Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The effect of incorporating carboxylic acid functionalities into 2,2′-bipyridine on the biological activity of the complexes formed: synthesis, structure, DNA/protein interaction, antioxidant activity and cytotoxicity†
Thangavel Sathiya Kamatchiaf,
Nataraj Chitrapriyab,
Sarvana Loganthan Ashok Kumar c,
Jang Yoon Jungc,
Horst Puschmannd,
Frank R. Fronczeke and
Karuppannan Natarajan*a
c,
Jang Yoon Jungc,
Horst Puschmannd,
Frank R. Fronczeke and
Karuppannan Natarajan*a
aDepartment of Chemistry, Bharathiar University, Coimbatore 641046, India. E-mail: k_natraj6@yahoo.com; Fax: +91 422 242238; Tel: +91 422 2428319
bDepartment of Chemistry, Yeungnam University, Gyeongsan City, Gyeong-buk 712-749, Republic of Korea
cDepartment of Chemistry, GRT Institute of Engineering Technology, Tiruttani 631209, India
dDepartment of Chemistry, Durham University, Durham, DH1 3KE, UK
eDepartment of Chemistry, Louisiana State University, Baton Rouge, LA 70803, USA
fDepartment of Chemistry, Sakthi College of Arts and Science for Women, Oddanchatram, Dindigul 624 619, India
First published on 14th March 2017
Abstract
In order to find out the influence of carboxylic acid functionalities (COOH) present at different positions in 2,2′-bipyridine on various biological activities such as DNA/protein binding, antioxidant activity and cytotoxicity, three new ruthenium(II) complexes [RuCl2(bpy)(S-DMSO)2] (1) (bpy = 2,2′-bipyridine), [RuCl2(H2L1)(S-DMSO)2] (2) (H2L1 = 2,2′-bipyridine-4,4′-dicarboxylic acid) and [RuCl2(H2L2)(S-DMSO)2] (3) (H2L2 = 2,2′-bipyridine-5,5′-dicarboxylic acid) have been synthesized and structurally characterized by analytical and spectral methods. The structures of 1 and 3 have been determined by single crystal X-ray diffraction studies, which revealed that both are a roughly regular octahedron with bipyridine/bipyridine dicarboxylic acid as neutral bidentate donors with the involvement of both the nitrogen atoms of the bipyridine ring. In vitro DNA binding studies of the complexes were carried out employing absorption titrations, fluorescence spectra, thermal melting, viscosity and circular dichroic measurements, which disclosed that all the complexes bind to CT-DNA via groove binding. The interactions of the complexes with bovine serum albumin (BSA) were also investigated using UV-visible, fluorescence and synchronous fluorescence spectroscopic measurements. The results indicated that the new complexes quench the intrinsic fluorescence of BSA protein in a static quenching mode. The assessment of free radical scavenging ability involving the DPPH radical, hydroxyl radical, nitric oxide radical, superoxide anion radical, and hydrogen peroxide and a metal chelating assay showed that the new complexes 2 and 3 possess excellent radical scavenging properties over 1 and standard antioxidants vitamin C and BHT. The in vitro cytotoxic activity of the new ruthenium complexes has been validated against HCT-15, HeLa, SKOV3, MCF7 and SKMel2 human cancer cells by SRB assay and cytotoxic selectivity has been examined against NIH 3T3 and HEK 293 normal cells by MTT assay and compared with that of the ruthenium anticancer drug NAMI A and standard platinum drug, cisplatin. The results indicated that the new complexes 2 and 3 displayed substantial cytotoxic specificity towards cancer cells only. Incorporation of a carboxylic acid group in the bipyridine moiety has resulted in showing differences in DNA/protein binding affinity, efficiency in antioxidant activity and cytotoxicity.
Introduction
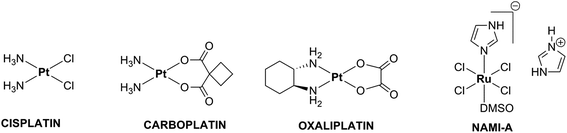
Cancer is one of the diseases that has tormented human beings throughout history, for which a wide variety of treatments have been implemented over the years, like surgical removal of tumors, radiation treatment and chemotherapy.1 Chemotherapy is done most effectively with platinum complexes and out of thousands of synthesized and evaluated Pt(II) complexes, only three of them, namely, cisplatin, carboplatin and oxaliplatin (Fig. 1) have only been approved for worldwide clinical practice and have enjoyed a huge clinical and commercial hit.2–4 Despite the widespread clinical use, chemotherapy with cisplatin and its analogues still has several drawbacks, in particular: (i) a relatively narrow spectrum of activity (ii) severe side-effects and (iii) acquired resistance.5 To overcome these problems, a search for new pharmaceutical agents featuring alternative metals has been undertaken.2,6 Among the several metals that are currently being investigated for their anticancer activity, ruthenium occupies a prominent position.6a,7 However, many of the ruthenium complexes are barely soluble in aqueous solution6a,7b,8 and the solubility of ruthenium compounds has been increased by incorporating dialkylsulfoxide derivatives in the complexes as in [trans-RuCl5(DMSO)Im][ImH] (NAMI-A) (Fig. 1), which is now recognized as the most successful ruthenium-based anticancer complex that has entered clinical trials.9 Hence, we attempted to synthesise new ruthenium complexes by incorporating the aforementioned key feature dimethylsulphoxide and also the DNA intercalating moiety bipyridine. Moreover, very little is known in the literature about the reactivity of Ru–chloride–DMSO complexes toward carboxylate or dicarboxylate ligands.10 But, it is reported that an appropriate attachment of the carboxylic acid (COOH) group in coordination complexes could modulate the solubility of the complex, cell transport and biological activity.11 This has inspired us to synthesize three new ruthenium complexes containing bipyridine and bipyridine dicarboxylic acids with DMSO as ancillary ligand.It is a well known fact that DNA and protein are the major pharmacological targets of anticancer drugs,12 and hence, the objective of the present work is to understand in detail the DNA binding mode of the new complexes with the aid of different techniques. It has been proved that free radicals such as superoxide anion (O2˙−), hydroxyl radical (OH˙) and hydrogen peroxide (H2O2) can induce DNA damage in humans. This kind of damage to DNA has also been shown to contribute to aging and various diseases including cardiovascular, cancer and chronic inflammation.13,14 This has prompted us to test the synthesized complexes as free radical scavengers against various free radicals. Further, the in vitro anticancer activities of the synthesized complexes were also evaluated on a panel of cancer and normal cell lines.
Experimental section
Materials and instrumentation
All chemicals were reagent grade and were used as received from commercial suppliers unless otherwise stated. Commercially available RuCl3·3H2O (Himedia) was used to prepare the starting complex. The starting complex cis-[RuCl2(DMSO)4] was prepared according to the method reported by Evans et al.15 2,2′-Bipyridine was purchased from Merck chemicals. 2,2′-Bipyridine-4,4′-dicarboxylic acid and 2,2′-bipyridine-5,5′-dicarboxylic acid were purchased from Sigma-Aldrich. Melting points were determined with Lab India instrument. Elemental analyses of carbon, hydrogen, nitrogen and sulphur were performed on Vario EL III Elementar elemental analyzer. Electronic absorption spectra of the complexes were recorded using JASCO 600 spectrophotometer and emission measurements were carried out by using a JASCO FP-6600 spectrofluorometer. Nicolet Avatar Model FT-IR spectrophotometer was used to record the IR spectra (4000–400 cm−1) of the free ligands and complexes as KBr pellets. 1H NMR spectra were recorded on Bruker AMX 500 at 500 MHz using tetramethylsilane as an internal standard. The chemical shifts are expressed in parts per million (ppm). Calf thymus DNA (CT-DNA) was purchased from Sigma and dissolved in 5 mM Tris–HCl buffer (pH 7.0) containing 100 mM NaCl and 1 mM EDTA. It was dialyzed several times against 5 mM Tris–HCl buffer. All experiments involving interactions of complexes with CT-DNA were carried out in Tris HCl buffer (pH 7.0). Bovine serum albumin (BSA) and ethidium bromide (EB) were obtained from Sigma-Aldrich and used as received. Antioxidant activity measurements were done using UV spectrophotometer (UV-1800, Shimadzu).Synthesis of new ruthenium(II) complexes
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1602 ν(C
C), 1602 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1085 ν(S
N), 1085 ν(S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)S-bonded. UV-visible (3% DMSO/H2O), λmax, nm (ε, dm3 mol−1 cm−1): 243 (38
O)S-bonded. UV-visible (3% DMSO/H2O), λmax, nm (ε, dm3 mol−1 cm−1): 243 (38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 941), 287 (58
941), 287 (58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800), 320 (34
800), 320 (34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 858), 343 (9400), 400 (6325). 1H NMR δH (CDCl3, ppm): 3.58, 3.55, 3.24, 2.68 (4 s, –CH3), 9.90 (1H, d, J = 8.0 Hz,
858), 343 (9400), 400 (6325). 1H NMR δH (CDCl3, ppm): 3.58, 3.55, 3.24, 2.68 (4 s, –CH3), 9.90 (1H, d, J = 8.0 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH6), 9.73 (1H, d, J = 8.0 Hz,
CH6), 9.73 (1H, d, J = 8.0 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH6′), 8.04 (1H, t, J = 5.5, J = 7.5 Hz,
CH6′), 8.04 (1H, t, J = 5.5, J = 7.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH5), 7.93 (1H, t, J = 8 Hz,
CH5), 7.93 (1H, t, J = 8 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH5′), 7.63 (1H, t, J = 7.0 Hz,
CH5′), 7.63 (1H, t, J = 7.0 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH4), 7.48 (1H, dd, J = 8.5 Hz,
CH4), 7.48 (1H, dd, J = 8.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH4′), 8.19 (1H, d, J = 7.0 Hz,
CH4′), 8.19 (1H, d, J = 7.0 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH3), 8.14 (1H, d, J = 7.5 Hz,
CH3), 8.14 (1H, d, J = 7.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH3′).
CH3′).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) chloroform–methanol) and attempts to isolate crystals suitable for single crystal XRD studies were unsuccessful. Yield: 0.085 g, 71%. Melting point: >300 °C. Elemental analyses calculated for C16H20N2O6S2Cl2Ru: C, 33.57; H, 3.52; N, 4.89; S, 11.20%; found: C, 33.51; H, 3.52; N, 4.89; S, 11.21%. FT-IR (cm−1) in KBr: 3387 ν(COOH), 1711 ν(C
5) chloroform–methanol) and attempts to isolate crystals suitable for single crystal XRD studies were unsuccessful. Yield: 0.085 g, 71%. Melting point: >300 °C. Elemental analyses calculated for C16H20N2O6S2Cl2Ru: C, 33.57; H, 3.52; N, 4.89; S, 11.20%; found: C, 33.51; H, 3.52; N, 4.89; S, 11.21%. FT-IR (cm−1) in KBr: 3387 ν(COOH), 1711 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1609 ν(C
O), 1609 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1075 ν(S
N), 1075 ν(S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)S-bonded. UV-visible (3% DMSO/H2O), λmax, nm (ε, dm3 mol−1 cm−1): 246 (34
O)S-bonded. UV-visible (3% DMSO/H2O), λmax, nm (ε, dm3 mol−1 cm−1): 246 (34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 375), 300 (54
375), 300 (54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 391), 331 (32
391), 331 (32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 116), 405 (6775). 1H NMR δH (CDCl3, ppm): 3.51, 3.28, 3.09, 2.42 (4 s, –CH3), 9.29 (1H, d, J = 8.5 Hz,
116), 405 (6775). 1H NMR δH (CDCl3, ppm): 3.51, 3.28, 3.09, 2.42 (4 s, –CH3), 9.29 (1H, d, J = 8.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH6), 7.76 (1H, d, J = 9.0 Hz,
CH6), 7.76 (1H, d, J = 9.0 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH6′), 7.98 (1H, dd, Jo = 7.5 Hz, Jm = 1.5 Hz,
CH6′), 7.98 (1H, dd, Jo = 7.5 Hz, Jm = 1.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH5), 7.07 (1H, dd, Jo = 8 Hz, Jm = 2.0 Hz,
CH5), 7.07 (1H, dd, Jo = 8 Hz, Jm = 2.0 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH5′), 8.59 (1H, d, Jm = 2.0 Hz,
CH5′), 8.59 (1H, d, Jm = 2.0 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH3), 8.43 (1H, d, Jm = 2.0 Hz,
CH3), 8.43 (1H, d, Jm = 2.0 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH3′), 12.74, 13.30 (2 s, –COOH).
CH3′), 12.74, 13.30 (2 s, –COOH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1642 ν(C
O), 1642 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1075 ν(S
N), 1075 ν(S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)S-bonded. UV-visible (3% DMSO/H2O), λmax, nm (ε, dm3 mol−1 cm−1): 254 (24
O)S-bonded. UV-visible (3% DMSO/H2O), λmax, nm (ε, dm3 mol−1 cm−1): 254 (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 691), 301 (53
691), 301 (53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 425), 330 (25
425), 330 (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250), 407 (3983). 1H NMR δH (CDCl3, ppm): 3.42, 3.36, 3.02, 2.38 (4 s, –CH3), 10.27 (1H, d, Jm = 2.0 Hz,
250), 407 (3983). 1H NMR δH (CDCl3, ppm): 3.42, 3.36, 3.02, 2.38 (4 s, –CH3), 10.27 (1H, d, Jm = 2.0 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH6), 10.07 (1H, d, Jm = 2.0 Hz,
CH6), 10.07 (1H, d, Jm = 2.0 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH6′), 8.86 (1H, d, J = 8.0 Hz,
CH6′), 8.86 (1H, d, J = 8.0 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH4), 8.81 (1H, d, J = 8.5 Hz,
CH4), 8.81 (1H, d, J = 8.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH4′), 8.63 (1H, dd, Jo = 8.0 Hz, Jm = 2.0 Hz,
CH4′), 8.63 (1H, dd, Jo = 8.0 Hz, Jm = 2.0 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH3), 8.50 (1H, dd, Jo = 8.0 Hz, Jm = 1.5 Hz,
CH3), 8.50 (1H, dd, Jo = 8.0 Hz, Jm = 1.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH3′), 13.49, 13.91 (2 s, –COOH).
CH3′), 13.49, 13.91 (2 s, –COOH).X-ray crystallography
X-ray diffraction measurements of complex 1 were performed on a Xcalibur, Sapphire3, Gemini ultra diffractometer. The crystal was kept at 120 K during data collection. Using Olex2,16 the structure was solved with the Olex2.solve17 structure solution program using charge flipping and refined with the SHELXL18 refinement package using least squares minimization. X-ray diffraction measurements of complex 3 were performed at 95 K on a Nonius Kappa CCD diffractometer equipped with graphite monochromated Mo Kα radiation and an Oxford Cryostream cryostat at temperature. The structures of the complexes were solved by direct methods and refinements were carried out by using full matrix least-squares techniques. The hydrogen atoms were generally visible in difference maps and were placed in idealized positions and treated as riding in the refinements, except for those on one water molecule, for which coordinates were refined. For the other two water molecules, H atoms were not located. The following computer programs were used: structure solution SIR-97,19 refinement SHELXL-97,18 molecular diagrams and ORTEP-3 (ref. 20) for Windows.DNA binding experiments
The concentrations of DNA and the new complexes 1–3 were determined spectrophotometrically using their extinction coefficients ε258 nm = 6700 M−1 cm−1, ε287 nm = 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1 cm−1, ε300 nm = 54
800 M−1 cm−1, ε300 nm = 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 391 M−1 cm−1 and ε301 nm = 53
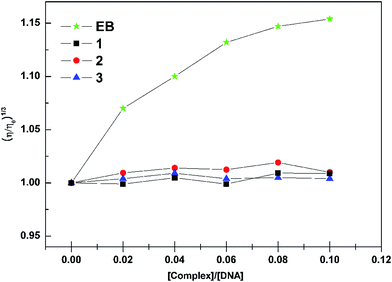
391 M−1 cm−1 and ε301 nm = 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 425 M−1 cm−1 respectively. The experiments were carried out in 5 mM Tris–HCl buffer (pH 7.0) at ambient temperature and the complexes were dissolved in 5 mM Tris–HCl buffer containing 3% DMSO. Changes in the fluorescence emission spectrum of the ethidium bromide–DNA complex were recorded under various complex concentrations. The fluorescence spectra in the fluorimeter were obtained at an excitation wavelength of 522 nm and an emission wavelength of 584 nm. Melting profiles were measured at 260 nm by a Cary 300 spectrophotometer. Readings were recorded for every 2 °C raise in temperature per minute. The viscosity measurement was carried out using an Ubbelohde viscometer immersed in a thermostatic water bath maintained at 25 ± 0.1 °C. DNA samples with approximately 200 base pairs in length were prepared by sonication in order to minimize complexities arising from DNA flexibility. Flow times were measured with a digital stopwatch; each sample was measured three times, and an average flow time was calculated. Relative viscosities for CT-DNA in the presence and absence of the complex were calculated from the relation η = (t − t0)/t0, where t is the observed flow time of DNA-containing solution and t0 is the flow time of Tris–HCl buffer alone. Data are presented as (η/η0)1/3 versus binding ratio, where η is the viscosity of CT-DNA in the presence of complex and η0 is the viscosity of CT-DNA alone.
425 M−1 cm−1 respectively. The experiments were carried out in 5 mM Tris–HCl buffer (pH 7.0) at ambient temperature and the complexes were dissolved in 5 mM Tris–HCl buffer containing 3% DMSO. Changes in the fluorescence emission spectrum of the ethidium bromide–DNA complex were recorded under various complex concentrations. The fluorescence spectra in the fluorimeter were obtained at an excitation wavelength of 522 nm and an emission wavelength of 584 nm. Melting profiles were measured at 260 nm by a Cary 300 spectrophotometer. Readings were recorded for every 2 °C raise in temperature per minute. The viscosity measurement was carried out using an Ubbelohde viscometer immersed in a thermostatic water bath maintained at 25 ± 0.1 °C. DNA samples with approximately 200 base pairs in length were prepared by sonication in order to minimize complexities arising from DNA flexibility. Flow times were measured with a digital stopwatch; each sample was measured three times, and an average flow time was calculated. Relative viscosities for CT-DNA in the presence and absence of the complex were calculated from the relation η = (t − t0)/t0, where t is the observed flow time of DNA-containing solution and t0 is the flow time of Tris–HCl buffer alone. Data are presented as (η/η0)1/3 versus binding ratio, where η is the viscosity of CT-DNA in the presence of complex and η0 is the viscosity of CT-DNA alone.
Protein binding studies
Binding of the complexes with bovine serum albumin (BSA) was studied from the fluorescence spectra recorded with an excitation wavelength of at 280 nm and the corresponding emission at 345 nm assignable to that of BSA. The excitation and emission slit widths and scan rates were maintained constant for all of the experiments. A stock solution of BSA was prepared in 50 mM phosphate buffer (pH = 7.2) and stored in the dark at 4 °C for further use. A concentrated stock solution of the complexes was prepared as mentioned for the DNA binding experiments, except that the phosphate buffer was used instead of a Tris–HCl buffer for all of the experiments. In all the experiments the concentration of BSA was kept constant at 1 μM and complexes 1–3 were varied from 0–30 μM. Titrations were manually done by using a micropipette for the addition of the complexes. For synchronous fluorescence spectra also, the same concentrations of BSA and the complexes were used and the spectra were measured at two different Δλ values (difference between the excitation and emission wavelengths of BSA), such as 15 and 60 nm.Antioxidant assays
The ability of ruthenium complexes to act as hydrogen donors or free radical scavengers was tested by conducting a series of in vitro antioxidant assays involving DPPH radical, hydroxyl radical, nitric oxide radical, hydrogen peroxide, superoxide anion radical, metal chelating assay and the results were compared with that of standard antioxidants including natural antioxidant vitamin C and synthetic antioxidant BHT (Butylated Hydroxy Toluene).The DPPH radical scavenging activity of the complexes was measured according to the method of Blois.21 The DPPH radical is a stable free radical and due to the presence of an odd electron, it shows a strong absorption band at 517 nm in visible spectrum. If this electron becomes paired off in the presence of a free radical scavenger, this absorption vanishes resulting in decolorization stoichiometrically with respect to the number of electrons taken up. Various concentrations of the experimental complexes were taken and the volumes were adjusted to 100 μL with methanol. About 5 mL of 0.1 mM methanolic solution of DPPH was added to the aliquots of samples and standards (BHT and vitamin C) and shaken vigorously. Negative control was prepared by adding 100 μL of methanol in 5 mL of 0.1 mM methanolic solution DPPH. The tubes were allowed to stand for 20 min at 27 °C. The absorbance of the sample was measured at 517 nm against the blank (methanol).
The hydroxyl radical scavenging activity of the complex has been investigated by using the Nash method.22 In vitro hydroxyl radicals were generated by Fe3+/ascorbic acid system. The detection of hydroxyl radicals was carried out by measuring the amount of formaldehyde formed from the oxidation reaction with DMSO. The formaldehyde produced was detected spectrophotometrically at 412 nm. In a typical experiment, a mixture of 1.0 mL of iron–EDTA solution (0.13% ferrous ammonium sulfate and 0.26% EDTA), 0.5 mL of EDTA solution (0.018%), and 1.0 mL of DMSO (0.85% DMSO (v/v) in 0.1 M phosphate buffer, pH 7.4) were sequentially added in the test tubes which contains fixed concentration of the test compounds. The reaction was initiated by adding 0.5 mL of ascorbic acid (0.22%) and was incubated at 80–90 °C for 15 min in a water bath. After incubation, the reaction was terminated by the addition of 1.0 mL of ice-cold trichloroacetic acid (17.5% w/v). Subsequently, 3.0 mL of Nash reagent was added to each tube and left at room temperature for 15 min. The intensity of the colour formed was measured spectrophotometrically at 412 nm against the reagent blank.
Assay of nitric oxide (NO˙) scavenging activity is based on the method,23 where sodium nitroprusside in aqueous solution at physiological pH spontaneously generates nitric oxide, which interacts with oxygen to produce nitrite ions. This can be estimated using Griess reagent. Scavengers of nitric oxide compete with oxygen leading to reduced production of nitrite ions. For the experiment, sodium nitroprusside (10 mM) in phosphate buffered saline was mixed with a fixed concentration of the complex, standards and incubated at room temperature for 150 min. After the incubation period, 0.5 mL of Griess reagent containing 1% sulfanilamide, 2% H3PO4 and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride was added. The absorbance of the chromophore formed was measured at 546 nm.
The ability of the complexes to scavenge hydrogen peroxide was determined using the method of Ruch et al.24 In a typical experiment, a solution of hydrogen peroxide (2.0 mM) was prepared in phosphate buffer (0.2 M, pH 7.4) and its concentration was determined spectrophotometrically from absorption at 230 nm with molar absorptivity 81 M−1 cm−1. The complexes (100 μg mL−1), BHT and vitamin C (100 μg mL−1) were added to 3.4 mL of phosphate buffer prepared above together with hydrogen peroxide solution (0.6 mL). An identical reaction mixture without the sample was taken as negative control. Absorbance of hydrogen peroxide at 230 nm was determined after 10 min against the blank (phosphate buffer).
The superoxide anion radical (O2−˙) scavenging assay is based on the capacity of the complexes to inhibit formazan formation by scavenging the superoxide radicals generated in riboflavin-light-NBT system.25 In a typical experiment, a 3 mL reaction mixture contained 50 mM sodium phosphate buffer (pH 7.6), 20 μg riboflavin, 12 mM EDTA, 0.1 mg NBT and 1 mL complex solution (20–100 μg mL−1). Reaction was started by illuminating the reaction mixture with different concentrations of complex for 90 s. Immediately after illumination, the absorbance was measured at 590 nm. The entire reaction assembly was enclosed in a box lined with aluminium foil. Identical tubes with reaction mixture kept in dark served as blanks.
The chelation with ferrous ions by the experimental complexes was estimated by the method of Dinis et al.26 Initially, about 100 μL of the samples and the standards were added to 50 μL solution of 2 mM FeCl2. The reaction was initiated by the addition of 200 μL of 5 mM ferrozine and the mixture was shaken vigorously and left standing at room temperature for 10 min. Absorbance of the solution was then measured spectrophotometrically at 562 nm against the blank (deionized water).
For the above six assays, all the tests were run in triplicate and the percentage activity was calculated with the help of the following equation
| Scavenging activity (%) = [(A0 − A1)/A0] × 100 |
When the inhibition of the tested compounds is 50%, the tested compound concentration is IC50.
In vitro anticancer activity evaluation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well and incubated at 37 °C, 5% CO2, 95% air and 100% relative humidity for 24 h prior to addition of complexes and the standard drugs NAMI A and cisplatin. The test compounds were dissolved in DMSO and diluted in respective medium containing 1% FBS. After 24 h, the medium was replaced with respective medium with 1% FBS containing the test compounds at various concentration and incubated at 37 °C, 5% CO2, 95% air and 100% relative humidity for 48 h. Triplicate was maintained and the medium without the test compounds was served as control. After 48 h, 10 μL of MTT (5 mg mL−1) in phosphate buffered saline (PBS) was added to each well and incubated at 37 °C for 4 h. The medium with MTT was then flicked off and the formed formazan crystals were dissolved in 100 μL of DMSO and then measured the absorbance at 570 nm using micro plate reader. The % cell inhibition was determined using the following formula, and a graph was plotted between % of cell inhibition and concentration. From this plot, the IC50 value was calculated.
000 cells per well and incubated at 37 °C, 5% CO2, 95% air and 100% relative humidity for 24 h prior to addition of complexes and the standard drugs NAMI A and cisplatin. The test compounds were dissolved in DMSO and diluted in respective medium containing 1% FBS. After 24 h, the medium was replaced with respective medium with 1% FBS containing the test compounds at various concentration and incubated at 37 °C, 5% CO2, 95% air and 100% relative humidity for 48 h. Triplicate was maintained and the medium without the test compounds was served as control. After 48 h, 10 μL of MTT (5 mg mL−1) in phosphate buffered saline (PBS) was added to each well and incubated at 37 °C for 4 h. The medium with MTT was then flicked off and the formed formazan crystals were dissolved in 100 μL of DMSO and then measured the absorbance at 570 nm using micro plate reader. The % cell inhibition was determined using the following formula, and a graph was plotted between % of cell inhibition and concentration. From this plot, the IC50 value was calculated.| % inhibition = [mean OD of untreated cells (control)/mean OD of treated cells (control)] × 100. |
Results and discussion
Synthesis and characterization of the complexes
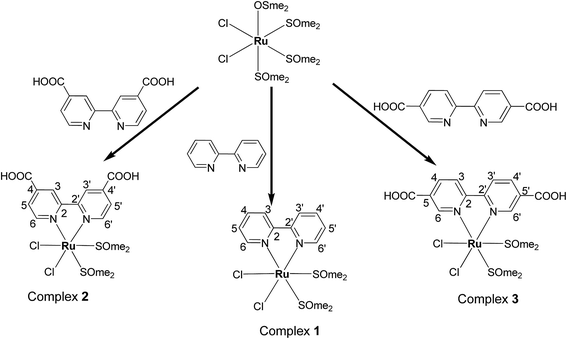
The straightforward reactions of the bipyridine (bpy) and bipyridine dicarboxylic acids (H2LI/H2L2) with cis-[RuCl2(DMSO)4] gave new complexes of the types [RuCl2(bpy)(S-DMSO)2] and [RuCl2(H2L)(S-DMSO)2] where H2L = H2L1/H2L2 as sketched out in Scheme 1. The complexes are diamagnetic corresponding to bivalent state of ruthenium (low-spin d6, S = 0). The complexes were stable to air and light, non-hygroscopic in nature and were remarkably soluble in CHCl3, CH2Cl2, CH3CN, DMF and DMSO. These complexes were synthesized in good yields and characterized by elemental analysis, IR and 1H NMR spectroscopic techniques. The retention of the IR band around 3375–3387 cm−1 in 2 and 3 confirms the non participation of COOH group of bipyridine dicarboxylic acid in coordination, which is consistent with the results of NMR and X-ray analysis. 1H NMR spectrum of the complex 1 is given in Fig. S1 (ESI†). It has been observed that a molecule of the ligands (bpy, H2L1 and H2L2) replaced an S-bonded DMSO and an O-bonded DMSO from the precursor complex cis-[RuCl2(O-DMSO)(S-DMSO)3]. The solid state structure of the complexes 1 and 3 were determined by single crystal X-ray crystallographic studies. It revealed that bipyridine and bipyridine dicarboxylic acid were coordinated to the metal ion as neutral bidentate NN donors.X-ray crystallography
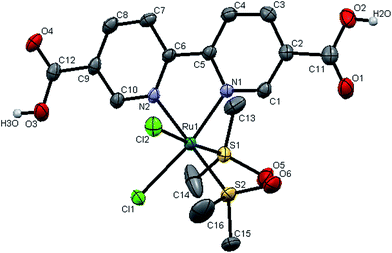
The molecular structures of complexes 1 and 3 were established by single crystal X-ray analysis. The ORTEP diagrams with the atom numbering scheme of 1 and 3 are displayed in Fig. 2 and 3 respectively. The relevant details concerning the data collection and structure refinement of the complexes were summarized in Table 1 and selected geometrical parameters (inter atomic distances and angles) are given Table S1 (ESI†). Numerous ruthenium complexes with two or three N–N ligands are known, but examples of mono (N–N) complexes remain scarce, because it is difficult to prevent ligand redistribution during synthesis. The precursor contains three S-bonded DMSO's and one O-bonded DMSO and the structural formula can be written as cis,fac-[RuCl2(DMSO)3(DMSO)]. The O-bonded DMSO is the most labile ligand in cis,fac-[RuCl2(DMSO)3(DMSO)] and is selectively replaced by stronger σ- and π-donors (L) leaving the geometry of the resulting complex unchanged forming cis,fac-[RuCl2(DMSO)3(L)]. With the chelating ligands (LL′), displacement of the more weakly held O-bonded DMSO occurs first followed by the displacement of a DMSO ligand cis to it.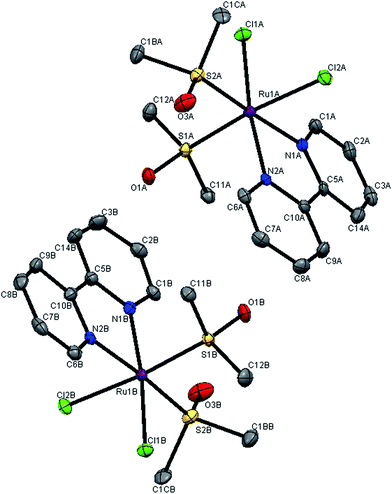 | ||
| Fig. 2 X-ray crystal structure with atom numbering scheme for complex 1 as thermal ellipsoids at 50% probability level. The hydrogen atoms have been omitted for clarity. | ||
| Complex 1 | Complex 3 | |
|---|---|---|
| CCDC deposit number | 869937 | 843881 |
| Empirical formula | C14H20Cl2N2O2RuS2 | C16H26Cl2N2O9RuS2 |
| Formula weight | 484.41 | 626.48 |
| Temperature/K | 120.0 (2) | 95.0 (5) |
| Crystal system | Orthorhombic | Monoclinic |
| Space group | Pca21 | P21/c |
| a/Å | 27.7264(7) | 13.794(2) |
| b/Å | 7.7631(3) | 12.924(2) |
| c/Å | 16.5025(7) | 14.1348(15) |
| α/° | 90 | 90 |
| β/° | 90 | 114.513(7) |
| γ/° | 90 | 90 |
| Volume/Å3 | 3552.1(2) | 2292.7(5) |
| Z | 8 | 4 |
| ρcalc mg mm−3 | 1.812 | 1.815 |
| m/mm−1 | 1.427 | 1.150 |
| F(000) | 1952 | 1272 |
| Crystal size/mm3 | 0.47 × 0.36 × 0.26 | 0.25 × 0.10 × 0.10 |
| 2θ range for data collection | 5.25 to 52° | 6.24 to 61° |
| Index ranges | −34 ≤ h ≤ 34 | −18 ≤ h ≤ 19 |
| −9 ≤ k ≤ 9 | −11 ≤ k ≤ 18 | |
| −20 ≤ l ≤ 20 | −19 ≤ l ≤ 18 | |
| Reflections collected | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 162 162 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 867 867 |
| Independent reflections | 6978 [R(int) = 0.0526] | 6251 [R(int) = 0.0208] |
| Data/restraints/parameters | 6978/1/423 | 6251/0/302 |
| Goodness-of-fit on F2 | 1.128 | 1.013 |
| Final R indexes [I ≥ 2σ(I)] | R1 = 0.0292 | R1 = 0.0479 |
| wR2 = 0.0703 | wR2 = 0.1064 | |
| Final R indexes [all data] | R1 = 0.0301 | R1 = 0.0633 |
| wR2 = 0.0709 | wR2 = 0.1151 | |
| Largest diff. peak/hole/e Å−3 | 2.31/−0.72 | 1.79/−1.32 |
Crystal structure of the complexes 1 and 3
The crystal structures of the complexes 1 and 3 were obtained by examining the nice crystals obtained in the course of slow evaporation. From unit cell dimensions, it is clear that the crystal system of complex 1 is orthorhombic belonging to the space group Pca21 whereas complex 3 is crystallized in monoclinic space group P21/c. In complex 1, there are two independent complex molecules in the asymmetric unit, related by an approximate inversion center near 1/8, 3/4, z, as shown in the ORTEP diagram 2. This is the most common location for local centers in this space group.27 For the new complexes, the coordination sphere around ruthenium centre constitutes distorted octahedron with coordination of bipyridine N–N atoms with bite angles N(1A)–Ru(1A)–N(2A) = 78.17(19)°, N(1B)–Ru(1B)–N(2B) = 78.84(19)° for complex 1 and N(1)–Ru(1)–N(2) = 78.7(1)° for complex 3. The two S-coordinated DMSO molecules and the two chloride ions in the coordination sphere were found to be cis pairs. The basal plane is constructed of two nitrogen atoms of the bipyridine ligand, a chloride and an S-bonded DMSO. The remaining apical coordination sites are filled up by a chloride and an S-bonded DMSO. The distortion in the complex from ideal octahedral geometry is due to the customary small bite angle of the NN chelate and the bending of somewhat bulky chloride and a DMSO ligands towards the chelate, which is evident from the angles N(1A)–Ru(1A)–N(2A) = 78.17(19)°, N(1B)–Ru(1B)–N(2B) = 78.84(19)° [smaller than S(1A)–Ru(1A)–S(2A) = 92.68(6)°; S(1B)–Ru(1B)–S(2B) = 93.80(6)° and Cl(1A)–Ru(1A)–Cl(2A) = 89.31(5)°; Cl(1B)–Ru(1B)–Cl(2B) = 90.39(5)°] and S(1A)–Ru(1A)–Cl(2A) = 174.17(6)°, S(1B)–Ru(1B)–Cl(2B) = 174.58(6)° in complex 1, where as N(1)–Ru(1)–N(2) = 78.70(1)° [smaller than S1–Ru(1)–S(2) = 92.94(4)° and Cl(1)–Ru(1)–Cl(2) = 89.55(4)°] and S(1)–Ru(1)–Cl(2) = 175.96(4)° in complex 3. The average Ru–Cl bond lengths in complex 1, Ru(1)–Cl(1) = 2.427 Å; Ru(1)–Cl(2) = 2.426 Å [Ru(1A)–Cl(1A) = 2.4229(14), Ru(1A)–Cl(2A) = 2.4310(15), Ru(1B)–Cl(1B) = 2.4209(15), Ru(1B)–Cl(2B) = 2.43091(16)] and in complex 3, Ru(1)–Cl(1) = 2.414 (1) Å; Ru(1)–Cl(2) = 2.4289 (9) Å is comparable with that of cis,fac-[RuCl2(DMSO)3(DMSO)] (average Ru–Cl = 2.42 Å)28 and other Ru(II)–DMSO–Cl complexes.29,30 The geometry of the coordinated DMSO is approximately tetrahedral with angles ranging from 111 to 119° in the two complexes. The Ru–S bond lengths [for 1, Ru(1A)–S(1A) = 2.2322(15); Ru(1A)–S(2A) = 2.2888(16); Ru(1B)–S(1B) = 2.2366(15); Ru(1B)–S(2B) = 2.2885(15) Å; for 3, Ru(1)–S(1) = 2.2258(9); Ru(1)–S(2) = 2.291(1) Å] are in the same range as observed in other Ru(II)–DMSO–Cl complexes.31–33 The S–O bond distance [for 1, S(1A)–O(1A) = 1.484(4), S(2A)–O(3A) = 1.489(5); S(1B)–O(1B) = 1.478(4), S(2B)–O(3B) = 1.477(5) Å; for 3, S(1)–O(5) = 1.493(3), S(2)–O(6) = 1.487(4) Å] and the S–C bond distance [1.784–1.790 Å in 1, 1.7745–1.797 Å in 3] are consistent with the values reported in the literature.31,34,35 The deviation of the bond angles in complex 1, S(1A)–Ru(1A)–S(2A) = 92.68(6)°; S(1B)–Ru1B–S2B = 93.80(6)° and in complex 3, S(1)–Ru(1)–S(2) = 92.68(4) from 90° is most probably due to steric repulsion between the DMSO molecules. The average Ru–N bond distance fall in the range of Ru–N = 2.082–2.086 Å in 1, and Ru–N = 2.083 Å in 3 and are consistent with the values reported in the literature.33,36,37DNA binding studies
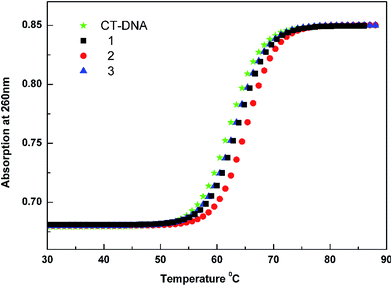
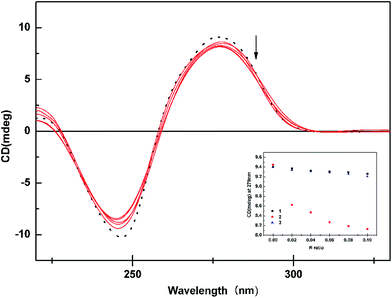
From the observed spectral data, the intrinsic binding constants for the complexes 1–3 were calculated using the eqn (1) at the π–π* absorption band and the values obtained are smaller than those that observed for intercalators.
| [DNA]/(εa − εf) = [DNA]/(εb − εf) + 1/(Kb(εb − εf)) | (1) |
In order to confirm the quenching mechanism in our cases, the fluorescence quenching was analyzed according to Stern–Volmer eqn (2) at different temperatures (Fig. S3 and Table S4, ESI†) and the quenching constants are given in Table 2.
| F0/F = 1 + Ksv[Q] | (2) |
| Complexes | 27 °C | 45 °C |
|---|---|---|
| 1 | 3.15 × 104 | 2.51 × 104 |
| 2 | 5.52 × 104 | 1.68 × 104 |
| 3 | 3.02 × 104 | 2.56 × 104 |
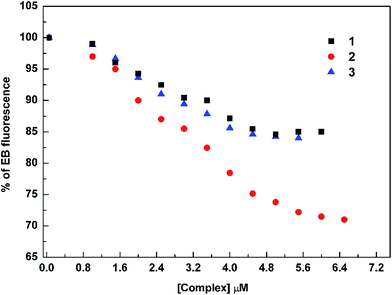
The results in Table 2 indicate that the probable quenching mechanism of fluorescence of EB–DNA by complexes 1–3 is by static quenching since in all cases Ksv has been seen to be inversely proportional to temperature.40
Protein binding studies
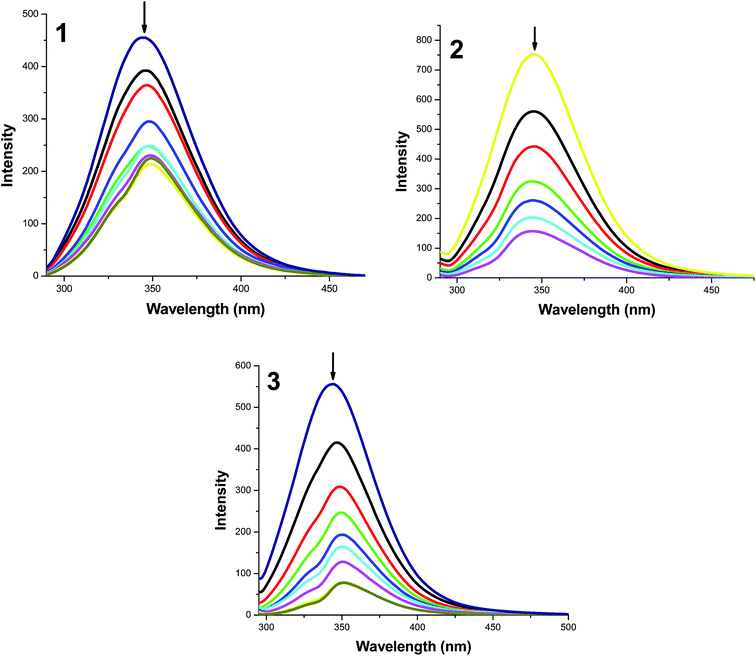
Since the interaction of biologically active compounds with proteins leads to either enhancement or loss of the biological properties of such compounds, it is important to study the interaction of any test compound with proteins. Hence, we have studied the interactions of our new complexes with BSA by means of UV-visible and fluorescence spectroscopy since BSA is one of the most extensively studied proteins, particularly because of its structural homology with human serum albumin (HSA). UV-visible spectra of BSA in the absence and presence of the complexes is shown in Fig. S4 (ESI†). It is seen from the Fig. S4† that the absorption intensity of BSA was enhanced as the complexes were added, and there was a small red shift of about 2, 7 and 2 nm for the compounds 1, 2 and 3 respectively. This result suggested not only the complex formation between BSA and the test compounds but also confirms a static quenching process.49Fluorescence quenching studies of BSA
In order to get more information on the binding of the compounds with BSA, fluorescence spectrum of BSA was studied upon the addition of the test compounds. Even though three fluorophores, namely, tryptophan, tyrosine and phenylalanine are present in BSA, the intrinsic fluorescence of BSA is mainly due to tryptophan alone50 and changes in the emission spectra of tryptophan can give information about protein conformational transitions, subunit associations, substrate binding, or denaturation. Therefore, the intrinsic fluorescence of BSA can provide considerable information on their structure and dynamics and is often employed in the study of protein folding and association reactions. Hence, the interaction of BSA with our complexes (1–3) was studied by fluorescence measurement at room temperature and from which the binding constants of the complexes were calculated. In a typical experiment, a solution of BSA (1 μM) was titrated with various concentrations of the complexes (0–35 μM). Fluorescence spectra were recorded in the range of 290–500 nm upon excitation at 280 nm. The changes observed on the fluorescence emission spectra of a solution of BSA on the addition of increasing amounts of complexes 1–3 are shown in Fig. 9. Addition of complexes to BSA produced a dramatic modification on the emission profile. The fluorescence of BSA was quenched effectively with the red shift of 5 nm in the case of complex 1 and blue and red shift of 8 and 2 nm in the emission maximum of the complexes 2 and 3 respectively. The observed difference in the initial fluorescence of BSA is mainly due to the fact that the active site in protein is buried in a hydrophobic environment. This result indicated a definite interaction of all of the test compounds with the BSA protein.To study the quenching process further, fluorescence quenching data were analyzed with the Stern–Volmer eqn (3) and Scatchard eqn (4). The ratio of the fluorescence intensity in the absence of (I0) and in the presence of (I) the quencher is related to the concentration of the quencher [Q] by a coefficient Ksv.
| I0/I = 1 + Ksv[Q] | (3) |
| Complexes | Ksv × M−1 | K × M−1 | n |
|---|---|---|---|
| BSA + 1 | 3.18 × 104 | 5.79 × 104 | 1.05 |
| BSA + 2 | 1.36 × 105 | 3.77 × 106 | 1.34 |
| BSA + 3 | 1.17 × 105 | 1.33 × 106 | 1.25 |
For the static quenching, when molecules bind independently to a set of equivalent sites on a macromolecule, the binding constant (K) and the number of binding sites (n) can be determined by the Scatchard eqn (4).
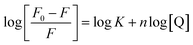 | (4) |
Synchronous fluorescence spectra
After having obtained the binding constant and binding number of the compounds with BSA, it is important to know about the conformational change of protein molecular environment in a vicinity of the fluorophore functional groups.51 The different nature of chromophores can be found from synchronous fluorescence spectroscopy by looking at the difference between excitation and emission wavelength (Δλ = λemi − λexc).52 A value of 15 nm for Δλ is characteristic of tyrosine residue, whereas a value of 60 nm is characteristic of tryptophan.53 This kind of variation in the tryptophan emission occurs due to protein conformational changes. In order to explore the structural change of BSA, we measured synchronous fluorescence spectra at Δλ = 15 nm (Fig. S7, ESI†) and Δλ = 60 nm (Fig. S8, ESI†) of BSA with complexes 1–3. The synchronous fluorescence spectral studies clearly suggested that the fluorescence intensities of both the tryptophan and tyrosine were affected with an increasing concentration of the complexes, which clearly indicated that the interaction of complexes with BSA affects the conformation of both the tryptophan and tyrosine micro regions. So, the strong interaction between the test compounds and BSA protein implied that these compounds can easily be stored in protein and can be released in desired targets.Bipyridine ligand systems are extremely versatile in that small structural changes can be readily made that lead to very different chemical and physical properties. The spectroscopic results show that the carboxylic acid functionalities at different positions on the bipyridine ligand can cause some interesting differences in their DNA/protein binding properties. Since the chelating part of the ligand and ancillary ligands is same in all the three complexes the difference in binding affinity depends on the position of the –COOH groups at bipyridine moiety. The substitution on 5,5′-position of pyridine rings of bipyridine ligand may cause steric constraints, when the complex approaches the DNA base pairs and protein, thus decreasing the binding affinity of complex with DNA/protein. In complex 2, the two –COOH substituents present at 4,4′-position of bipyridine ligand may bring some flexibility to the complex or furnish slightly tapered shape to chelating ligand which would help to approach the DNA/protein easily. This will lead to the formation of favourable H-bonding interactions with DNA groove/protein surface and two –COOH groups, thus resulting in the complex 2 bound to the grooves of DNA/protein more strongly. Surprisingly, the complexes 1 and 3 exhibit similar binding affinity although complex 1 does not have carboxylic acid functionality, which may be due to the less accessibility of the complex 3 towards DNA/protein and contribution of the ancillary ligands of bipyridine into the binding. It is remarkable that even seemingly minor changes in the ligand architecture and electronic structure can lead to profound effects on DNA/protein binding. These results have confirmed that the affinity magnitudes of the complexes toward DNA/protein may be controlled and tuned by playing with the position of the substituent on the chelating ligand and this strategy may be valuable in understanding the DNA/protein binding properties of the complexes containing bipyridine and bipyridine dicarboxylic acids as well as laying a foundation for the rational design of novel, powerful agents for probing and targeting nucleic acids.
Antioxidant activity studies
Since the experiments carried out so far revealed that the new ruthenium complexes exhibit reasonable DNA and protein binding affinity, it is considered worthwhile to test their ability to quench the free radicals and study their antioxidant properties. Usually free radicals are generated in many bioorganic redox processes and they induce oxidative damage in various components of the body (lipids, proteins and DNA) and they have been implicated in chronic diseases such as cancer, hypertension, Parkinson disease, Alzheimer, cardiac infarction, atherosclerosis, rheumatism, cataracts etc.54,55 Efforts to counteract the damage caused by the free radicals are gaining acceptance as an origin for novel therapeutic approaches and the field of preventive medicine is experiencing an upsurge of interest in medically useful antioxidants.The in vitro antioxidant properties of ruthenium complexes have attracted a lot of interest, but the radical scavenging activity is limited to hydroxyl radical.56 Hence, we carried out experiments to investigate the free radical scavenging ability of the new ruthenium complexes against a panel of free radicals, with a hope to develop potential antioxidants and therapeutic reagents. The IC50 value of all the complexes (Table 4) obtained from different types of assay experiments strongly supports that the new complexes possess good antioxidant activities, which are much better than that of the standard antioxidants vitamin C and BHT.
| Compounds | IC50a (μM) | |||||
|---|---|---|---|---|---|---|
| DPPH˙ | OH˙ | NO˙ | O2−˙ | H2O2 | Metal chelation | |
| a Fifty percent inhibitory concentration of the test compounds against free radicals. | ||||||
| 1 | 21.89 ± 0.92 | 59.24 ± 0.13 | 75.96 ± 0.30 | 57.06 ± 0.93 | 208.49 ± 5.19 | 117.80 ± 0.72 |
| 2 | 9.66 ± 0.82 | 44.42 ± 0.11 | 60.27 ± 0.21 | 35.86 ± 1.28 | 165.62 ± 0.69 | 7.11 ± 0.40 |
| 3 | 9.44 ± 1.21 | 43.56 ± 0.15 | 56.38 ± 0.20 | 26.36 ± 0.38 | 123.09 ± 0.72 | 6.65 ± 0.69 |
| Vitamin C | 147.6 ± 4.2 | 232.8 ± 1.9 | 215.8 ± 2.7 | 221.4 ± 1.2 | 238.5 ± 3.6 | — |
| BHT | 86.2 ± 1.8 | 163.4 ± 0.7 | 154.3 ± 2.4 | 131.6 ± 1.5 | 149.8 ± 4.3 | — |
Antioxidants exert their effect by different mechanisms such as scavenging or inhibiting free radicals by the donation of an electron or proton (H+) or chelation of metal ions that otherwise may lead to free radical formation. From the radical scavenging data (Table 4), it can be seen that complexes 2 and 3 showed better radical scavenging activity than complex 1. This might be due to the presence of free carboxylic acid groups in the bipyridine moiety of 2 and 3 thus making those complexes efficient hydrogen donors to stabilize the unpaired electrons and thereby scavenging the free radicals. Out of the five radical species chosen to examine, the DPPH radical scavenging power of the tested complexes was the most (9.44 ± 1.21 μM), and the hydrogen peroxide scavenging ability was the least (208.49 ± 5.19 μM). The antioxidant ability of any compound besides being related with the hydrogen atom transfer reaction could also be due to its capacity to chelate metal ions and/or inhibit oxidative enzymes. Earlier investigations have shown that the participation of perferryl complex (ADP–Fe3+–O2−˙) in the initiation and propagation of lipid peroxidation, indicating the requirement of oxygen and free iron.57 Hence, it is inferred that the presence of molecules with the ability to chelate metal ions could reduce the reactive species which will lead to the protection of lipid membranes against peroxidation. Hence, we studied the metal chelating capacity of our compounds with Fe2+ ion which showed that the complexes exhibited moderate to high metal chelating activity which might be due to the chelation of Fe2+ ion by the uncoordinated COOH group. A plausible mechanism for metal chelating and DPPH scavenging activity of the complexes is given in Fig. S9 (ESI†). The appropriate attachment of COOH group (H+ donation and COO− chelation) is only the responsible factor, which makes 2 and 3 superior to 1 in all the radical scavenging assays including metal chelating activity. Though the structural differences between the two compounds 2 and 3 are not very pronounced, the complex 3 displayed better antioxidant activity than 2 in of all assays, suggesting that the possible intramolecular hydrogen bonding formation in 2 may hinder the donation of H+ in neutralizing the free radicals. The metal chelating and DPPH radical scavenging ability of the new complexes are better than those that were previously reported by us for other ruthenium(II) complexes comprising dicarboxylic acids.58,11c
Anticancer activity studies
Since the results of antioxidant activity experiments revealed that the new complexes exhibit good antioxidant activity, we switched over our study to anticancer activity evaluation because nowadays it has been strongly suspected that cancer may be one of those degenerative disease induced by free radicals. Further, substantial evidence supports the active involvement of free radicals in the development of several pathological conditions including neurodegenerative and cardiovascular diseases, diabetes, cancer or even normal aging. To study cytotoxic activity of the new complexes on cancer cells, the SRB test was used. Moreover, since the balance between the therapeutic potential and toxic side effects of a compound is very important when evaluating its usefulness as a pharmacological drug, experiments were also designed to investigate the in vitro cytotoxic selectivity of synthesized ruthenium complexes against normal cells by MTT assay. The cytotoxic activity study was carried with the complexes 1–3, using five different cancer cell lines HeLa, HCT-15, SKOV3, MCF7, SKMel2 and two normal cell lines NIH 3T3, HEK 293. The effects of the test compounds on the viability of these cells were evaluated after an exposure period of 48 h. The cells were treated with different concentrations of the test compounds. The test compounds were dissolved in DMSO and the blank samples containing same volume of DMSO were taken as controls to identify the activity of the solvent in the cytotoxicity experiment. In parallel, the influence of ruthenium anticancer drug NAMI A and widely used platinum anticancer drug, cisplatin have also been assayed as a positive control. The IC50 values for three new ruthenium complexes 1–3, NAMI A and cisplatin for selected cell lines are shown in Table 5. In the case of all the cancer cells tested, it is interesting to observe that while complex 1 is not active, complexes 2 and 3 show some cytotoxic activity against cell lines, although the activities are lower than those exhibited by cisplatin which is very common for ruthenium based anticancer agents.40 However, while comparing the cytotoxicity of the new complexes with NAMI A, they displayed better activity corresponding to inhibition over NAMI A in almost all the cancer cells tested. The lack of cytotoxic activity of 1 was expected in view of its specific structure i.e. the absence of COOH; by contrast, the activity of 2 and 3 toward the cancer cell lines is quite striking in view of the presence of the carboxylic acid arm in the bipyridine ligand. As evaluated by their IC50 values, 2 and 3 displayed better cytotoxic activity against SKOV3 cells when compared to the other cancer cells screened. 1 did not show any significant activity even up to 500 μM concentration on HeLa and MCF7 cells. The results of MTT assay also indicated that the IC50 values of complexes 2 and 3 against NIH 3T3 and HEK 293 cells are found to be above 1000 μM, which confirmed that the complexes are very specific on cancer cells and even less toxic compared to NAMI A (IC50(NIH 3T3) = 570 μM; IC50(HEK 293) = 533 μM) and cisplatin (IC50(NIH 3T3) = 175 μM; IC50(HEK 293) = 115 μM). Considering the specific structures of 1 and 2, 3, it appears that the carboxyl moiety of 2 and 3 may play a critical role for the observed cytotoxic activity and selectivity on the tested cell lines. The COOH group could not easily penetrate the hydrophobic cellular membrane of noncancerous cells but could readily bind to certain biomolecules over expressed on surfaces of the rapidly growing cancer cells. The activity results suggest a variation of cell sensitivity in the cell lines studied and was in the sequence of 2 ∼ 3 > 1, which is consistent with the trends of their radical scavenging abilities. Another generalization is that there is no substantial variation in the cytotoxic activities when the position of COOH in the bipyridine moiety of the complexes was changed. Though the new ruthenium complexes did not show significant in vitro cytotoxicity, further studies are needed to assess their antiproliferative activity in vivo and to elucidate the actual mechanism of the anti-tumor activity. But the cytotoxicity and selectivity of the synthesized complexes may be improved by introducing more carboxylic acid functionalities, other functional groups like sulphonic acid, ester, methoxy, amine, DNA intercalating terpyridine, phenanthroline moieties and one or more ruthenium ions in the form of bi or polynuclear ruthenium complexes.| Complexes | IC50a (μM) | ||||||
|---|---|---|---|---|---|---|---|
| SRB assay | MTT assay | ||||||
| HCT-15 | HeLa | SKOV3 | MCF7 | SKMel2 | NIH 3T3 | HEK 293 | |
| a Fifty percent inhibitory concentration after exposure for 48 h. | |||||||
| 1 | 376.23 ± 2.20 | >500 | 241.76 ± 1.22 | >500 | 357.22 ± 3.50 | 235 ± 1 | 197 ± 2 |
| 2 | 227.74 ± 0.08 | 380.34 ± 0.58 | 192.98 ± 1.47 | 258.00 ± 0.55 | 279.42 ± 0.39 | >1000 | >1000 |
| 3 | 208.12 ± 0.59 | 291 ± 0.11 | 189.47 ± 0.14 | 227.74 ± 1.47 | 235.76 ± 1.60 | >1000 | >1000 |
| NAMI A | >500 | >500 | >500 | >500 | >500 | 570 ± 4 | 533 ± 3 |
| Cisplatin | 45.49 ± 0.40 | 54.00 ± 0.7 | 32.56 ± 1.36 | 18.73 ± 0.28 | 25.53 ± 0.3 | 175 ± 2 | 115 ± 5 |
Conclusion
The present contribution describes the synthesis and characterization of two types of three new ruthenium(II) complexes. One is composed by bipyridine moiety with COOH and the other is only with the bipyridine moiety and two chlorides and dimethylsulphoxides are common to both. In both the cases, ruthenium is bonded to the nitrogen atoms of the bipyridine ring, which is confirmed by single crystal X-ray crystallographic studies. The groove binding of the mentioned complexes with DNA was deduced by taking account of relevant UV-visible absorption spectra, fluorescence spectra, DNA melting, viscosity measurements and circular dichroism. The protein binding properties of the complexes were examined by the fluorescence spectra and a greater binding affinity was observed for the complexes 2 and 3 over 1. The incorporation and position of COOH group in the bipyridine ring played a significant role in modulating the DNA/protein binding behaviours of the complexes. Moreover, the results obtained from various antioxidant assays and cytotoxic studies showed that the complexes 2 and 3 showed good radical scavenging ability, moderate cytotoxic activity and greater cytotoxic selectivity over 1. It is seen from the results that the introduction of COOH group in the bipyridine ring markedly increased the antitumor and antioxidant efficiency of the new complexes. We can envision this to open up new avenues for the designing and screening of the suitable ruthenium complexes containing free COOH group for anticancer activity studies.Electronic supplementary information
1H NMR spectrum of complex 1 (Fig. S1†); selected geometrical parameters for complexes 1 and 3 (Table S1†); plot of [DNA]/(εa − εf) vs. [DNA] for the titration of CT-DNA with complexes 1–3 (Fig. S2†); correlation equation and R2 value of the complexes 1–3 for plot of [DNA]/(εa − εf) vs. [DNA] (Table S2†); correlation equation and R2 value for EB–DNA fluorescence quenching by complexes 1–3 (Table S3†); Stern–Volmer plots for EB–DNA quenching by the ruthenium complexes at different temperatures (Fig. S3†); correlation equation and R2 value of 1–3 for Stern–Volmer plots for EB–DNA quenching by the ruthenium complexes at different temperatures (Table S4†); correlation equation and R2 value of EB and complexes on viscosity of DNA (Table S5†); UV-visible absorption spectra of BSA in the absence and presence of the complexes 1–3 (Fig. S4†); plot of I0/I vs. log[Q] (Fig. S5†); correlation equation and R2 value of complexes 1–3 for plot of I0/I vs. log[Q] (Table S6†); plot of log[(F0 − F)/F] vs. log[Q] (Fig. S6†); correlation equation and R2 value of the complexes 1–3 for plot of log[(F0 − F)/F] vs. log[Q] (Table S7†); synchronous spectra of BSA in the presence of increasing amounts of the complexes 1–3 for a wavelength difference of Δλ = 15 nm (Fig. S7†); synchronous spectra of BSA in the presence of increasing amounts of the complexes 1–3 for a wavelength difference of Δλ = 60 nm (Fig. S8†); plausible mechanisms for DPPH radical scavenging and metal chelating activity for complex 3 (Fig. S9†); CCDC reference numbers 869937 and 843881.Acknowledgements
Council of Scientific and Industrial Research, New Delhi, India, for the award of Senior Research Fellowship to T. Sathiya Kamatchi is gratefully acknowledged. We would like to thank Dr P. Kalaivani for her help in carrying out the protein binding studies. Acknowledgment is also made to Mr S. Saravanan and Dr T. Parimelazhagan, Department of Botany, Bharathiar University, India for their help in radical scavenging assays.References
- (a) B. A. Chabner and T. G. Roberts, Nat. Rev. Cancer, 2005, 5, 65–72 CrossRef CAS PubMed; (b) J. Bernier, E. J. Hall and A. Giaccia, Nat. Rev. Cancer, 2004, 4, 737–747 CrossRef CAS PubMed.
- M. Galanski, V. B. Arion, M. A. Jakupec and B. K. Keppler, Curr. Pharm. Des., 2003, 9, 2078–2089 CrossRef CAS PubMed.
- M. A. Jakupec, M. Galanski and B. K. Keppler, Rev. Physiol., Biochem. Pharmacol., 2003, 146, 1–53 CAS.
- M. Galanski, M. A. Jakupec and B. K. Keppler, Curr. Med. Chem., 2005, 12, 2075–2094 CrossRef CAS PubMed.
- (a) G. Daugaard and U. Abildgaard, Cancer Chemother. Pharmacol., 1989, 25, 1–9 CrossRef CAS PubMed; (b) V. Pinzani, F. Bressolle, I. J. Haug, M. Galtier, J. P. Blayac and P. Balmes, Cancer Chemother. Pharmacol., 1994, 35, 1–9 CrossRef CAS PubMed; (c) D. Screnci and M. J. McKeage, J. Inorg. Biochem., 1999, 77, 105–110 CrossRef CAS PubMed; (d) L. Troy, K. McFarland, S. Littman-Power, B. J. Kelly, E. T. Walpole, D. Wyld and D. Thomson, Psycho Oncol., 2000, 9, 29–39 CrossRef CAS; (e) M. Markman, Expert Opin. Drug Saf., 2003, 2, 597–607 CrossRef CAS PubMed; (f) P. C. Bruijnincx and P. J. Sadler, Curr. Opin. Chem. Biol., 2008, 12, 197–206 CrossRef CAS PubMed; (g) K. Barabas, R. Milner, D. Lurie and C. Adin, Vet. Comp. Oncol., 2008, 6, 1–18 CrossRef CAS PubMed; (h) P. Borst, S. Rottenberg and J. Jonkers, Cell Cycle, 2008, 7, 1353–1359 CrossRef CAS PubMed.
- (a) M. J. Clarke, F. C. Zhu and D. R. Frasca, Chem. Rev., 1999, 99, 2511–2533 CrossRef CAS PubMed; (b) R. H. Fish and G. Jaouen, Organometallics, 2003, 22, 2166–2177 CrossRef CAS.
- (a) C. S. Allardyce and P. J. Dyson, Platinum Met. Rev., 2001, 45, 62–69 CAS; (b) M. J. Clarke, Coord. Chem. Rev., 2003, 236, 209–233 CrossRef CAS; (c) Y. K. Yan, M. Melchart, A. Habtemariam and P. J. Sadler, Chem. Commun., 2005, 38, 4764–4776 RSC; (d) P. J. Dyson and G. Sava, Dalton Trans., 2006, 16, 1929–1933 RSC; (e) M. Melchart and P. J. Sadler, in Bioorganometallics: Biomolecules, Labeling, Medicine, ed. G. Jaouen, Wiley-VCH, New York, 2006, pp. 39–62 Search PubMed; (f) A. Levina, A. Mitra and P. A. Lay, Metallomics, 2009, 1, 458–470 RSC; (g) I. Bratsos, T. Gianferrara, E. Alessio, C. G. Hartinger, M. A. Jakupec and B. K. Keppler, in Bioinorganic Medicinal Chemistry, ed. E. Alessio, Wiley-VCH, Weinheim, 2011, pp. 151–174 Search PubMed; (h) S. B. Fricker, Dalton Trans., 2007, 4903–4917 RSC.
- I. Bratsos, S. Jedner, T. Gianferrara and E. Alessio, Chimia, 2007, 61, 692–697 CrossRef CAS.
- (a) F. Frausin, V. Scarcia, M. Cocchietto and G. Sava, J. Pharmacol. Exp. Ther., 2005, 313, 227–233 CrossRef CAS PubMed; (b) B. Serli, E. Zangrando, T. Gianferrara, L. Yellowlees and E. Alessio, Coord. Chem. Rev., 2003, 245, 73–83 CrossRef CAS; (c) G. Sava, R. Gagliardi, A. Bergamo, E. Alessio and G. Mestroni, Anticancer Res., 1999, 19, 969–972 CAS.
- E. Alessio, Chem. Rev., 2004, 104, 4203–4242 CrossRef CAS PubMed.
- (a) C. M. Giandomenico, M. J. Abrams, B. A. Murrer, J. F. Vollano, M. I. Rheinheimer, S. B. Wyer, G. E. Bossard and J. D. Higgins, Inorg. Chem., 1995, 34, 1015–1021 CrossRef CAS PubMed; (b) M. Galanski and B. K. Keppler, Inorg. Chem., 1996, 35, 1709–1711 CrossRef CAS PubMed; (c) T. Sathiya Kamatchi, N. Chitrapriya, H. Lee, F. R. Fronczek and K. Natarajan, Eur. J. Med. Chem., 2013, 59, 253–264 CrossRef CAS PubMed.
- (a) H. Y. Mei and J. K. Barton, Proc. Natl. Acad. Sci. U. S. A., 1988, 85(5), 1339–1343 CrossRef CAS PubMed; (b) V. Brabec, Prog. Nucleic Acid Res. Mol. Biol., 2002, 71, 1–68 CAS; (c) B. M. Zeglis, V. C. Pierre and J. K. Barton, Chem. Commun., 2007, 44, 4565–4579 RSC; (d) C. Gaiddon, P. Jeannequin, P. Bischoff, M. Pfeffer, C. Sirlin and J. P. Loeffler, J. Pharmacol. Exp. Ther., 2005, 315(3), 1403–1411 CrossRef CAS PubMed; (e) R. L. Hayward, Q. C. Schornagel, R. Tente, J. S. Macpherson, R. E. Aird and S. Guichard, Cancer Chemother. Pharmacol., 2005, 55(6), 577–583 CrossRef CAS PubMed; (f) S. Chatterjee, S. Kundu, A. Bhattacharyya, C. G. Hartinger and P. J. Dyson, J. Biol. Inorg Chem., 2008, 13(7), 1149–1155 CrossRef CAS PubMed; (g) B. Wu, M. S. Ong, M. Groessl, Z. Adhireksan, C. G. Hartinger, P. J. Dyson and C. A. Davey, Chem.–Eur. J., 2011, 17, 3562–3566 CrossRef CAS PubMed.
- K. B. Beckman and B. N. Ames, Phys. Rev., 1998, 78, 447–453 Search PubMed.
- B. Halliwel and J. M. C. Gutteridge, Free Radicals in Biology and Medicine, Clarendon Press, Oxford, 2nd edn, 1989, pp. 416–494 Search PubMed.
- I. P. Evans, A. Spencer and G. Wilkinson, J. Chem. Soc., Dalton Trans., 1973, 2, 204–209 RSC.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, OLEX2: a complete structure solution, refinement and analysis program, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- L. J. Bourhis, O. V. Dolomanov, R. J. Gildea, J. A. K. Howard and H. Puschmann, olex2.solve, 2011 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- M. C. Altomare, M. Burla, G. L. Camalli, C. Cascarano, A. Giacovazzo, A. G. G. Guagliardi, G. Moliterni and R. Polidori, J. Appl. Crystallogr., 1999, 32, 115–119 CrossRef.
- L. J. Farrugia, J. Appl. Crystallogr., 1997, 30, 565–566 CrossRef CAS.
- M. S. Blois, Nature, 1958, 29, 1199–1200 CrossRef.
- T. Nash, J. Biochem., 1953, 55, 416–421 CrossRef CAS.
- L. C. Green, D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok and S. R. Tannenbaum, Anal. Biochem., 1982, 126, 131–138 CrossRef CAS PubMed.
- R. J. Ruch, S. J. Cheng and J. E. Klaunig, Carcinogenesis, 1989, 10, 1003–1008 CrossRef CAS PubMed.
- C. Beauchamp and I. Fridovich, Anal. Biochem., 1971, 44, 276–287 CrossRef CAS PubMed.
- T. C. P. Dinis, V. M. C. Madeira and L. M. Almeida, Arch. Biochem. Biophys., 1994, 315, 161–169 CrossRef CAS PubMed.
- R. E. Marsh, V. Schomaker and F. H. Herbstein, Acta Crystallogr., Sect. B: Struct. Sci., 1998, 54, 921–924 Search PubMed.
- E. Alessio, G. Mestroni, G. Nardin, W. M. Attia, M. Calligaris, G. Sava and S. Zorzet, Inorg. Chem., 1988, 27, 4106–4113 CrossRef.
- M. Trivedi, Y. K. Sharma, R. Nagarajan and N. P. Rath, J. Mol. Struct., 2010, 975, 335–342 CrossRef CAS.
- J. Mola, I. Romero, M. Rodriguez, F. Bozoglian, A. Poater, M. Sola, T. Parella, J. Benet-Buchholz, X. Fontrodona and A. Llobet, Inorg. Chem., 2007, 46(25), 10707–10716 CrossRef CAS PubMed.
- E. Reisner, V. B. Arion, A. Rufinska, I. Chiorescu, W. F. Schmid and B. K. Keppler, Dalton Trans., 2005, 21(14), 2355–2364 RSC.
- M. B. Cingi, M. Lanfranchi, M. A. Pellinghelli and M. Tegoni, Eur. J. Inorg. Chem., 2000, 703–711 CrossRef.
- C. Tan, S. Hu, J. Liu and L. Ji, Eur. J. Med. Chem., 2011, 46, 1555–1563 CrossRef CAS PubMed.
- I. Bratsos, B. Serli, E. Zangrando, N. Katsaros and E. Alessio, Inorg. Chem., 2007, 46, 975–992 CrossRef CAS PubMed.
- M. Calligaris, Coord. Chem. Rev., 2004, 248, 351–375 CrossRef CAS.
- I. Bratsos, D. Urankar, E. Zangrando, P. Genova-Kalou, J. Kosmrlj, E. Alessio and I. Turel, Dalton Trans., 2011, 40, 5188–5199 RSC.
- C. Sens, M. Rodriguez, I. Romero, A. Llobet, T. Parella, B. P. Sullivan and J. Benet-Buchholz, Inorg. Chem., 2003, 42, 2040–2048 CrossRef CAS PubMed.
- D. J. Patel, Acc. Chem. Res., 1979, 12, 118–125 CrossRef CAS.
- (a) G. Z. Chen, X. Z. Haong, J. C. Xu, Z. Z. Zhang and Z. B. Wang, The methods of Fluorescence Analysis, Beijing Science Press, 2nd edn, 1990, pp. 2–112 Search PubMed; (b) Y. Lu, Y. L. Wang, S. H. Gao, G. K. Wang, C. L. Yan and D. J. Chen, J. Lumin., 2009, 129, 1048–1054 CrossRef CAS; (c) S. Ashoka, J. Seetharamappa, P. B. Kandagal and S. M. T. Shaikh, J. Lumin., 2006, 121, 179–186 CrossRef CAS.
- J. Chen, X. Y. Jiang, X. Q. Chen and Y. Chen, J. Mol. Struct., 2008, 876, 121–126 CrossRef CAS.
- (a) M. J. Han, L. H. Gao and K. Z. Wang, New J. Chem., 2006, 30, 208–214 RSC; (b) Y. Z. Ma, H. J. Yin and K. Z. Wang, J. Phys. Chem. B, 2009, 113, 111039–111047 Search PubMed.
- S. Satyanarayana, J. C. Dabrowiak and J. B. Chaires, Biochemistry, 1993, 32, 2573–2584 CrossRef CAS PubMed.
- Y. Wang, G. Lin, J. Hong, T. Lu, L. Li, N. Okabe and M. Odoko, Inorg. Chim. Acta, 2009, 362, 377–384 CrossRef CAS.
- E. C. Long and J. K. Barton, Acc. Chem. Res., 1990, 23, 271–273 CrossRef CAS.
- D. Suh and J. B. Chaires, Bioorg. Med. Chem., 1995, 3(6), 723–728 CrossRef CAS PubMed.
- L. Lerman, J. Mol. Biol., 1961, 3, 18–30 CrossRef CAS PubMed.
- B. Norden and F. Tjerneld, Biopolymers, 1982, 21, 1713–1734 CrossRef CAS PubMed.
- P. T. Selvi and M. Palaniandavar, Inorg. Chim. Acta, 2002, 337, 420–428 CrossRef CAS.
- H. Y. Liu, Z. H. Xu, X. H. Liu, P. X. XI and Z. Z. Zeng, Chem. Pharm. Bull., 2009, 57, 1237–1242 CrossRef CAS PubMed.
- A. Sulkowska, J. Mol. Struct., 2002, 614, 227–232 CrossRef CAS.
- G. Z. Chen, X. Z. Huang, J. G. Xu, Z. B. Wang and Z. Z. Zhang, Method of Fluorescent Analysis, Science Press, Beijing, 2nd edn, 1990, vol. 126, ch. 4, p. 123 Search PubMed.
- J. N. Miller, Recent advances in molecular luminescence analysis, Proc. Anal. Div. Chem. Soc., 1979, 16, 203–208 CAS.
- J. H. Tang, F. Luan and X. G. Chen, Bioorg. Med. Chem., 2006, 49, 3210–3217 CrossRef PubMed.
- K. Tsai, T. G. Hsu, K. M. Hsu, H. Cheng, T. Y. Liu, C. F. Hsu and C. W. Kong, Free Radical Biol. Med., 2001, 31, 1465–1472 CrossRef CAS PubMed.
- C. S. Rivas, J. C. Espin and H. Wichers, Phytochem. Anal., 2000, 11, 330–338 CrossRef.
- (a) H. L. Huang, Y. J. Liu, C. H. Zeng, J. H. Yao, Z. H. Liang, Z. Z. Li and F. H. Wu, J. Mol. Struct., 2010, 966, 136–143 CrossRef CAS; (b) Y. J. Liu, C. H. Zeng, Z. H. Liang, J. H. Yao, H. L. Huang, Z. Z. Li and F. H. Wu, Eur. J. Med. Chem., 2010, 45, 3087–3095 CrossRef CAS PubMed; (c) X. L. Hong, H. Li and C. H. Peng, J. Mol. Struct., 2011, 990, 197–203 CrossRef CAS; (d) Z. Z. Li, Z. H. Liang, H. L. Huang and Y. J. Liu, J. Mol. Struct., 2011, 1001, 36–42 CrossRef CAS; (e) Y. J. Liu, Z. H. Liang, Z. Z. Li, J. H. Yao and H. L. Huang, J. Organomet. Chem., 2011, 696, 2728–2735 CrossRef CAS.
- K. Kogure, H. Sassa, K. Abe, K. Kitahara, Y. Sano, H. Kawano, Y. Nakagawa and H. Terada, Biol. Pharm. Bull., 1998, 21, 180–183 CAS.
- T. Sathiya Kamatchi, N. Chitrapriya, S. K. Kim, F. R. Fronczek and K. Natarajan, Dalton Trans., 2012, 41, 2066–2077 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S9 and Tables S1–S7. CCDC 869937 and 843881. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra00425g |
| This journal is © The Royal Society of Chemistry 2017 |