Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Preparation of protic ionic liquids containing cyclic oligosiloxane frameworks
T. Hiroharaa,
T. Kaib,
J. Ohshitab and
Y. Kaneko *a
*a
aGraduate School of Science and Engineering, Kagoshima University, 1-21-40 Korimoto, Kagoshima 890-0065, Japan. E-mail: ykaneko@eng.kagoshima-u.ac.jp; Fax: +81 99 285 7794; Tel: +81 99 285 7794
bGraduate School of Engineering, Hiroshima University, Higashi-Hiroshima, Hiroshima 739-8527, Japan
First published on 8th February 2017
Abstract
Protic ionic liquids (PILs) containing cyclic oligosiloxanes with ammonium side-chain groups (Am-CyS-IL and 2Am-CyS-IL) were successfully prepared by the hydrolytic condensation of 3-aminopropyldimethoxymethylsilane and 3-(2-aminoethylamino)propyldimethoxymethylsilane, respectively, in a water/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 v/v) mixed solution of bis(trifluoromethanesulfonyl)imide (HNTf2). The differential scanning calorimetry (DSC) curves of Am-CyS-IL and 2Am-CyS-IL exhibited baseline shifts attributable to glass transition temperatures (Tg) of −2 °C and 9 °C, respectively, and endothermic peaks at their melting temperatures (Tm) were not detected for both compounds, indicating their amorphous structures. In addition, fluidity was visually observed for each compound at ∼35 °C and ∼45 °C, respectively. On the basis of these results, we concluded that Am-CyS-IL and 2Am-CyS-IL were PILs. To investigate the influence of the kind of siloxane framework on the nature of the ionic liquid, we prepared polyhedral oligomeric silsesquioxanes (POSSs) containing the same side-chain groups and counteranions (Am-POSS and 2Am-POSS) from 3-aminopropyltrimethoxysilane and 3-(2-aminoethylamino)propyltrimethoxysilane, respectively, using an HNTf2 catalyst. Am-POSS had a Tg of 15 °C and a Tm of 193 °C, and 2Am-POSS had a Tg of 32 °C and a Tm of 208 °C, which were estimated by DSC analyses. Furthermore, fluidity was not observed below 150 °C for both compounds, indicating that these POSS compounds were not PILs. These results suggest that the cyclic oligosiloxane frameworks are an important factor for the preparation of siloxane-based PILs exhibiting fluidity at low temperature. In addition, the present PILs containing cyclic oligosiloxane frameworks exhibited relatively high decomposition temperatures (Td; Am-CyS-IL: Td5 = 351 °C and Td10 = 362 °C, 2Am-CyS-IL: Td5 = 294 °C and Td10 = 309 °C).
19 v/v) mixed solution of bis(trifluoromethanesulfonyl)imide (HNTf2). The differential scanning calorimetry (DSC) curves of Am-CyS-IL and 2Am-CyS-IL exhibited baseline shifts attributable to glass transition temperatures (Tg) of −2 °C and 9 °C, respectively, and endothermic peaks at their melting temperatures (Tm) were not detected for both compounds, indicating their amorphous structures. In addition, fluidity was visually observed for each compound at ∼35 °C and ∼45 °C, respectively. On the basis of these results, we concluded that Am-CyS-IL and 2Am-CyS-IL were PILs. To investigate the influence of the kind of siloxane framework on the nature of the ionic liquid, we prepared polyhedral oligomeric silsesquioxanes (POSSs) containing the same side-chain groups and counteranions (Am-POSS and 2Am-POSS) from 3-aminopropyltrimethoxysilane and 3-(2-aminoethylamino)propyltrimethoxysilane, respectively, using an HNTf2 catalyst. Am-POSS had a Tg of 15 °C and a Tm of 193 °C, and 2Am-POSS had a Tg of 32 °C and a Tm of 208 °C, which were estimated by DSC analyses. Furthermore, fluidity was not observed below 150 °C for both compounds, indicating that these POSS compounds were not PILs. These results suggest that the cyclic oligosiloxane frameworks are an important factor for the preparation of siloxane-based PILs exhibiting fluidity at low temperature. In addition, the present PILs containing cyclic oligosiloxane frameworks exhibited relatively high decomposition temperatures (Td; Am-CyS-IL: Td5 = 351 °C and Td10 = 362 °C, 2Am-CyS-IL: Td5 = 294 °C and Td10 = 309 °C).
Introduction
Ionic liquids (ILs) are generally defined as molten salts below 100 °C or 150 °C. ILs are classified as aprotic ILs (AILs) or protic ILs (PILs) depending on the absence or presence of protons. PILs are prepared by a simple neutralization (proton transfer) reaction between a Brønsted acid and a Brønsted base.1,2 Therefore, they have the advantages of being easier to prepare and cheaper compared with AILs. So far, PILs have been widely studied due to their remarkable potential as electrolytes for fuel cells,3 lithium-ion batteries4 and capacitors,5 as biomass extraction solvents,6 and for carbon dioxide captures.7,8 In particular, because PILs have relatively good thermal stability, they are expected to be applied as thermostable proton conductors (solid electrolytes) for fuel cells. However, the decomposition temperatures (Td) of PILs on pyrolysis are generally lower than those of AILs because of proton transfer from the cation back to the anion to reform the original acid and base neutral species.1So far, in the field of AILs, ILs containing siloxane frameworks have been reported.9–14 Siloxane compounds exhibit excellent thermal stability due to the high binding energy of their Si–O–Si linkages. An AIL containing polyhedral oligomeric silsesquioxane (POSS), a siloxane framework, was first prepared by Chujo, Tanaka, and co-worker.9 This POSS-AIL contained carboxylate anion side chains and imidazolium cations as counterions. Zhang et al. have also reported the preparation of an AIL containing POSS with imidazolium cations side chains and dodecyl sulfate anions as counterions.10 Meanwhile, we prepared a highly thermostable POSS-AIL containing imidazolium cationic side-chains and bis(trifluoromethanesulfonyl)imide (NTf2) anions as counterions by a simple hydrolytic condensation method.11 In addition, AILs containing randomly structured oligomeric silsesquioxanes with imidazolium11 and quaternary ammonium12 side-chain groups were also prepared. More recently, a room-temperature POSS-AIL containing two types of cationic side-chain groups, i.e. imidazolium and quaternary ammonium groups, and NTf2 anions as counterions was prepared by a similar hydrolytic condensation method.13 Furthermore, as an example of AILs containing other types of siloxane frameworks, we prepared imidazolium salt type AILs containing cyclic siloxanes.14 In these siloxane-based AILs prepared by us, the use of a superacid catalyst is essential for structural control and indicating IL nature. The Td values of the aforementioned AILs containing siloxane frameworks were higher than those of AILs with side-chain structures of the corresponding siloxane-based AILs. Therefore, PILs containing siloxane frameworks are expected to exhibit higher thermal stability. However, to the best of our knowledge, the preparation of PILs containing siloxane frameworks has not yet been reported.
To successfully prepare such siloxane-based PILs, we referred to our previous study of the facile preparation of a single-structured cyclic tetrasiloxane containing ammonium groups by the hydrolytic condensation of 3-aminopropyldiethoxymethylsilane using aqueous superacid trifluoromethanesulfonic acid (HOTf) as a catalyst.15 However, this cyclic tetrasiloxane was not an IL, i.e. its melting temperature (Tm) is ca. 310 °C. In general, it has been reported that Tm and/or flow temperatures of ILs containing NTf2 anions are lower than those containing OTf anions with the same cationic species.14,16
In this study, when the hydrolytic condensation of dialkoxysilanes containing primary and/or secondary amino groups, such as 3-aminopropyldimethoxymethylsilane (APDMMS) and 3-(2-aminoethylamino)propyldimethoxymethylsilane (AEAPDMMS), were investigated using superacid HNTf2 as a catalyst, we found that PILs containing cyclic oligosiloxane frameworks were successfully prepared. Here we report the preparation, characterizations and thermal properties of these PILs, and a comparison of the properties with POSSs containing the same side-chain groups and counterions.
Experimental
Materials
APDMMS, AEAPDMMS, 3-aminopropyltrimethoxysilane (APTMS) and 3-(2-aminoethylamino)propyltrimethoxysilane (AEAPTMS) were purchased from Tokyo Chemical Industry Co., Ltd. (Japan). Other reagents and solvents were purchased from Wako Pure Chemical Industries, Ltd. (Japan). All reagents and solvents were used without further purification.Preparation of PILs containing cyclic oligosiloxane with one ammonium group per repeating unit (Am-CyS-IL)
A water/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 v/v) mixed solution of HNTf2 (0.5 mol L−1, 15 mL, 7.5 mmol) was added to APDMMS (purity: 97%, 0.505 g, 3.0 mmol) with stirring at room temperature. After the resulting solution was further stirred for 2 h, this solution was heated at ∼60 °C in an open container until the solvent evaporated. Subsequently, the resulting viscous product was maintained at 100 °C for ∼2 h, dissolved in methanol (1 mL) at room temperature, and then this methanol solution was poured into chloroform (30 mL). The chloroform-insoluble part was isolated by decantation, washed with chloroform (∼30 mL × 3), and then dried by heating at 150 °C for ∼10 h to yield 1.019 g of a viscous product (Am-CyS-IL; yield, ∼85%; the ideal chemical formula of one repeating unit of the product [CH3SiO(CH2)3NH3·(CF3SO2)2N, FW = 398.4] was used for this determination). 1H NMR (400 MHz, DMSO-d6): δ 7.80–7.43 (3H, br, –NH3), δ 2.83–2.64 (2H, br, –CH2NH3), δ 1.61–1.41 (2H, br, –SiCH2CH2CH2NH3), δ 0.63–0.37 (2H, br, –SiCH2–), δ 0.19 to −0.03 (3H, br, –SiCH3). 29Si NMR (79.5 MHz, DMSO-d6): δ −19.3 to −19.8 (D2, cyclic tetramer), δ −21.6 to −21.9 (D2, cyclic pentamer), δ −22.2 to −22.5 (D2, cyclic hexamer).
19 v/v) mixed solution of HNTf2 (0.5 mol L−1, 15 mL, 7.5 mmol) was added to APDMMS (purity: 97%, 0.505 g, 3.0 mmol) with stirring at room temperature. After the resulting solution was further stirred for 2 h, this solution was heated at ∼60 °C in an open container until the solvent evaporated. Subsequently, the resulting viscous product was maintained at 100 °C for ∼2 h, dissolved in methanol (1 mL) at room temperature, and then this methanol solution was poured into chloroform (30 mL). The chloroform-insoluble part was isolated by decantation, washed with chloroform (∼30 mL × 3), and then dried by heating at 150 °C for ∼10 h to yield 1.019 g of a viscous product (Am-CyS-IL; yield, ∼85%; the ideal chemical formula of one repeating unit of the product [CH3SiO(CH2)3NH3·(CF3SO2)2N, FW = 398.4] was used for this determination). 1H NMR (400 MHz, DMSO-d6): δ 7.80–7.43 (3H, br, –NH3), δ 2.83–2.64 (2H, br, –CH2NH3), δ 1.61–1.41 (2H, br, –SiCH2CH2CH2NH3), δ 0.63–0.37 (2H, br, –SiCH2–), δ 0.19 to −0.03 (3H, br, –SiCH3). 29Si NMR (79.5 MHz, DMSO-d6): δ −19.3 to −19.8 (D2, cyclic tetramer), δ −21.6 to −21.9 (D2, cyclic pentamer), δ −22.2 to −22.5 (D2, cyclic hexamer).
Preparation of PILs containing cyclic oligosiloxane with two ammonium groups per repeating unit (2Am-CyS-IL)
A water/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 v/v) mixed solution of HNTf2 (0.5 mol L−1, 30 mL, 15 mmol) was added to AEAPDMMS (purity: 97%, 0.638 g, 3.0 mmol) with stirring at room temperature. The subsequent procedures were the same as those described above for the preparation of Am-CyS-IL, and 1.896 g of a viscous product (2Am-CyS-IL) was obtained (yield, ∼87%; the ideal chemical formula of one repeating unit of the product [CH3SiO(CH2)3NH2(CH2)2NH3·2(CF3SO2)2N, FW = 722.7] was used for this determination). 1H NMR (400 MHz, DMSO-d6): δ 8.56–8.14 (2H, br, –NH2CH2CH2NH3), δ 7.99–7.60 (3H, br, –NH2CH2CH2NH3), δ 3.20–2.97 (4H, br, –NH2CH2CH2NH3), δ 2.97–2.75 (2H, br, –SiCH2CH2CH2NH2–), δ 1.69–1.38 (2H, br, –SiCH2CH2CH2NH2–), δ 0.70–0.36 (2H, br, –SiCH2–), δ 0.27 to −0.01 (3H, br, –SiCH3). 29Si NMR (79.5 MHz, DMSO-d6): δ −19.1 to −20.1 (D2, cyclic tetramer), δ −21.3 to −21.8 (D2, cyclic pentamer), δ −22.1 to −22.3 (D2, cyclic hexamer).
19 v/v) mixed solution of HNTf2 (0.5 mol L−1, 30 mL, 15 mmol) was added to AEAPDMMS (purity: 97%, 0.638 g, 3.0 mmol) with stirring at room temperature. The subsequent procedures were the same as those described above for the preparation of Am-CyS-IL, and 1.896 g of a viscous product (2Am-CyS-IL) was obtained (yield, ∼87%; the ideal chemical formula of one repeating unit of the product [CH3SiO(CH2)3NH2(CH2)2NH3·2(CF3SO2)2N, FW = 722.7] was used for this determination). 1H NMR (400 MHz, DMSO-d6): δ 8.56–8.14 (2H, br, –NH2CH2CH2NH3), δ 7.99–7.60 (3H, br, –NH2CH2CH2NH3), δ 3.20–2.97 (4H, br, –NH2CH2CH2NH3), δ 2.97–2.75 (2H, br, –SiCH2CH2CH2NH2–), δ 1.69–1.38 (2H, br, –SiCH2CH2CH2NH2–), δ 0.70–0.36 (2H, br, –SiCH2–), δ 0.27 to −0.01 (3H, br, –SiCH3). 29Si NMR (79.5 MHz, DMSO-d6): δ −19.1 to −20.1 (D2, cyclic tetramer), δ −21.3 to −21.8 (D2, cyclic pentamer), δ −22.1 to −22.3 (D2, cyclic hexamer).
Preparation of POSS with one ammonium group per repeating unit (Am-POSS)
An aqueous HNTf2 solution (0.5 mol L−1, 15 mL, 7.5 mmol) was added to APTMS (purity: 96%, 0.560 g, 3.0 mmol) with stirring at room temperature. The subsequent procedures were the same as those described above for the preparation of Am-CyS-IL, and 1.150 g of a solid substance (Am-POSS) was obtained (yield, ∼98%; the ideal chemical formula of one repeating unit of the product [SiO1.5(CH2)3NH3·(CF3SO2)2N, FW = 391.4] was used for this determination). 1H NMR (400 MHz, DMSO-d6): δ 7.70–7.46 (3H, br, –NH3), δ 2.84–2.63 (2H, br, –CH2NH3), δ 1.63–1.40 (2H, br, –SiCH2CH2CH2NH3), δ 0.74–0.52 (2H, br, –SiCH2–). 29Si NMR (79.5 MHz, DMSO-d6): δ −66.5 (T8), δ −68.5 (T10).Preparation of POSS with two ammonium groups per repeating unit (2Am-POSS)
A aqueous HNTf2 solution (0.5 mol L−1, 30 mL, 15 mmol) was added to AEAPTMS (purity: 95%, 0.702 g, 3.0 mmol) with stirring at room temperature. The subsequent procedures were the same as those described above for the preparation of Am-CyS-IL, and 2.110 g of a solid substance (2Am-POSS) was obtained (yield, ∼98%; the ideal chemical formula of one repeating unit of the product [SiO1.5(CH2)3NH2(CH2)2NH3·2(CF3SO2)2N, FW = 715.6] was used for this determination). 1H NMR (400 MHz, DMSO-d6): δ 8.48–8.23 (2H, br, –NH2CH2CH2NH3), δ 7.96–7.69 (3H, br, –NH2CH2CH2NH3), δ 3.20–2.91 (4H, br, –NH2CH2CH2NH3), δ 2.91–2.68 (2H, br, –SiCH2CH2CH2NH2–), δ 1.69–1.33 (2H, br, –SiCH2CH2CH2NH2–), δ 0.78–0.40 (2H, br, –SiCH2–). 29Si NMR (79.5 MHz, DMSO-d6): δ −67.3 (T8), δ −69.4 (T10).Measurements
1H and 29Si NMR spectra were recorded using a JEOL ECX-400 spectrometer (JEOL Ltd.). The atomic ratios of Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S in the products were confirmed by energy-dispersive X-ray (EDX) analyses using an XL30 scanning electron microscope (FEI Co.). Matrix-assisted laser desorption ionization time-of-flight mass spectral (MALDI-TOF MS) analyses of the products were performed using a Shimadzu Voyager Biospectrometry Workstation Ver. 5.1 (SHIMADZU Co.) positive ion mode with 2,5-dihydroxybenzoic acid (DHB) as the matrix. Attenuated total reflectance infrared (ATR-IR) spectra were recorded using a JASCO FTIR-4200 spectrometer (JASCO Co.). Differential scanning calorimetry (DSC) analyses were performed using a DSC-60 Plus (SHIMADZU Co.). The sample was placed in an aluminium capsule and cooled to −140 °C at a rate of 20 °C min−1 under a nitrogen flow (50 mL min−1), and then heated from −140 °C to 250 °C at the same rate. Glass transition temperatures (Tg) and Tm were determined as the onset of baseline shifts and as the tops of endothermic peaks, respectively, in the third set of curves (from −140 °C to 250 °C at a rate of 20 °C min−1) to eliminate the heat histories of the samples. X-ray diffraction (XRD) patterns were recorded at a scanning speed of 2θ = 6.6° min−1 using an X'Pert Pro diffractometer (PANalytical) with Ni-filtered Cu Kα radiation (0.15418 nm). Thermogravimetric analyses (TGA) were performed on an Exstar TG/DTA6200 (Seiko Instruments) at a heating rate of 10 °C min−1 up to 1000 °C under a nitrogen flow (250 mL min−1). The decomposition temperatures were determined from the 5% (Td5) and 10% (Td10) weight losses.
S in the products were confirmed by energy-dispersive X-ray (EDX) analyses using an XL30 scanning electron microscope (FEI Co.). Matrix-assisted laser desorption ionization time-of-flight mass spectral (MALDI-TOF MS) analyses of the products were performed using a Shimadzu Voyager Biospectrometry Workstation Ver. 5.1 (SHIMADZU Co.) positive ion mode with 2,5-dihydroxybenzoic acid (DHB) as the matrix. Attenuated total reflectance infrared (ATR-IR) spectra were recorded using a JASCO FTIR-4200 spectrometer (JASCO Co.). Differential scanning calorimetry (DSC) analyses were performed using a DSC-60 Plus (SHIMADZU Co.). The sample was placed in an aluminium capsule and cooled to −140 °C at a rate of 20 °C min−1 under a nitrogen flow (50 mL min−1), and then heated from −140 °C to 250 °C at the same rate. Glass transition temperatures (Tg) and Tm were determined as the onset of baseline shifts and as the tops of endothermic peaks, respectively, in the third set of curves (from −140 °C to 250 °C at a rate of 20 °C min−1) to eliminate the heat histories of the samples. X-ray diffraction (XRD) patterns were recorded at a scanning speed of 2θ = 6.6° min−1 using an X'Pert Pro diffractometer (PANalytical) with Ni-filtered Cu Kα radiation (0.15418 nm). Thermogravimetric analyses (TGA) were performed on an Exstar TG/DTA6200 (Seiko Instruments) at a heating rate of 10 °C min−1 up to 1000 °C under a nitrogen flow (250 mL min−1). The decomposition temperatures were determined from the 5% (Td5) and 10% (Td10) weight losses.
Results and discussion
Preparation and characterizations of Am-CyS-IL and 2Am-CyS-IL
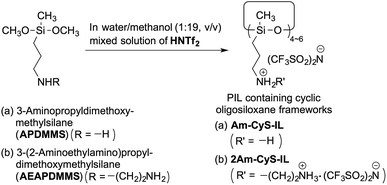
A PIL containing a cyclic oligosiloxane with one ammonium group per repeating unit, Am-CyS-IL, was prepared according to the following procedure (Scheme 1a): APDMMS was stirred in a water/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 v/v) mixed solution of HNTf2 (0.5 mol L−1) at room temperature for 2 h. Here, an excess amount of HNTf2 was required (the feed molar ratio of HNTf2/APDMMS was 2.5). The resulting solution was heated at ca. 60 °C in an open container until the solvent evaporated. The resulting crude product was further heated at 100 °C for 2 h, washed with chloroform, and then dried at 150 °C for ∼10 h to obtain Am-CyS-IL. Am-CyS-IL was soluble in high-polarity solvents, such as water, methanol and acetone, but insoluble in low-polarity solvents, such as chloroform, toluene and hexane.
19 v/v) mixed solution of HNTf2 (0.5 mol L−1) at room temperature for 2 h. Here, an excess amount of HNTf2 was required (the feed molar ratio of HNTf2/APDMMS was 2.5). The resulting solution was heated at ca. 60 °C in an open container until the solvent evaporated. The resulting crude product was further heated at 100 °C for 2 h, washed with chloroform, and then dried at 150 °C for ∼10 h to obtain Am-CyS-IL. Am-CyS-IL was soluble in high-polarity solvents, such as water, methanol and acetone, but insoluble in low-polarity solvents, such as chloroform, toluene and hexane.
 | ||
| Scheme 1 Preparation of PILs containing cyclic oligosiloxane frameworks: (a) Am-CyS-IL and (b) 2Am-CyS-IL. | ||
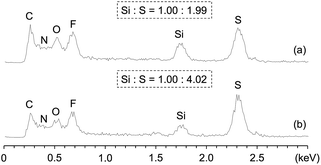
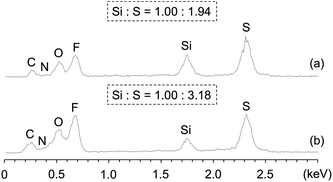
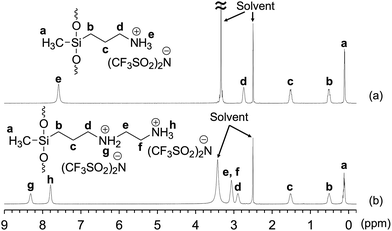
The 1H NMR spectrum of Am-CyS-IL in DMSO-d6 exhibited signals attributable to 3-aminopropyl and methyl groups, but signals due to the methoxy groups of APDMMS were not observed (Fig. 1a), indicating that the APDMMS reagent was not present in the product. The EDX pattern of Am-CyS-IL revealed that the Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S atomic ratio was ca. 1.00
S atomic ratio was ca. 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.99, indicating that the molar ratio of the ammonium cation to the NTf2 anion in Am-CyS-IL was ca. 1
1.99, indicating that the molar ratio of the ammonium cation to the NTf2 anion in Am-CyS-IL was ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
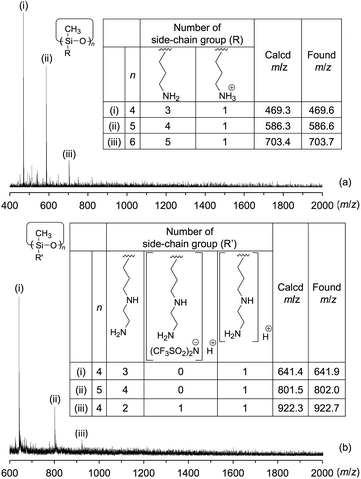
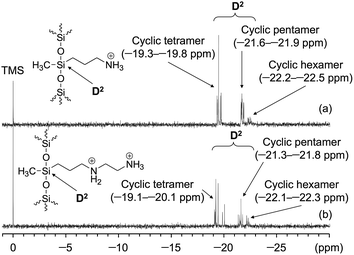
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 2a). In the MALDI-TOF MS analysis of Am-CyS-IL, several peaks were observed that corresponded to the masses of cyclic siloxane tetramer, pentamer and hexamer (Fig. 3a). The 29Si NMR spectrum of Am-CyS-IL in DMSO-d6 at 40 °C showed three multiple signals in the D2 region (−19.3 to −19.8 ppm for cyclic tetrasiloxane, −21.6 to −21.9 ppm for cyclic pentasiloxane, and −22.2 to −22.5 ppm for cyclic hexasiloxane) and no signals in the D1 region at ca. −15 ppm (Fig. 4a). Furthermore, the aforementioned 1H NMR spectrum contained multiple signals, a, attributable to methyl groups at 0.20 to −0.01 ppm (Fig. 1a). These results indicate that Am-CyS-IL was a mixture of cyclic tetra-, penta-, and hexasiloxanes, with some stereoisomers.
1 (Fig. 2a). In the MALDI-TOF MS analysis of Am-CyS-IL, several peaks were observed that corresponded to the masses of cyclic siloxane tetramer, pentamer and hexamer (Fig. 3a). The 29Si NMR spectrum of Am-CyS-IL in DMSO-d6 at 40 °C showed three multiple signals in the D2 region (−19.3 to −19.8 ppm for cyclic tetrasiloxane, −21.6 to −21.9 ppm for cyclic pentasiloxane, and −22.2 to −22.5 ppm for cyclic hexasiloxane) and no signals in the D1 region at ca. −15 ppm (Fig. 4a). Furthermore, the aforementioned 1H NMR spectrum contained multiple signals, a, attributable to methyl groups at 0.20 to −0.01 ppm (Fig. 1a). These results indicate that Am-CyS-IL was a mixture of cyclic tetra-, penta-, and hexasiloxanes, with some stereoisomers.
 | ||
| Fig. 1 1H NMR spectra of (a) Am-CyS-IL and (b) 2Am-CyS-IL in DMSO-d6. Chemical shifts were referenced to DMSO (δ 2.5). | ||
 | ||
| Fig. 4 29Si NMR spectra of (a) Am-CyS-IL and (b) 2Am-CyS-IL in DMSO-d6 at 40 °C. Chemical shifts were referenced to tetramethylsilane (TMS; δ 0.0). | ||
A PIL containing a cyclic oligosiloxane with two ammonium groups per repeating unit, 2Am-CyS-IL, was prepared using almost the same procedure as that of Am-CyS-IL but using another organodialkoxysilane containing two amino groups, AEAPDMMS, as a starting material (Scheme 1b). 2Am-CyS-IL was soluble in high-polarity solvents, such as water, methanol and acetone, but insoluble in low-polarity solvents, such as chloroform, toluene and hexane.
The 1H NMR (Fig. 1b) and EDX (Fig. 2b) results of 2Am-CyS-IL indicated that the AEAPDMMS reagent was not present in the product, and that the molar ratio of ammonium cations to NTf2 anions in 2Am-CyS-IL was ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The MALDI-TOF MS results indicated the existence of a mixture of cyclic siloxane tetramer and pentamer (Fig. 3b). Furthermore, the 29Si NMR spectrum of 2Am-CyS-IL showed three multiple signals in the D2 region (−19.1 to −20.1 ppm for cyclic tetrasiloxane, −21.3 to −21.8 ppm for cyclic pentasiloxane, and −22.1 to −22.4 ppm for cyclic hexasiloxane) and no signals in the D1 region at ca. −15 ppm (Fig. 4b), supporting the formation of cyclic siloxanes. In addition, these compounds had some stereoisomers, as demonstrated by the 1H NMR spectrum with multiple signals, a, due to the methyl groups at 0.27 to −0.01 ppm (Fig. 1b) and the 29Si NMR spectrum with three multiple signals, as described above (Fig. 4b).
1. The MALDI-TOF MS results indicated the existence of a mixture of cyclic siloxane tetramer and pentamer (Fig. 3b). Furthermore, the 29Si NMR spectrum of 2Am-CyS-IL showed three multiple signals in the D2 region (−19.1 to −20.1 ppm for cyclic tetrasiloxane, −21.3 to −21.8 ppm for cyclic pentasiloxane, and −22.1 to −22.4 ppm for cyclic hexasiloxane) and no signals in the D1 region at ca. −15 ppm (Fig. 4b), supporting the formation of cyclic siloxanes. In addition, these compounds had some stereoisomers, as demonstrated by the 1H NMR spectrum with multiple signals, a, due to the methyl groups at 0.27 to −0.01 ppm (Fig. 1b) and the 29Si NMR spectrum with three multiple signals, as described above (Fig. 4b).
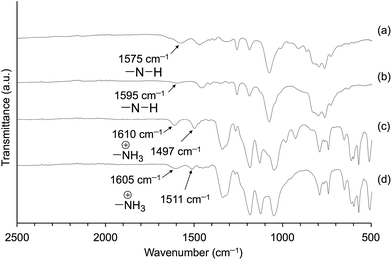
To confirm the formation of ionic pairs of the resulting products (Am-CyS-IL and 2Am-CyS-IL), we compared the ATR-IR spectra of these products and their starting materials (APDMMS and AEAPDMMS). The IR spectra of APDMMS and AEAPDMMS showed absorption peaks attributable to the bending vibration of amino groups at 1575 and 1595 cm−1, respectively (Fig. 5a and b). Conversely, those of Am-CyS-IL and 2Am-CyS-IL exhibited two typical absorption peaks attributable to the bending vibration of ammonium cations at 1610 and 1497 cm−1 for Am-CyS-IL (Fig. 5c) and at 1605 and 1511 cm−1 for 2Am-CyS-IL (Fig. 5d). In addition, these spectra did not exhibit absorption peaks due to the bending vibration of amino groups. Therefore, we concluded that cationic species (ammonium cations) were included in Am-CyS-IL and 2Am-CyS-IL. Meanwhile, because the NTf2 anion is extremely stable, NTf2 must be present as the anionic species in these compounds.
Thermal properties of Am-CyS-IL and 2Am-CyS-IL
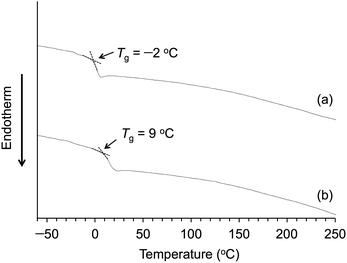
The DSC analyses of Am-CyS-IL and 2Am-CyS-IL were performed at a heating rate of 20 °C min−1 under a nitrogen flow (50 mL min−1). The baseline shifts assigned to Tg were observed at −2 °C for Am-CyS-IL (Fig. 6a) and at 9 °C for 2Am-CyS-IL (Fig. 6b). Conversely, endothermic peaks due to Tm were not detected for both compounds (Fig. 6a and b), indicating that Am-CyS-IL and 2Am-CyS-IL were amorphous compounds. In addition, sharp diffraction peaks were not observed in the XRD patterns of Am-CyS-IL and 2Am-CyS-IL (Fig. 7a and b), supporting their amorphous or poorly crystalline structures.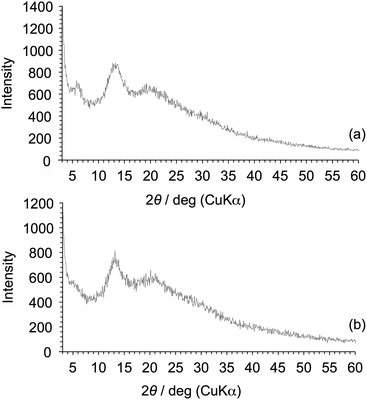 | ||
| Fig. 7 XRD patterns of (a) Am-CyS-IL and (b) 2Am-CyS-IL. The amount of each product on the glass was ca. 1.0 mg cm−2. | ||
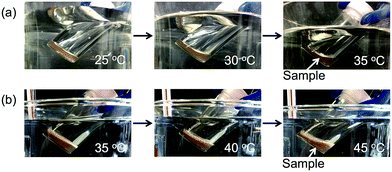
The flow temperatures of Am-CyS-IL and 2Am-CyS-IL were confirmed as follows. Samples in glass vessels were maintained in a horizontal position at 150 °C for 15 min, and then the vessels were cooled to room temperature, still in the horizontal position. Next, the vessels were maintained in a horizontal position at various temperatures (varied in 5 °C intervals) for 15 min, and then held for 15 min while tilting at each temperature. During this procedure, Am-CyS-IL and 2Am-CyS-IL showed obvious fluidity at ∼35 °C (Fig. 8a) and ∼45 °C (Fig. 8b), respectively. On the basis of these results, we concluded that Am-CyS-IL and 2Am-CyS-IL were PILs.
To investigate the amount of water in these PILs, the weight losses at 150 °C were evaluated by TGA (Fig. 9a and b). Consequently, these values were 1.1% for Am-CyS-IL and 0.3% for 2Am-CyS-IL, indicating that these PILs contain a little moisture. However, because these samples were dried at 150 °C for 10 h after synthesis, it is presumed that they slightly absorbed moisture in the environment.
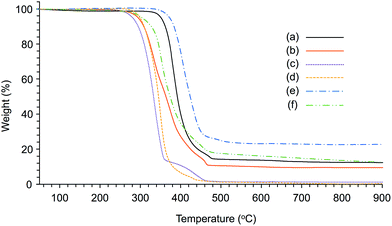 | ||
| Fig. 9 TGA thermograms of (a) Am-CyS-IL, (b) 2Am-CyS-IL, (c) propylamine–NTf2 salt, (d) N-propylethylenediamine–NTf2 salt, (e) Am-POSS and (f) 2Am-POSS under a nitrogen flow. | ||
The thermal stabilities of Am-CyS-IL and 2Am-CyS-IL against pyrolysis were investigated by TGA at a heating rate of 10 °C min−1 up to 1000 °C under a nitrogen flow (250 mL min−1). The Td5 and Td10 values were 351 and 362 °C, respectively, for Am-CyS-IL (Fig. 9a). These values were higher than those of the propylamine–NTf2 salt (281 and 295 °C), having the side-chain structure of Am-CyS-IL, with a Tg of −65 °C and a Tm of 28 °C (Fig. 9c). These results indicate that the thermal stability of Am-CyS-IL was enhanced by the incorporation of the cyclic oligosiloxane frameworks. The cyclic oligosiloxane frameworks could suppress molecular tumbling, resulting in the prevention of degradation.9 On the other hand, the Td5 and Td10 values of 2Am-CyS-IL were 294 and 309 °C (Fig. 9b), which are almost the same as those of the N-propylethylenediamine–NTf2 salt (299 and 309 °C), having the side-chain structure of 2Am-CyS-IL, with a Tg of −29 °C and a Tm of 85 °C (Fig. 9d). Because the side-chain groups of 2Am-CyS-IL are long, the suppression of molecular tumbling would not work. The weights of the residues of Am-CyS-IL (ca. 12%; Fig. 9a) and 2Am-CyS-IL (ca. 10%; Fig. 9b) at 900 °C were almost the same as the theoretical SiO2 yields (15% and 8%, respectively).
Influence of structures of siloxane frameworks on IL nature
To investigate the influence of the structures of the siloxane frameworks on the nature of the ILs, we prepared POSS compounds containing the same side-chain groups and counteranions (Am-POSS and 2Am-POSS). In our previous studies, POSS compounds containing ammonium side-chain groups were prepared by the hydrolytic condensation of APTMS,17 AEAPTMS18 and an APTMS/AEAPTMS mixture18 using aqueous superacid HOTf. Therefore, to obtain POSS compounds containing the same counteranions as Am-CyS-IL and 2Am-CyS-IL, the hydrolytic condensation of APTMS and AEAPTMS was performed using HNTf2 as a catalyst, respectively (Scheme 2).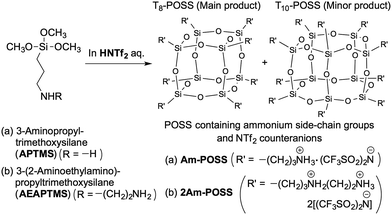 | ||
| Scheme 2 Preparation of POSSs containing ammonium side-chain groups and NTf2 counteranions: (a) Am-POSS and (b) 2Am-POSS. | ||
POSSs with one ammonium group (Am-POSS) and two ammonium groups (2Am-POSS) per repeating unit were prepared according to the following procedure (Scheme 2): APTMS and AEAPTMS were stirred in 0.5 mol L−1 aqueous HNTf2 solutions at room temperature for 2 h, respectively. Here, excess amounts of HNTf2 were required (feed molar ratio of HNTf2/amino group was 2.5). The subsequent procedures were the same as those described above for the preparation of Am-CyS-IL and 2Am-CyS-IL.
Characterization of Am-POSS and 2Am-POSS was performed using 1H NMR (Fig. 10), EDX (Fig. 11) and 29Si NMR (Fig. 12) measurements. Especially, the 29Si NMR results indicated that these compounds were mixtures of cage-like octasilsesquioxane (T8-POSS) and cage-like decasilsesquioxane (T10-POSS) (Fig. 12). However, the molar ratio of ammonium cations to NTf2 anions in 2Am-POSS was ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.79, i.e. a non-equimolar ratio (Fig. 11b). This is probably because it is difficult for HNTf2 to approach the amino side-chain groups in 2Am-POSS due to their bulky nature.
0.79, i.e. a non-equimolar ratio (Fig. 11b). This is probably because it is difficult for HNTf2 to approach the amino side-chain groups in 2Am-POSS due to their bulky nature.
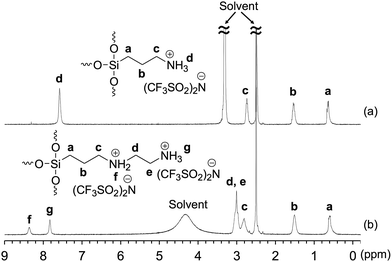 | ||
| Fig. 10 1H NMR spectra of (a) Am-POSS and (b) 2Am-POSS in DMSO-d6. Chemical shifts were referenced to DMSO (δ 2.5). | ||
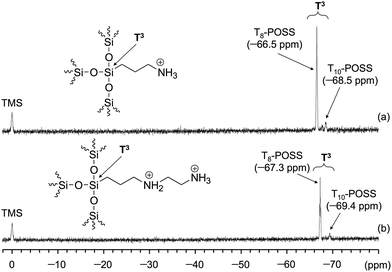 | ||
| Fig. 12 29Si NMR spectra of (a) Am-POSS and (b) 2Am-POSS in DMSO-d6 at 40 °C. Chemical shifts were referenced to TMS (δ 0.0). | ||
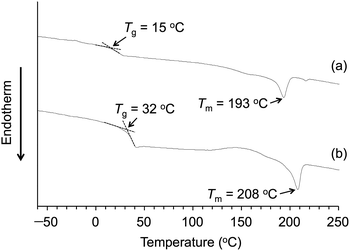
The DSC curve of Am-POSS showed a baseline shift due to Tg at 15 °C and an endothermic peak due to Tm at 193 °C (Fig. 13a). In addition, the XRD pattern of Am-POSS showed several sharp diffraction peaks (Fig. 14a), indicating that Am-POSS was a crystalline compound. Furthermore, this POSS compound did not exhibit fluidity at 150 °C (Fig. 15a), indicating that it was not an IL. On the other hand, the DSC curve of 2Am-POSS exhibited a baseline shift due to Tg at 32 °C and an endothermic peak due to Tm at 208 °C (Fig. 13b). Additionally, the XRD pattern of 2Am-POSS also supported the formation of a crystalline structure, i.e. several sharp diffraction peaks were detected (Fig. 14b). Furthermore, this compound did not show fluidity at 150 °C (Fig. 15b). Based on these results, we concluded that the cyclic oligosiloxane frameworks with flexible structures compared with POSS are important for the preparation of siloxane-based PILs exhibiting fluidity at low temperature.
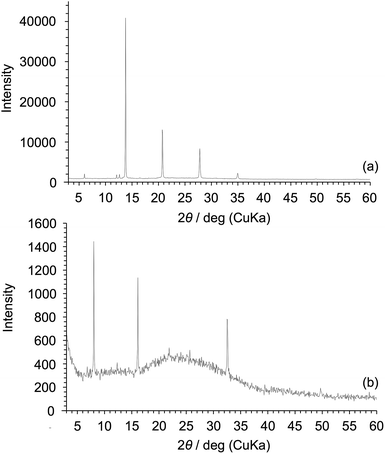 | ||
| Fig. 14 XRD patterns of (a) Am-POSS and (b) 2Am-POSS. The amount of each product on the glass was ca. 1.0 mg cm−2. | ||
Incidentally, Am-POSS and 2Am-POSS exhibited high thermal stabilities in the TGA analyses. The Td5 and Td10 values were 372 and 384 °C for Am-POSS (Fig. 9e) and 304 and 327 °C for 2Am-POSS (Fig. 9f).
Conclusions
In this study, we found that PILs containing cyclic oligosiloxane frameworks, Am-CyS-IL and 2Am-CyS-IL, could be successfully prepared by the hydrolytic condensation of APDMMS and AEAPDMMS using a water/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 v/v) mixed solution of HNTf2. The DSC curves of Am-CyS-IL and 2Am-CyS-IL exhibited baseline shifts attributable to Tg at −2 °C and 9 °C, respectively, and endothermic peaks at Tm were not detected for both compounds, indicating their amorphous structures. In addition, fluidity was visually observed for each compound at ∼35 °C and ∼45 °C, respectively, indicating that Am-CyS-IL and 2Am-CyS-IL were PILs. For comparison, POSSs containing the same side-chain groups and counteranions, Am-POSS and 2Am-POSS, were prepared from APTMS and AEAPTMS, respectively, using an HNTf2 catalyst. Am-POSS had Tg of 15 °C and Tm of 193 °C, and 2Am-POSS had Tg of 32 °C and Tm of 208 °C. Furthermore, fluidity was not observed below 150 °C for either compound, indicating that these POSS compounds were not PILs. These results suggest that the cyclic oligosiloxane frameworks are an important factor for the preparation of siloxane-based PILs exhibiting fluidity at low temperature. The present PILs containing cyclic oligosiloxane frameworks exhibited relatively high thermal stabilities (Am-CyS-IL: Td5 = 351 °C and Td10 = 362 °C, 2Am-CyS-IL: Td5 = 294 °C and Td10 = 309 °C).
19 v/v) mixed solution of HNTf2. The DSC curves of Am-CyS-IL and 2Am-CyS-IL exhibited baseline shifts attributable to Tg at −2 °C and 9 °C, respectively, and endothermic peaks at Tm were not detected for both compounds, indicating their amorphous structures. In addition, fluidity was visually observed for each compound at ∼35 °C and ∼45 °C, respectively, indicating that Am-CyS-IL and 2Am-CyS-IL were PILs. For comparison, POSSs containing the same side-chain groups and counteranions, Am-POSS and 2Am-POSS, were prepared from APTMS and AEAPTMS, respectively, using an HNTf2 catalyst. Am-POSS had Tg of 15 °C and Tm of 193 °C, and 2Am-POSS had Tg of 32 °C and Tm of 208 °C. Furthermore, fluidity was not observed below 150 °C for either compound, indicating that these POSS compounds were not PILs. These results suggest that the cyclic oligosiloxane frameworks are an important factor for the preparation of siloxane-based PILs exhibiting fluidity at low temperature. The present PILs containing cyclic oligosiloxane frameworks exhibited relatively high thermal stabilities (Am-CyS-IL: Td5 = 351 °C and Td10 = 362 °C, 2Am-CyS-IL: Td5 = 294 °C and Td10 = 309 °C).
Acknowledgements
We acknowledge the support of Prof. Y. Suda, Dr M. Wakao and Dr H. Shinchi of the Graduate School of Science and Engineering, Kagoshima University (Japan) in MALDI-TOF MS measurements. This work was supported by JSPS KAKENHI (Grant-in-Aid for Challenging Exploratory Research) Number 15K13711.References and Notes
- T. L. Greaves and C. J. Drummond, Chem. Rev., 2008, 108, 206 CrossRef CAS PubMed.
- T. L. Greaves and C. J. Drummond, Chem. Rev., 2015, 115, 11379 CrossRef CAS PubMed.
- S. Y. Lee, A. Ogawa, M. Kanno, H. Nakamoto, T. Yasuda and M. Watanabe, J. Am. Chem. Soc., 2010, 132, 9764 CrossRef CAS PubMed.
- S. Menne, J. Pires, M. Anouti and A. Balducci, Electrochem. Commun., 2013, 31, 39 CrossRef CAS.
- L. Mayrand-Provencher, S. Lin, D. Lazzerini and D. Rochefort, J. Power Sources, 2010, 195, 5114 CrossRef CAS.
- E. C. Achinivu, R. M. Howard, G. Li, H. Gracz and W. A. Henderson, Green Chem., 2014, 16, 1114 RSC.
- R. Vijayraghavan, S. J. Pas, E. I. Izgorodina and D. R. MacFarlane, Phys. Chem. Chem. Phys., 2013, 15, 19994 RSC.
- T. J. Simons, T. Verheyen, E. I. Izgorodina, R. Vijayaraghavan, S. Young, A. K. Pearson, S. J. Pas and D. R. MacFarlane, Phys. Chem. Chem. Phys., 2016, 18, 1140 RSC.
- K. Tanaka, F. Ishiguro and Y. Chujo, J. Am. Chem. Soc., 2010, 132, 17649 CrossRef CAS PubMed.
- J. Tan, D. Ma, X. Sun, S. Feng and C. Zhang, Dalton Trans., 2013, 42, 4337 RSC.
- T. Ishii, T. Enoki, T. Mizumo, J. Ohshita and Y. Kaneko, RSC Adv., 2015, 5, 15226 RSC.
- T. Ishii, T. Mizumo and Y. Kaneko, Bull. Chem. Soc. Jpn., 2014, 87, 155 CrossRef CAS.
- A. Harada, S. Koge, J. Ohshita and Y. Kaneko, Bull. Chem. Soc. Jpn., 2016, 89, 1129 CrossRef CAS.
- T. Kubo, S. Koge, J. Ohshita and Y. Kaneko, Chem. Lett., 2015, 44, 1362 CrossRef CAS.
- S. Kinoshita, S. Watase, K. Matsukawa and Y. Kaneko, J. Am. Chem. Soc., 2015, 137, 5061 CrossRef CAS PubMed.
- Y. R. Mirzaei, B. Twamley and J. M. Shreeve, J. Org. Chem., 2002, 67, 9340 CrossRef CAS PubMed.
- Y. Kaneko, M. Shoiriki and T. Mizumo, J. Mater. Chem., 2012, 22, 14475 RSC.
- T. Tokunaga, M. Shoiriki, T. Mizumo and Y. Kaneko, J. Mater. Chem. C, 2014, 2, 2496 RSC.
| This journal is © The Royal Society of Chemistry 2017 |