanini, M. A. Fernandez-Rodriguez, V. V. Naik, A. Frutiger, W. Richtering, T. Ellenbogen, N. H. Voelcker and L. Isa, Nano Lett., 2016, 16, 157–163 CrossRef PubMed.
- M. Richter, M. Hunnenmorder and R. V. Klitzing, Colloid Polym. Sci., 2014, 292, 2439–2452 CAS.
- R. H. Pelton and P. Chibante, Colloids Surf., 1986, 20, 247–256 CrossRef CAS.
- J. S. Kim, N. Singh and L. A. Lyon, Angew. Chem., Int. Ed., 2006, 45, 1446–1449 CrossRef CAS PubMed.
- R. Rengarajan, D. Mittleman, C. Rich and V. Colvin, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2005, 71, 016615 CrossRef PubMed.
- F. H. Kaatz, A. Bultheel and T. Egami, J. Mater. Sci., 2009, 44, 40–46 CrossRef CAS.
- H. Kawaguchi, Polym. Int., 2014, 63, 925–932 CrossRef CAS.
- S. Wellert, M. Richter, T. Hellweg, R. von Klitzing and Y. Hertle, Z. Phys. Chem., 2015, 229, 1225–1250 CrossRef CAS.
- A. Burmistrova, M. Richter, C. Uzum and R. von Klitzing, Colloid Polym. Sci., 2011, 289, 613–624 CAS.
- M. Destribats, M. Eyharts, V. Lapeyre, E. Sellier, I. Varga, V. Ravaine and V. Schmitt, Langmuir, 2014, 30, 1768–1777 CrossRef CAS PubMed.
- M. Destribats, V. Lapeyre, M. Wolfs, E. Sellier, F. Leal-Calderon, V. Ravaine and V. Schmitt, Soft Matter, 2011, 7, 7689–7698 RSC.
- G. Zhang, D. Wang, Z.-Z. Gu, J. Hartmann and H. Möhwald, Chem. Mater., 2005, 17, 5268–5274 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra00338b |
| This journal is © The Royal Society of Chemistry 2017 |