Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
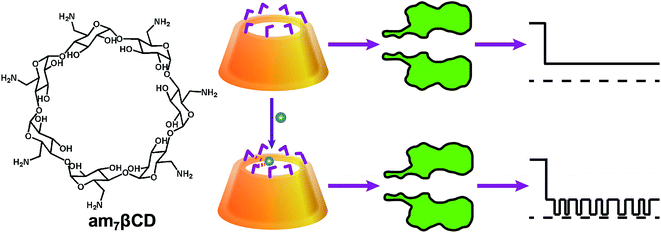
Nanopore sensor for copper ion detection using a polyamine decorated β-cyclodextrin as the recognition element
Yanli Guo *,
Feifei Jian and
Xiaofeng Kang
*,
Feifei Jian and
Xiaofeng Kang *
*
Key Laboratory of Synthetic and Natural Functional Molecular Chemistry, College of Chemistry & Materials Science, Northwest University, Xi'an 710127, P. R. China. E-mail: guoyl@nwu.edu.cn; kangxf@nwu.edu.cn; Fax: +86-029-81535026; Tel: +86-029-81535026
First published on 7th March 2017
Abstract
A novel and simple nanopore sensing method has been developed for the detection of CuII ions using polyamine decorated cyclodextrin as the recognition element. The strong binding affinity between CuII and the amino groups of cyclodextrin inside an α-hemolysin pore causes the new current blockade events. The event frequency is linear for concentrations of CuII in the range 0.08–20 μM. The detection limit is as low as 12 nM. More significantly, the sensing system is highly specific for CuII and does not respond to other metal ions with concentrations up to 10 fold that of CuII. The applicability of this sensor has also been verified by the analysis of CuII ions in running water, suggesting the potential application of this sensing system.
1. Introduction
Nanopore stochastic sensing based on single-molecule recognition is an emerging analytical technique on account of having high sensitivity and being rapid and low-cost.1,2 The principle of nanopore sensing is to monitor the ionic current modulation caused by analytes driving through nanopores under a fixed applied voltage.3 The characteristics of current modulation signatures, including frequency, amplitude and dwell time, depend upon the size, charged status and concentration of the analytes. In this way, nanopore sensing has been used successfully to detect a wide variety of substances, ranging from tiny metal ions4–6 to organic molecules7–9 and even biological macromolecules such as nucleic acids,10–12 protein13–15 and peptides.16,17 Furthermore, protein nanopores have shown attractive prospects as a next-generation DNA sequencing platform.18So far, sensing metal ions with an α-hemolysin (αHL) nanopore can be realized in two ways. One is to mutate the protein to construct binding sites in the lumen of αHL. Choi and Mach used an αHL mutant pore with four cysteine mutations to detect AgI and CdII.19 Braha's group reported simultaneous detection of ZnII, CoII and CdII using an engineered pore containing histidine residues.20 Similar research has been conducted by Kasianowicz et al.21 Another method is based on the interaction of metal ions and DNA or peptides. PbII was successfully detected by inducing the conformational change of the G4 DNA aptamer.22 Wen and colleagues designed specific T-rich DNA oligomers as probes to detect HgII ions.4 Subsequently, they extended this strategy for the detection of PbII and BaII ions.5 Furthermore, a polyhistidine peptide chain was exploited to detect CuII based on the chelating reaction by Wang et al.23 However, effective sensors with good selectivity, reproducibility and maneuverability are rare. There are still some problems that remain to be solved. First, it is difficult to operate and imitate for the method of modifying the nanopore interior due to the complexity of mutagenesis and separation of protein nanopore. Second, strong background disturbance exists in detecting low concentration metal ions, limiting the sensitivity of the method. Therefore, there is an upsurge of need in the development of a new approach to overcome these limitations.
In the present work, we used the polyamine decorated cyclodextrins as recognition element in αHL pore for the highly sensitive and selective detection of CuII ion, which is a well-known heavy metal and plays a vital role in many biological processes.24–26 Its concentration will directly affect people's health.27,28 As is well known, cyclodextrins could be lodged noncovalently within the lumen of the αHL pore and produced a substantial and incomplete channel block.29 The host–guest inclusion of cyclodextrins with analytes could generate additional transient reductions in the current, which permitted the analytes to be identified and quantified.30 Modified β-cyclodextrins (βCDs) have been also covalently attached to the pore to differentiate deoxynucleotides with over 99% confidence.31 Moreover, chiral discrimination was achieved by αHL pore equipped with the βCD adapter.32 However, previous studies involving cyclodextrins have been mainly focused on organic molecules,33,34 no attention has been paid to the applications for metal ions. Herein, we detect CuII for the first time with αHL nanopore containing a functionalized cyclodextrin. In this method, mutating the protein to construct binding sites for the CuII is not necessary, which simplifies the fabrication of nanopore sensors. More importantly, we validated the practicality of this method for the detection of CuII in environmental samples through analyses of running water.
2. Experimental section
2.1. Reagents and materials
The (WT)7, (M113F)7 and (M113R)7 αHL protein were synthesized and purified according to the methods reported from documents.7,35 All the metal salts, CuCl2 (>99%), HgCl2 (>99%), CuCl2 (>99%), ZnCl2 (>99%), MgCl2 (>99%), CdCl2 (>99%), Co(NO3)2 (>99%), Ni(NO3)2 (>99%), Mn(NO3)2 (>99%), TbCl3 (>99%), GdCl3 (>99%), Dy(CH3COO)2 (>99%), purchased from Aladdin and were prepared at concentrations of 10.0 mM each. Heptakis-(6-deoxy-6-amino)-β-cyclodextrin (am7βCD) (>99%) was obtained from Cycloab (Budapest, Hungary). Both NaCl (>99%) and Tris (>99%), used to prepare electrolyte solution, were obtained from Kermel Chemical Reagents Co., Ltd. (Tianjin, China). It should be noted that the pH of the electrolyte solutions adjusted by using hydrochloric acid. All reagents were dissolved in ultrapure water. Teflon film (25 μm) was ordered from Goodfellow Corp. (Malvern, PA, USA). The 1,2-diphytanoyl-sn-glycero-3-phosphocholine (DPhPC) used for planar bilayer lipid formation was gained from Avanti Polar Lipids (Alabaster, AL, USA). Running water sample was collected from Northwest University (Xian, China). The sample was filtered through a 0.22 μm membrane (Shanghai Xin Ya Purification Equipment Co., Ltd., China) prior to the detection.2.2. Single channel recording
Single channel electrical measurements were carried out by using traditional methods. For simplify, planar lipid bilayer membranes of DPhPC were typically created using the method of Montal and Mueller36 on an aperture 120–150 μm in diameter in a Teflon film which separates two identical compartments. Each compartment was filled with 1500 μL of buffer solution (cis buffer and trans buffer). Both sides of aperture were pretreated with a 10% v/v hexadecane/pentane mixture before the addition of buffer solution. Then the compartments were both injected with 900 μL buffer solution to the level just below the aperture and lipid solution was added to buffer. When the pentane was evaporated, solvent-free lipid monolayer formed at the solution–air interface. The remaining 600 μL buffer solution was introduced to compartments until the level of buffer rose above the aperture. Unless otherwise stated, (M113F)7 pores were added to the grounded cis compartment (0.05–0.2 ng mL−1). After the successful insertion of a single αHL pore, copper ions and am7βCD were added to the cis and trans side respectively. The single channel current was detected with two freshly prepared Ag/AgCl electrodes, collected with a patch clamp amplifier (Axopatch 200B, Axon Instruments, Foster city, CA, USA) and filtered with a low-pass Bessel filter with a corner frequency of 5 kHz and then digitized with a Digidata 1440A A/D converter (Axon Instruments) at a sampling frequency of 20 kHz.2.3. Data analysis
Single channel current recordings were performed and analyzed with pClamp 10.3 (Axon Instruments). Origin 8.5 (Microcal, Northampton, MA) was employed for histogram construction, curve fitting and graph presentation. Both the values of τon (the mean interevent interval) and τoff (the mean dwell time) for am7βCD–CuII complexes, were obtained from the dwell time histograms by fitting the distributions to single exponential functions by the Levenberg–Marquardt procedure.16 The current blockades were produced by the fitted Gaussian distributions.3. Results and discussion
3.1. The principle of the CuII detection
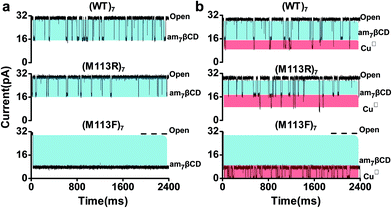
Inspired by the successful applications of copper complexes of polyamines decorated cyclodextrin37 and the wealth of information about cyclodextrins interacted with αHL nanopore,38 we designed that a simple but efficient CuII ions sensor which could be operate easily under mild conditions. As shown in Scheme 1, we used β-cyclodextrin with the seven primary hydroxyls replaced with amino groups (heptakis-(6-deoxy-6-amino)-β-cyclodextrin; am7βCD) as recognition element, which possess a range of nitrogen donor atoms and are very effective ligands for CuII ions with high specificities. Without CuII ions, am7βCD adapter which entered into the lumen of the αHL pore only produced one kind of events. In sharp contrast, upon addition of CuII ions to electrolyte solution, they would coordinate with am7βCD molecules and form am7βCD–CuII complexes, which resulted in new additional current blockade events having significantly different signatures from those in the absence of CuII ions. It permitted the CuII ions to be readily recognized.To demonstrate this hypothesis, our initial experiments were carried out at pH 8 under identical conditions with different αHL nanopore containing an am7βCD adapter as the detector. As displayed in Fig. 1, significant differences in event signature were observed before and after addition of CuII ions for three protein nanopores. It is clear that dwell time of am7βCD was quite different (3.97 ± 0.18 ms) in (WT)7 and (1.03 ± 0.09 ms) in (M113R)7. In the case of (M113F)7 pore, the am7βCD adapter almost permanently locked into a state of 77.2 ± 0.5% block. It has been reported that sensing with molecular adapter would be enhanced if the adapter did not every so often dissociate from the pore.39 As a result, the (M113F)7 protein could provide an enhanced resolution for CuII ions recognition compared with that observed in other αHL pore.
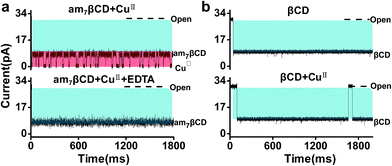
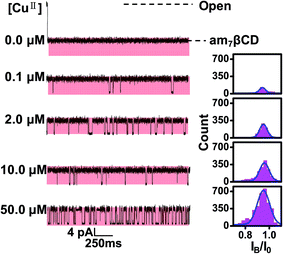
To further prove that the new type of events are related to the formation of am7βCD–CuII complexes, control experiments were examined. Firstly, when EDTA was introduced to the solution, we found that these events originated from am7βCD were eliminated, which demonstrated a strong association between CuII ions and these events (Fig. 2a). Further, am7βCD in nanopore stochastic sensing was replaced with natural βCD. The results showed that no additional events was observed in the current trace, confirming the role that am7βCD–CuII complexes play in the CuII ions detection (Fig. 2b).
3.2. Optimization of detection conditions
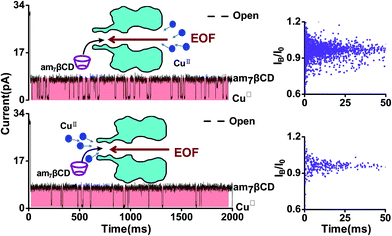
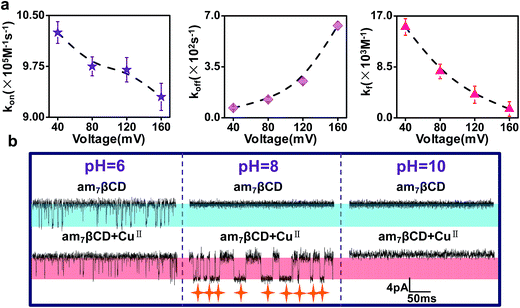
In order to achieve high sensitivity and selectivity of detection of CuII ions, the optimal detection conditions are essential. To begin with, we compared the signals where CuII ions were added from the trans side of the bilayer and the case where CuII ions were added from the cis side of the bilayer (Fig. 3). In general, the transport of the charged analytes through the nanopore was dominated by electrophoresis. Since CuII ions have positive charges, we anticipated the CuII ions to traverse more easily the nanopore from trans to cis side instead of from cis to trans when positive voltage was applied to the trans chamber. However, the experimental results showed that the event frequency of CuII ions from trans side was 10 times than from cis side, in apparent contradiction with the assumption. In fact, there is a combined action of diffusion, electrophoresis, and electroosmosis in the transport of charged molecules through nanopore. A few experimental studies have demonstrated that the electroosmotic flow (EOF) provided an important or even dominant, contribution to the analytes transport, especially for small neutral molecules40,41 or molecules with a low net charge,42–44 under appropriate experimental conditions. Our experiment phenomenon also was interpreted as EOF being the main force driving electrically. The αHL protein nanopore exhibits a selectivity to anions45 and am7βCD molecule is positively charged46 at pH 8. Previous studies have demonstrated that anion selective of αHL becomes more anion selective when natural or the positively charged βCD adapter is lodged within the channel lumen.47 Therefore, the (M113F)7 pore equipped with an cationic adapter, am7βCD, was still anion selective. EOF is consist with the direction of anion flow under applied positive voltages. As a consequence, when CuII ions were introduced into the cis chamber, current blockade events occurred preferentially. While following the direction of cation flow and oppositing to EOF, only a few blockades were observed for CuII ions added from the trans side of the bilayer.As previously documented, the transmembrane voltage is an important factor for determining the translocation of analytes.48,49 In addition to providing a novel approach for sensing CuII ions, our sensor permits the determination of association and dissociation rate constants, separately, and formation constants for am7βCD–CuII complexes. The rate constants of am7βCD–CuII for association and dissociation were calculated by corresponding formula, that is, kon = 1/(τon[CuII]) and koff = 1/τoff. To better illustrate voltage with CuII ions binding events, we did the experiment in which CuII ion was kept constant at 20 μM, while transmembrane voltage was changed. As expected, the frequency of event decreased with increasing applied positive voltage, which corresponded to decreases in the association rate constants (Fig. 4a, left). On the contrary, the dissociation rate constant koff increased with voltage, suggesting that the rather high voltage was harmful for the association of CuII ions (Fig. 4a, middle). Equilibrium formation constants were calculated by using Kf = kon/koff for am7βCD–CuII and differed by over 10-fold under the current experimental conditions (Fig. 4a, right). The most extreme values were produced at the voltage of 40 mV and 160 mV. For instance, CuII ions were bound 10.7 times more strongly at 40 mV (Kf = 1.5 ± 0.1 × 104 M−1) than at 160 mV (Kf = 1.4 ± 0.1 × 103 M−1). These data implied that CuII binding event was favored at lower values of voltage. So as to improve the CuII ions detection sensitivity, we used +40 mV as the applied voltage in the remaining experiments.
Based on the fact that certain properties of the αHL protein pore are dependent, to a great extent, on pH, including the ion selectivity, conductance, charge state and magnitude of single-channel noise.49–51 Thus, we carried out the CuII ion detection experiments with am7βCD at three different pH values (Fig. 4b). The results showed that, in the absence of CuII ions, only one current blockade level was observed at pH 8 and 10. And there was no additional background noise arised from the am7βCD state, indicating that am7βCD was firmly held in the β barrel of protein nanopore under these conditions. In contrast, there were a lot of substates during occupied by am7βCD in pH 6 buffer solution, which might be attributed to the conformational changes of am7βCD in the lumen of the αHL pore.38 On the other hand, the addition of 20 μM CuII ions to the buffer solution of pH 8 produced a large number of markedly new blockade events having a mean residual current of 0.49 ± 0.12 pA and a mean dwell time of 14.82 ± 1.13 ms. By contrast, no additional current block was detected when CuII ions was applied to the buffer solution, whatever the buffer solution pH 6 or 10. Apart from the interference from the background noise, the possible reason why the event signatures of CuII ions were not detected at pH = 6 was that amino groups of the cyclodextrin were completely protonated.46 When the buffer solution pH increased to 10, CuII ions were almost hydrolysis entirely. Therefore, the electrolyte solution of pH 8 was selected for the following experiments.
3.3. Detection sensitivity and selectivity for CuII
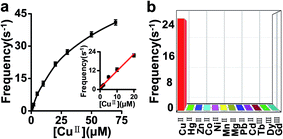
To test the sensitivity of this sensor, CuII ions at various concentrations were examined under the optimum experimental conditions. As illustrated in Fig. 5 and 6a, the event frequency increased with increasing concentration of added CuII ions. Linear regression analysis showed good linearity between the frequency and CuII concentration ranging from 80 nM to 20 μM with a correlation coefficient of 0.98 (Fig. 6a, inset). The detection limit of CuII ions could be as low as 12 nM (S/N = 3), which was lower than previously reported values (LOD = 40 nM).23 Subsequently, under the same conditions, we evaluated the selectivity of this sensing platform toward CuII (20 μM) relative to other kinds of metal ions including PbII, CdII, MgII, CoII, MnII, NiII, ZnII, HgII, DyIII, GdIII and TbIII (each 200 μM). These metal ions, especially CoII, NiII, ZnII ions, were well known to be able to interact with amino groups. Apparently, the results revealed that no obvious response could be observed upon the addition of other ions (Fig. 6b). Compared with other nanopore sensors for metal ions, our sensor is more selective toward CuII ions over the tested interference ions.3.4. Detection of CuII in running water
To the end, after a number of screenings of experimental conditions, the applicability of this methodology for detecting CuII ions in a real sample was further evaluated. The content of CuII ions in the sample of running water were detected and the concentration is 0.36 ± 0.05 μM. What's more, as shown in Table 1, the measured values for samples with known amounts of CuII ions showed good recoveries of 91.4–108.6%. The results demonstrated that this nanopore sensor had strong anti-interference ability and could be applied for the detection in real samples.| Sample | Added CuII (μM) | Measured (μM) | Recovery (%) |
|---|---|---|---|
| Running water 1 | 0 | 0.35 | — |
| Running water 2 | 0.50 | 0.82 | 91.4 |
| Running water 3 | 2.00 | 2.37 | 105.7 |
| Running water 4 | 4.00 | 4.38 | 108.6 |
4. Conclusions
In summary, we have developed a simple, selective and sensitive nanopore sensor for detection of CuII ions by employing am7βCD molecule as recognition element. Based on the chelating reaction between am7βCD and CuII ions, this sensor has been successfully applied to determination of a wide range of CuII ions from 80 nM to 20 μM. Compared with other nanopore sensors, our sensing system exhibits relatively low detection limit and better selectivity for CuII ions. More importantly, analyses of running water samples revealed that this approach has potential for detection of CuII ions in real environmental samples.Acknowledgements
This work was supported by the National Nature Science Foundation of China (No. 21175105, 21375104 and 21327806), the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20126101110015) and the Natural Science Foundation of Shaanxi Province of China (No. 2014JM2050).References
- J. E. Reiner, A. Balijepalli, J. W. Robertson, J. Campbell, J. Suehle and J. J. Kasianowicz, Chem. Rev., 2012, 112, 6431–6451 CrossRef CAS PubMed.
- B. M. Venkatesan and R. Bashir, Nat. Nanotechnol., 2011, 6, 615–624 CrossRef CAS PubMed.
- J. J. Kasianowicz, E. Brandin, D. Branton and D. W. Deamer, Proc. Natl. Acad. Sci., 1996, 93, 13770–13773 CrossRef CAS.
- S. Wen, T. Zeng, L. Liu, K. Zhao, Y. Zhao, X. Liu and H. C. Wu, J. Am. Chem. Soc., 2011, 133, 18312–18317 CrossRef CAS PubMed.
- C. Yang, L. Liu, T. Zeng, D. Yang, Z. Yao, Y. Zhao and H. C. Wu, Anal. Chem., 2013, 85, 7302–7307 CrossRef CAS PubMed.
- G. Liu, L. Zhang, D. Dong, Y. Liu and J. Li, Anal. Methods, 2016, 8, 7040–7046 RSC.
- S. Cheley, L. Q. Gu and H. Bayley, Chem. Biol., 2002, 9, 829–838 CrossRef CAS PubMed.
- A. J. Boersma, K. L. Brain and H. Bayley, ACS Nano, 2012, 6, 5304–5308 CrossRef CAS PubMed.
- H. C. Wu and H. Bayley, J. Am. Chem. Soc., 2008, 130, 6813–6819 CrossRef CAS PubMed.
- M. Ayub, S. W. Hardwick, B. F. Luisi and H. Bayley, Nano Lett., 2013, 13, 6144–6150 CrossRef CAS PubMed.
- Y. L. Ying, J. Zhang, R. Gao and Y. T. Long, Angew. Chem., Int. Ed., 2013, 52, 13154–13161 CrossRef CAS PubMed.
- A. Meller, L. Nivon, E. Brandin, J. Golovchenko and D. Branton, Proc. Natl. Acad. Sci., 2000, 97, 1079–1084 CrossRef CAS.
- J. Nivala, L. Mulroney, G. Li, J. Schreiber and M. Akeson, ACS Nano, 2014, 8, 12365–12375 CrossRef CAS PubMed.
- M. Pastorizagallego, M. F. Breton, F. Discala, L. Auvray, J. M. Betton and J. Pelta, ACS Nano, 2014, 8, 11350–11360 CrossRef CAS PubMed.
- D. Rotem, L. Jayasinghe, M. Salichou and H. Bayley, J. Am. Chem. Soc., 2012, 134, 2781–2787 CrossRef CAS PubMed.
- L. Movileanu, J. P. Schmittschmitt, J. M. Scholtz and H. Bayley, Biophys. J., 2005, 89, 1030–1045 CrossRef CAS PubMed.
- H. Y. Wang, Y. L. Ying, Y. Li, H. B. Kraatz and Y. T. Long, Anal. Chem., 2011, 83, 1746–1752 CrossRef CAS PubMed.
- S. Cornelis, Y. Gansemans, L. Deleye, D. Deforce and N. F. Van, Sci. Rep., 2017, 7, 41759 CrossRef CAS PubMed.
- L. S. Choi, T. Mach and H. Bayley, Biophys. J., 2013, 105, 356–364 CrossRef CAS PubMed.
- O. Braha, L. Q. Gu, L. Zhou, X. Lu, S. Cheley and H. Bayley, Nat. Biotechnol., 2000, 18, 1005–1007 CrossRef CAS PubMed.
- J. Kasianowicz, B. Walker, M. Krishnasastry and H. Bayley, MRS Online Proc. Libr., 1993, 330, 217–223 CrossRef.
- H. Y. Wang, Z. Y. Song, H. S. Zhang and S. P. Chen, Microchim. Acta, 2016, 183, 1003–1010 CrossRef CAS.
- G. Wang, L. Wang, Y. Han, S. Zhou and X. Guan, Biosens. Bioelectron., 2014, 53, 453–458 CrossRef CAS PubMed.
- A. Badarau and C. Dennison, J. Am. Chem. Soc., 2011, 133, 2983–2988 CrossRef CAS PubMed.
- R. V. Rathod, S. Bera, S. Man and D. Mondal, RSC Adv., 2016, 6, 34608–34615 RSC.
- E. Gaggelli, H. Kozlowski, D. Valensin and G. Valensin, Chem. Rev., 2006, 106, 1995–2044 CrossRef CAS PubMed.
- J. Liu and Y. Lu, J. Am. Chem. Soc., 2007, 129, 9838–9839 CrossRef CAS PubMed.
- S. Lutsenko, A. Gupta, J. L. Burkhead and V. Zuzel, Arch. Biochem. Biophys., 2008, 476, 22–32 CrossRef CAS PubMed.
- L. Q. Gu, O. Braha, S. Conlan, S. Cheley and H. Bayley, Nature, 1999, 398, 686–690 CrossRef CAS PubMed.
- Y. Astier, O. Braha and H. Bayley, J. Am. Chem. Soc., 2006, 128, 1705–1710 CrossRef CAS PubMed.
- J. Clarke, H. C. Wu, L. Jayasinghe, A. Patel, S. Reid and H. Bayley, Nat. Nanotechnol., 2009, 4, 265–270 CrossRef CAS PubMed.
- X. F. Kang, S. Cheley, X. Y. Guan and H. Bayley, J. Am. Chem. Soc., 2006, 128, 10684–10685 CrossRef CAS PubMed.
- L. Q. Gu, S. Cheley and H. Bayley, Science, 2001, 291, 636–640 CrossRef CAS PubMed.
- J. Gupta, Q. Zhao, G. Wang, X. Kang and X. Guan, Sens. Actuators, B, 2013, 176, 625–631 CrossRef CAS.
- L. Wang, Y. Han, S. Zhou, G. Wang and X. Guan, ACS Appl. Mater. Interfaces, 2014, 6, 7334–7339 CAS.
- M. Montal and P. Mueller, Proc. Natl. Acad. Sci., 1972, 69, 3561–3566 CrossRef CAS.
- G. Impellizzeri, G. Maccarrone, E. Rizzarelli, G. Vecchio, R. Corradini and R. Marchelli, Angew. Chem., Int. Ed., 1991, 30, 1348–1349 CrossRef.
- L. Q. Gu, S. Cheley and H. Bayley, J. Gen. Physiol., 2001, 118, 481–494 CrossRef CAS PubMed.
- H. C. Wu, Y. Astier, G. Maglia, E. Mikhailova and H. Bayley, J. Am. Chem. Soc., 2007, 129, 16142–16148 CrossRef CAS PubMed.
- F. Piguet, F. Discala, M. F. Breton, J. Pelta, L. Bacri and A. Oukhaled, J. Phys. Chem. Lett., 2014, 5, 4362–4367 CrossRef CAS PubMed.
- S. P. Bhamidimarri, J. D. Prajapati, D. B. B. Van, M. Winterhalter and U. Kleinekathöfer, Biophys. J., 2016, 110, 600–611 CrossRef CAS PubMed.
- L. Mereuta, M. Roy, A. Asandei, J. K. Lee, Y. Park, I. Andricioaei and T. Luchian, Sci. Rep., 2014, 4, 3885 Search PubMed.
- A. Asandei, M. Chinappi, J. K. Lee, S. C. Ho, L. Mereuta, Y. Park and T. Luchian, Sci. Rep., 2015, 5, 10419 CrossRef CAS PubMed.
- A. Asandei, I. Schiopu, M. Chinappi, H. S. Chang, Y. Park and T. Luchian, ACS Appl. Mater. Interfaces, 2016, 8, 13166–13179 CAS.
- B. Egwolf, Y. Luo, D. E. Walters and B. Roux, J. Phys. Chem. B, 2010, 114, 2901–2909 CrossRef CAS PubMed.
- B. Hamelin, L. Jullien, F. Guillo, J. M. Lehn, A. Jardy, L. D. Robertis and H. Driguez, J. Phys. Chem., 1995, 99, 17877–17885 CrossRef CAS.
- L. Q. Gu, S. M. Dalla, J. B. Vincent, G. Vigh, S. Cheley, O. Braha and H. Bayley, Proc. Natl. Acad. Sci., 2000, 97, 3959–3964 CrossRef CAS.
- O. Braha, J. Webb, L. Q. Gu, K. Kim and H. Bayley, ChemPhysChem, 2005, 6, 889–892 CrossRef CAS PubMed.
- L. Q. Gu and H. Bayley, Biophys. J., 2000, 79, 1967–1975 CrossRef CAS PubMed.
- P. G. Merzlyak, M. F. Capistrano, A. Valeva, J. J. Kasianowicz and O. V. Krasilnikov, Biophys. J., 2005, 89, 3059–3070 CrossRef CAS PubMed.
- O. V. Krasilnikov, M. P. Capistrano, L. N. Yuldasheva and R. A. Nogueira, J. Membr. Biol., 1997, 156, 157–172 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |