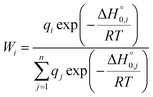Open Access Article
Open Access ArticleDimetallofullerene M2@C100 or carbide cluster fullerene M2C2@C98 (M = La, Y, and Sc): which ones are more stable?†
Lei Mua,
Xiaodi Baoa,
Shumei Yanga and
Xianglei Kong *ab
*ab
aThe State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071, China. E-mail: kongxianglei@nankai.edu.cn
bCollaborative Innovation Center of Chemical Science and Engineering, Nankai University, Tianjin 300071, China
First published on 14th March 2017
Abstract
The geometric and thermodynamic stability of the M2C100 (M = La, Y, and Sc) series was systematically investigated using density functional theory calculations on the level of B3LYP/6-31G(d) ∼ Lanl2dz. In all the cases, M2@D5(285913)-C100 isomers are the lowest-energy species. However, carbide endohedral fullerenes M2C2@C1(230933)-C98 present excellent thermodynamic stabilities, except for those with La metal. The main product in electric arc experiments at temperatures lower than 3500 K for La2C100 should be La2@D5(285913)-C100, which was successfully synthesized previously; for Y and Sc, the predicted main products in these experiments should be M2C2@C1(230933)-C98. Further analysis of the geometric structures of the M2C100 series showed that the dimetallofullerenes M2@C100 have greater effects on the shapes of cages than M2C2@C98. These results provide some valuable guidance for the synthesis and characterization of large endohedral fullerenes including La, Y or Sc.
Introduction
Endohedral dimetallofullerenes were firstly considered to exist in the form of M2@C2n.1–9 However, based on 13C NMR spectroscopic studies, Wang et al. found that the previously suggested endohedral metallofullerene (EMF) of Sc2@C86 was in fact, Sc2C2@C84.10 Since then, many types of carbide cluster fullerenes have been widely reported. It is well known that EMFs of M2C2n may exist in two distinguished forms: classical dimetallofullerenes, M2@C2n, or carbide cluster fullerenes M2C2@C2n−2.11–16 Among the reported carbide cluster fullerenes, Sc plays a very important role; for example, the reported carbide cluster fullerenes with two Sc atoms include Sc2C2@C84,8,10,17 Sc2C2@C82,12,18 Sc2C2@C80 (ref. 4 and 11) and Sc2C2@C68.15 Recently, a family of Y2C2@C2n was isolated by Dorn et al., and some of them were determined by 13C NMR spectroscopy,9 whereas the isolation and crystallographic characterization of La2C2@C2n is less reported.19,20 On the other hand, many dimetallofullerenes of M2@C2n have also been identified; for example, structures of Sc2@C66,21 Sc2@C82 (ref. 22) and Y2@C82 (ref. 23) have been determined by 13C NMR spectroscopy. Dimetallofullerenes of La2@C2n including La2@C72,24 La2@C78 (ref. 25) and La2@C80 (ref. 26) have also been synthesized and characterized.Recently, endohedral fullerenes with large cage sizes (n > 90) have attracted significant research interest;27–30 for example, the largest fullerene cage that has been identified by X-ray is Sm2@D3d(822)-C104, which shows a nanotubular shape,27 and a series of EMFs containing two gadolinium atoms with cages from C90 to C124 have been observed by Yang et al.28 However, the effective isolation and structural characterization of these EMFs are still very challenging; for example, Christine M. Beavers et al. discovered an extensive series of soluble dilanthanum endohedral fullerenes from La2C90 to La2C138, but only a very few of them have been isolated in pure form and characterized by single-crystal X-ray diffraction.29
Due to the difficulty in synthesis, separation and structural assignment of these species, theoretical study has been widely performed to help to predict or determine the structure, stability and properties of these species.31–36 There are at least two challenges in these calculations that should be mentioned. First, the number of different cage isomers for a particular cage size quickly increases with the cage size. Since the non-IPR cages can be greatly stabilized by the encaged metal atoms in EMFs, they should be considered in many cases. Yang et al. performed a systematic investigation on the structures of Dy2@C100 including IPR and non-IPR isomers (with a total number of 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 755) and found that the D5(285913)-C100 cage was the most promising candidate for encapsulation.31 Second, for EMFs having the form of M2C2n, both dimetallofullerenes M2@C2n and carbide cluster fullerenes M2C2@C2n−2 should be considered and compared. For example, in order to deduce the most stable isomers of M2C98 (M = Sc, Y, La, Gd, Lu), Zheng et al. performed systematic studies on the series by density functional theory (DFT) methods, and found that the metal-carbide endohedral fullerenes are more stable.34
755) and found that the D5(285913)-C100 cage was the most promising candidate for encapsulation.31 Second, for EMFs having the form of M2C2n, both dimetallofullerenes M2@C2n and carbide cluster fullerenes M2C2@C2n−2 should be considered and compared. For example, in order to deduce the most stable isomers of M2C98 (M = Sc, Y, La, Gd, Lu), Zheng et al. performed systematic studies on the series by density functional theory (DFT) methods, and found that the metal-carbide endohedral fullerenes are more stable.34
In order to better understand the structures of large-sized EMFs, herein, we conduct a comprehensive analysis on M2C100 (M = La, Y, Sc) with DFT methods. The geometric structure and thermodynamic stability of M2C100 species, including dimetallofullerenes of M2@C100 (based on the full screening of 285913 C100 cages) and carbide cluster fullerenes of M2C2@C98 (based on full screening of 231017 C98 cages), have been systematically investigated. Interestingly, our results show that compared with the previous results of M2C98 obtained by Zheng et al.,34 the increase in the cage by only a single unit of C2 does affect their energy priorities for corresponding dimetallofullerenes and carbide cluster fullerenes, implying the difficulty in predicting their structures and energies for EMFs with larger cages.
Computational details
It is known that each encapsulated metal atom of La, Y or Sc will donate three electrons to the fullerene cage in M2@C2n isomers;34–36 thus, the geometry optimization of M2@C2n isomers (M = La, Y, Sc) was set based on the optimization results of C1006−. Since non-IPR fullerenes can be stabilized by the inside metal ions, the total of 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 755 isomers, including all 450 IPR (isolated-pentagon-rule) isomers and 24
755 isomers, including all 450 IPR (isolated-pentagon-rule) isomers and 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 305 non-IPR isomers with less than two adjacent pentagons, were considered here. The 5 most stable isomers of C1006− were taken from the previous results reported by Yang et al.31 For M2C2@C98 (M = La, Y, Sc), the encapsulated M2C2 clusters maintain a + 4 valence state and thus, their geometry optimizations were performed based on the optimization results of C984−. A total of 17
305 non-IPR isomers with less than two adjacent pentagons, were considered here. The 5 most stable isomers of C1006− were taken from the previous results reported by Yang et al.31 For M2C2@C98 (M = La, Y, Sc), the encapsulated M2C2 clusters maintain a + 4 valence state and thus, their geometry optimizations were performed based on the optimization results of C984−. A total of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 941 carbon cages, including all 259 IPR and 17
941 carbon cages, including all 259 IPR and 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 232 non-IPR cages with less than two adjacent pentagons, were first calculated at the AM1 level (Table S1†). Then, the ten lowest-energy C984− (Table S2†) cages and five lowest-energy C1006− cages were further optimized at the level of B3LYP/6-31G. After that, optimized metalloclusters M2 (M = La, Y, Sc) or M2C2 (M = La, Y, Sc) were put into the corresponding C1006− and C984− cages in different directions. All these isomers of M2@C100 and the six most stable isomers of M2C2@C98 were at last optimized at the B3LYP/6-31G(d) ∼ Lanl2dz level and further verified by vibrational analysis on the same level. For all these species, electronic energies were calculated at 0 K with zero-point energy corrections and free energies were calculated at 298 K. All DFT calculations were carried out using the Gaussian 09 program package.37
232 non-IPR cages with less than two adjacent pentagons, were first calculated at the AM1 level (Table S1†). Then, the ten lowest-energy C984− (Table S2†) cages and five lowest-energy C1006− cages were further optimized at the level of B3LYP/6-31G. After that, optimized metalloclusters M2 (M = La, Y, Sc) or M2C2 (M = La, Y, Sc) were put into the corresponding C1006− and C984− cages in different directions. All these isomers of M2@C100 and the six most stable isomers of M2C2@C98 were at last optimized at the B3LYP/6-31G(d) ∼ Lanl2dz level and further verified by vibrational analysis on the same level. For all these species, electronic energies were calculated at 0 K with zero-point energy corrections and free energies were calculated at 298 K. All DFT calculations were carried out using the Gaussian 09 program package.37
Relative concentrations (Wi) of the ith isomer at different temperatures were calculated using the following equation:38
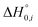 are the partition function and the relative heat of formation at absolute zero temperature of the ith isomer, respectively. Chirality contributions were also taken into account by doubling their partition functions for enantiomeric pairs, and rotational–vibrational partition functions were calculated from the optimized structural and vibrational data obtained at the level of B3LYP/6-31G(d) ∼ Lanl2dz without frequency scaling.
are the partition function and the relative heat of formation at absolute zero temperature of the ith isomer, respectively. Chirality contributions were also taken into account by doubling their partition functions for enantiomeric pairs, and rotational–vibrational partition functions were calculated from the optimized structural and vibrational data obtained at the level of B3LYP/6-31G(d) ∼ Lanl2dz without frequency scaling.
Results and discussion
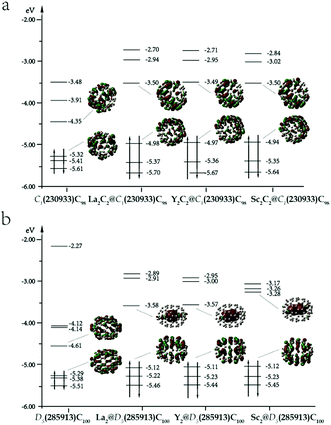
Table 1 shows the relative energies and HOMO–LUMO gaps of M2C100 isomers (M = La, Y, Sc) optimized at the level of B3LYP/6-31G(d) ∼ Lanl2dz. All the isomers were calculated based on closed-shell electron configurations. The results show that for La, the two isomers with lowest energies are La2@D5(285913)-C100 and La2@C2(285864)-C100, in which the latter has an energy of 17.9 kcal mol−1 higher than that of the former. Among the carbide cluster fullerenes, the isomer with the lowest energy is La2C2@C1(230933)-C98, which is 34.6 kcal mol−1 higher in energy than La2@D5(285913)-C100. A comparison among their relative energies is also shown in Fig. S1a,† where it can be seen that the energies of the La2@C100 isomers are generally lower, compared to those of La2C2@C98. It is also revealed in Table 1 that the isomer La2@D5(285913)-C100 has a considerably large HOMO–LUMO gap (1.54 eV), indicating its prominent chemical stability. For the IPR-violating fullerene of La2@C1(230600)-C98, its relative energy is found to be much higher, compared to those of IPR. To further confirm the results, a different pseudopotential of ECP46MHF was also applied,39 and the results were similar. The energy of La2C2@C1(230933)-C98 is 40.2 kcal mol−1 higher than that of La2@D5(285913)-C100 and their gaps are 0.1 eV and 0.02 eV lower than the results obtained with the pseudopotential of Lanl2dz for the two species, respectively.| Spiral IDc | IPR IDd | PAe | Sym.f | ΔEg (La) | Gaph (La) | ΔEg (Y) | Gaph (Y) | ΔEg (Sc) | Gaph (Sc) |
|---|---|---|---|---|---|---|---|---|---|
| a Optimization was performed on the basis of B3LYP/6-31G(d) ∼ Lanl2dz.b Optimization of the isomers of M2@C98-230925 (M = La, Y) failed.c Based on IUPAC standards to encode all of the carbon cages.d Based on IUPAC standards to encode the carbon cages abiding by IPR rules.e The number of adjacent pentagons.f Symmetry of the original empty carbon cage, which is also applied in the nomenclature of the EMFs (after the symbol of @).g Relative energy units in kcal mol−1.h Units in eV. | |||||||||
| C98-230924 | 166 | 0 | C2 | 45.0 | 1.05 | 18.4 | 1.16 | 9.6 | 1.13 |
| C98-230925 | 167 | 0 | C2v | — | — | — | — | 10.5 | 1.57 |
| C98-230926 | 168 | 0 | C1 | 45.2 | 1.25 | 17.9 | 1.25 | 8.2 | 1.26 |
| C98-230933 | 175 | 0 | C1 | 34.6 | 1.48 | 10.7 | 1.48 | 0.9 | 0.90 |
| C98-230979 | 221 | 0 | C2 | 52.0 | 1.50 | 24.0 | 1.36 | 13.9 | 1.38 |
| C98-230600 | 1 | C1 | 63.8 | 1.04 | 31.0 | 1.05 | 15.2 | 1.44 | |
| C100-285793 | 330 | 0 | C2 | 21.8 | 1.08 | 17.0 | 1.19 | 14.4 | 1.41 |
| C100-285858 | 395 | 0 | D2d | 22.8 | 1.26 | 18.6 | 1.34 | 13.9 | 1.61 |
| C100-285864 | 401 | 0 | C2 | 17.9 | 0.88 | 19.6 | 1.10 | 19.6 | 1.22 |
| C100-285868 | 405 | 0 | C1 | 26.4 | 0.84 | 20.7 | 0.89 | 14.8 | 1.32 |
| C100-285913 | 450 | 0 | D5 | 0 | 1.54 | 0 | 1.54 | 0 | 1.85 |
To evaluate the overall thermodynamic stability of these EMFs at relatively high temperatures, their temperature-relative concentration curves were calculated and are shown in Fig. 1a. All thermodynamic properties were evaluated using the harmonic approximation to calculate the partition function. Errors caused by anharmonicity are not considered here. Under temperatures lower than 2000 K, the relative concentration of La2@D5(285913)-C100 dominates the distribution. With increasing temperature, the concentration decreases and is surpassed by the isomer of La2C2@C1(230933)-C98 at 3500 K. At about 3500 K, the relative concentration of La2C2@C1(230933)-C98 ascends to 35% and prevails with the increase in temperature. As a result, dimetallofullerenes display distinct stability at low temperature, but carbide cluster fullerenes are more stable when the temperature is higher than 3500 K. The result suggests that the isomer of La2@D5(285913)-C100 may exist and accounts for an important component in the synthesis, which matches well with the experimental results reported by Beavers et al.29 In their electric arc experiments, a series of EMFs from La2C90 to La2C138 was discovered, and the most abundant product was found to be La2@D5(285913)-C100, which was isolated in pure form and characterized by X-ray diffraction. The consistent results also prove that our theoretical calculation results performed here are reliable and credible.
Based on a similar calculation strategy, further studies with Y and Sc atoms were also carried out. These results are also shown in Table 1. Y2@D5(285913)-C100 and Sc2@D5(285913)-C100 are the lowest-energy isomers of Y2@C100 and Sc2@C100, respectively. For carbide cluster fullerenes, the isomers with the lowest energies still have the IPR cages of C1(230933)-C98. However, the energy differences between pure EMFs and metal carbide EMFs for different metal atoms are different. The energy of Y2C2@C1(230933)-C98 was found to be 10.7 kcal mol−1 higher than that of Y2@D5(285913)-C100, and the value decreases to 0.90 kcal mol−1 in the case of Sc2C100. A comparison among the relative energies of all calculated isomers of M2@C100 and M2C2@C98 (M = Y, Sc) is shown in Table S4,† where their differences are clearly reflected. For Y2C100, the energies of Y2@C100 isomers and those of Y2C2@C98 are very close, and they change their energy orders sequentially. However, the energies for corresponding Sc2C2@C98 isomers are generally lower than those of Sc2@C100, except for the most stable isomer of Sc2@D5(285913)-C100. Triplet states of some isomers are also considered (Table S4†) and their energies are obviously higher than those of singlet states.
Relative concentrations of M2C100 (M = Y, Sc) isomers under different temperatures are shown in Fig. 1b and c, respectively. Y2@D5(285913)-C100, the lowest-energy structure in the Y2C100 series is prevalent under low temperature below 500 K. With temperature increasing, its relative concentration descends sharply and is surpassed by Y2C2@C1(230933)-C98 at 1100 K. At about 1700 K, the relative concentration of Y2C2@C1(230933)-C98 ascends to its maximum yield of 65%. Though its concentration decreases after 1700 K, it still occupied the main fraction. Y2C2@C1(230933)-C98 together with the other three carbide cluster fullerenes share the largest contributions when the temperature is higher than 3500 K. For the Sc2C100 system (Fig. 1c), the relative concentration of Sc2@D5(285913)-C100, which is the lowest-energy structure at 0 K, can be totally neglected under general temperature. In contrast, the relative concentration of Sc2C2@C1(230933)-C98 decreases to about 55% at 2000 K and keeping declining with increasing temperature, but is still significantly higher than other isomers. Like the Y2C100 system, carbide cluster fullerene isomers are dominant in the high temperature region. The results reflect a similar trend to the M2C98 (M = Y, Sc) series, in which M2C2@C2(230933)-C98 are the most stable isomers because of their thermodynamic and kinetic stabilities.
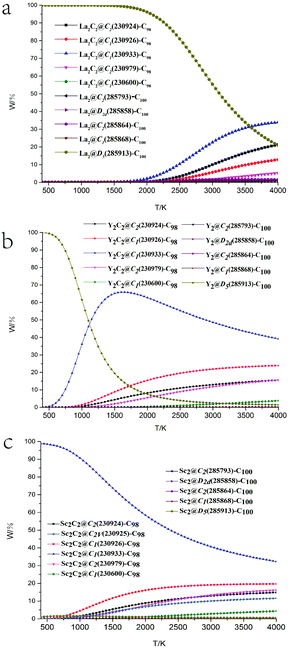
Chen et al. and Deng et al. discussed the possible geometric shapes of carbide clusters with different M–M distances,43,44 and revealed that Sc2C2 was a planar, twisted unit. The structures of the most stable isomers of M2C2@C98 and M2@C100 (M = La, Y, Sc) are shown in Fig. 2, and some of their structural parameters are listed in Table 2. For M2C2@C98 isomers, the Sc2C2 cluster tends to retain a linear structure, while La2C2 tends to form a butterfly-shaped structure. As shown in Table 2, the average La–C–C angle is about 83°, while that of Sc–C–C is about 162°, and the dM-cage values for La and Sc are 2.65 Å and 2.29 Å, respectively. They all have very similar C–C distances (∼1.26 Å) in the cages, indicating the existence of a triple bond between the two carbon atoms, which is also consistent with the NBO bonding analysis (Table S8†). These results are very similar to the previous results of M2C2@C96 (M = La, Y, Sc) reported by Zheng et al.34 On the other hand, the longest distances of the surfaces of three cages are 9.71, 9.70 and 9.69 Å, which are all very close to the corresponding value for the empty cage (9.71 Å), showing that the encapsulation of M2C2 has an insignificant effect on the shape of the cage. For M2@C100 isomers, the dM–M values are much larger than those in M2C2@C98 isomers. The metal atoms are oriented with the longest metal–metal distances to minimize the electrostatic repulsion between them. The calculated dLa-cage and dLa–La are 2.50 and 5.63 Å, respectively, which are very close to the experimentally reported values of 2.45 and 5.74 Å.35 However, for Y and Sc, their dM-cage values decrease and dM–M values increase correspondingly, suggesting that the interactions between the Sc atoms and the nearby hexagons are the strongest, compared to the cases of Y and La. The longest distances between the two carbon atoms along the axis are 10.59 Å for La, 10.44 Å for Y and 10.43 Å for Sc, respectively. These distances are all much longer than that of the empty cage (10.28 Å), indicating that the cages are elongated after the metal clusters of M2 are encapsulated.
| M–C–C angle (deg) | dM–Ma (Å) | dC–Cb (Å) | dM–Cc (Å) | dM–caged (Å) | Le (Å) | |
|---|---|---|---|---|---|---|
| a Distance between two metal atoms.b Distance between carbide atoms.c Distance between metal atoms and neighbouring carbon atoms of carbine fragment.d Distance between metal atoms and the nearest carbon atoms of the fullerene cage.e Maximum distance of two carbon atoms on the cage. Values of L for the empty cages of C98 and C100 are 9.71 Å and 10.28 Å, respectively. | ||||||
| La2C2@C1(230933)-C98 | 84.4, 82.5 | 4.58 | 1.26 | 2.54, 2.55 | 2.65 | 9.71 |
| Y2C2@C1(230933)-C98 | 108.2, 131.4 | 4.95 | 1.26 | 2.28, 2.30 | 2.47 | 9.70 |
| Sc2C2@C1(230933)-C98 | 158.8, 164.2 | 5.32 | 1.25 | 2.14, 2.15 | 2.29 | 9.69 |
| La2@D5(285913)-C100 | — | 5.63 | — | — | 2.50 | 10.59 |
| Y2@D5(285913)-C100 | — | 6.05 | — | — | 2.34 | 10.44 |
| Sc2@D5(285913)-C100 | — | 6.41 | — | — | 2.18 | 10.43 |
NBO charge distributions of M2C2 or M2 entrapped in cages C1(230933)-C98 or D5(285913)-C100 were employed and are shown in Fig. S2.† For all structures, carbon cages and encapsulated carbon atoms present negative charge states and metal atoms present positive charge states. The carbon atoms in carbide clusters are much more negatively charged than those on cages. It has also been found that the electrons are more centralized at the adjacent pentagon pole. The strong electronic interaction between the metal ion and the pentagon also helps to significantly stabilize the whole EMF.
In order to investigate the electronic structures of the thermodynamically favorable isomers, frontier molecular orbitals of M2C2@C98 and M2@C100 (M = La, Y, Sc) are presented in Fig. 3. In M2C2@C1(230933)-C98 isomers, all HOMO and LUMO orbitals have similar energy levels. The HOMO–LUMO gap of the C1(230933)-C98 empty cage is enlarged when the cage encapsulates M2C2 clusters. In other words, entrapping M2C2 clusters can obviously make the C1(230933)-C98 cage more stable, and the type of metal element is independent of electronic structures. Similar results have been found for the isomers of M2@D5(285913)-C100 (Fig. 3b). In these species, the HOMO is mainly localized on the carbon cage. However, the LUMO is completely localized on the two encaged metals. It shows a very unique situation in these isomers, in which the metal ions with 3+ oxidation state undergoing strongly repulsive Coulomb interaction might still have metallic interactions over a very long distance of 5–6 Å. The results also indicate that such structures might have a very stable and less reactive carbon cage.
 | ||
| Fig. 3 Main frontier molecular orbitals of the most stable isomers of M2@C100 (bottom) and M2C2@C98 (top) (M = La, Y, and Sc) and corresponding cages. | ||
These results can be compared with the previous results of M2C98 (M = La, Y. Sc) reported by Zheng et al.34 Table 3 shows the relative energies of the most stable isomers at 0 K. It is revealed that for M2C98 and M2C100 species, the classical dimetallofullerenes have a lower energy for La, but a higher energy for Sc. On the other hand, with the size of the carbon cage increasing, the advantage of the dimetallofullerene gradually increases. In order to make the results more reliable, the method of M06-2X/6-31G(d) ∼ Lanl2dz40–42 was also applied to the lowest-energy isomers of M2@C100 and M2C2@C98 (Tables S6–S8†). Although the energy values are different, the tendency remains the same. However, considering the temperature effect on the distribution of all possible isomers, it should be noticed that the most stable species at higher temperatures, such as 3500 K, are still governed by metal-carbide EMFs, except in the case of La2C100. The result also implies that dimetallofullerenes might be more readily synthesized experimentally for carbon cages with larger sizes (n > 100). A systematic calculation on M2C102 may help us to see if the suggestion is correct. The relevant work is ongoing in our lab.
| M2C2@C2n−2 | M2@C2n | ΔEb |
|---|---|---|
| a Energies of M2C98 species were taken directly from ref. 33.b Relative energies (in kcal mol−1) were calculated by ΔE = E(M2@C2n) − E(M2C2@C2n−2). | ||
| La2C2@C96-191809 | La2@C98-168785 | −4.8 |
| Y2C2@C96-191809 | Y2@C98-168785 | 17.1 |
| Sc2C2@C96-191809 | Sc2@C98-230924 | 19.7 |
| La2C2@C98-230933 | La2@C100-285913 | −34.6 |
| Y2C2@C98-230933 | Y2@C100-285913 | −10.7 |
| Sc2C2@C98-230933 | Sc2@C100-285913 | −0.9 |
Conclusions
Theoretical investigations have been performed on EMFs of M2C100 (M = La, Y, Sc) by DFT calculation. In all cases, isomers of M2@D5(285913)-C100 are the lowest-energy species. However, statistical thermodynamic analysis shows that the most stable isomers under high temperatures (for example, T = 3000 K) should be metal-carbide endohedral fullerenes, except for La metal. Based on these results, the main product in electric arc experiments for La2C100 should be La2@D5(285913)-C100, which has been proven by the successful synthesis and X-ray crystallographic characterization conducted by Christine M. Beavers et al. in 2011.29 For Sc2C100, the main products should be Sc2C2@C1(230933)-C98, and for Y2C100, the main products should be Y2C2@C1(230933)-C98, although some classical dimetallofullerenes might also be synthesized in the process. Further analysis on the geometric structures of M2C2@C98 and M2@C100 showed that the positions of M2 or M2C2 clusters rely very much on metal atoms. The effects of the encaged clusters on energies and shapes of the cages are also compared. Although both M2 and M2C2 clusters can make outside cages more stable, the M2C2 clusters have a lesser effect on the shapes of the cages than M2 clusters. The analyses on NBO charge distributions and frontier molecular orbitals reveal a strong electrostatic interaction between metal atoms and cages. It is also revealed that the LUMO orbital is mainly distributed on the metal atoms in the species of M2@C100. Simulated IR spectra of main M2C100 isomers are shown in Fig. S4.† These results not only provide some valuable information for the experimental characterization and synthesis of large EMF species of M2C100, but can also help us to determine structures of larger EMFs of M2C2n (2n > 100, M = La, Y, Sc).Acknowledgements
Financial support from the National Natural Science Foundation of China (No. 21475065, 21627801) is gratefully acknowledged.Notes and references
- A. A. Popov, S. F. Yang and L. Dunsch, Chem. Rev., 2013, 113, 5989–6113 CrossRef CAS PubMed.
- X. Lu, L. Feng, T. Akasaka and S. Nagase, Chem. Soc. Rev., 2012, 41, 7723–7760 RSC.
- H. Zheng, X. Zhao, W. W. Wang, T. Yang and S. Nagase, J. Chem. Phys., 2012, 137, 14308 CrossRef PubMed.
- H. Kurihara, X. Lu, Y. Iiduka, N. Mizorogi, Z. Slanina, T. Tsuchiya, T. Akasaka and S. Nagase, J. Am. Chem. Soc., 2011, 133, 2382–2385 CrossRef CAS PubMed.
- X. Lu, K. Nakajima, Y. Iiduka, H. Nikawa, N. Mizorogi, Z. Slanina, T. Tsuchiya, S. Nagase and T. Akasaka, J. Am. Chem. Soc., 2011, 133, 19553 CrossRef CAS PubMed.
- X. Lu, K. Nakajima, Y. Iiduka, H. Nikawa, T. Tsuchiya, N. Mizorogi, Z. Slanina, S. Nagase and T. Akasaka, Angew. Chem., Int. Ed., 2012, 51, 5889 CrossRef CAS PubMed.
- B. Cao, M. Hasegawa, K. Okada, T. Tomiyama, T. Okazaki, K. Suenaga and H. Shinohara, J. Am. Chem. Soc., 2001, 123, 9679–9680 CrossRef CAS PubMed.
- H. Kurihara, X. Lu, Y. Iiduka, H. Nikawa, M. Hachiya, N. Mizorogi, Z. Slanina, T. Tsuchiya, S. Nagase and T. Akasaka, Inorg. Chem., 2012, 51, 746 CrossRef CAS PubMed.
- J. Zhang, T. Fuhrer, W. Fu, J. Ge, D. W. Bearden, J. L. Dallas, J. C. Duchamp, K. L. Walker, H. Champion, H. F. Azurmendi, K. Harich and H. C. Dorn, J. Am. Chem. Soc., 2012, 134, 8487–8493 CrossRef CAS PubMed.
- C. R. Wang, T. Kai, T. Tomiyama, T. Yoshida, Y. Kobayashi, E. Nishibori, M. Takata, M. Sakata and H. Shinohara, Angew. Chem., Int. Ed., 2001, 40, 397 CrossRef CAS PubMed.
- H. Kurihara, X. Lu, Y. Iiduka, H. Nikawa, N. Mizorogi, Z. Slanina, T. Tsuchiya, S. Nagase and T. Akasaka, J. Am. Chem. Soc., 2012, 134, 3139 CrossRef CAS PubMed.
- Y. Yamazaki, K. Nakajima, T. Wakahara, T. Tsuchiya, M. O. Ishitsuka, Y. Maeda, T. Akasaka, M. Waelchli, N. Mizorogi and H. Nagase, Angew. Chem., Int. Ed. Engl., 2008, 47, 7905 CrossRef CAS PubMed.
- T. Yumura, Y. Sato, K. Suenaga and S. Iijima, J. Phys. Chem. B, 2005, 109, 20251 CrossRef CAS PubMed.
- Y. Iiduka, T. Wakahara, K. Nakajima, T. Nakahodo, T. Tsuchiya, Y. Maeda, T. Akasaka, K. Yoza, M. T. H. Liu, N. Mizorogi and S. Nagase, Angew. Chem., Int. Ed., 2007, 46, 5562 CrossRef CAS PubMed.
- Z. Q. Shi, X. Wu, C. R. Wang, X. Lu and H. Shinohara, Angew. Chem., Int. Ed., 2006, 45, 2107 CrossRef CAS PubMed.
- K. Tan and X. Lu, Chem. Commun., 2005, 4444 RSC.
- M. Krause, M. Hulman, H. Kuzmany, O. Dubay, G. Kresse, K. Vietze, G. Seifert, C. Wang and H. Shinohara, Phys. Rev. Lett., 2004, 93, 137403 CrossRef CAS PubMed.
- Y. Iiduka, T. Wakahara, K. Nakajima, T. Tsuchiya, T. Nakahodo, Y. Maeda, T. Akasaka, N. Mizorogi and S. Nagase, Chem. Commun., 2006, 2057 RSC.
- W. Cai, L. Bao, S. Zhao, Y. Xie, T. Akasaka and X. Lu, J. Am. Chem. Soc., 2015, 137, 10292 CrossRef CAS PubMed.
- W. Cai, F. Li, L. Bao, Y. Xie and X. Lu, J. Am. Chem. Soc., 2016, 138, 6670 CrossRef CAS PubMed.
- C. R. Wang, T. Kai, T. Tomiyama, T. Yoshida, Y. Kobayashi, E. Nishibori, M. Takata, M. Sakata and H. Shinohara, Nature, 2000, 408, 426 CrossRef CAS PubMed.
- H. Kurihara, X. Lu, Y. Iiduka, N. Mizorogi, Z. Slanina, T. Tsuchiya, S. Nagase and T. Akasaka, Chem. Commun., 2012, 48, 1290 RSC.
- T. Inoue, T. Tomiyama, T. Sugai, T. Okazaki, T. Suematsu, N. Fujii, H. Utsumi, K. Nojima and H. Shinohara, J. Phys. Chem. B, 2004, 108, 7573 CrossRef CAS.
- H. Kato, A. Taninaka, T. Sugai and H. Shinohara, J. Am. Chem. Soc., 2003, 125, 7782 CrossRef CAS PubMed.
- B. P. Cao, T. Wakahara, T. Tsuchiya, M. Kondo, Y. Maeda, G. M. A. Rahman, T. Akasaka, K. Kobayashi, S. Nagase and K. Yamamoto, J. Am. Chem. Soc., 2004, 126, 9164 CrossRef CAS PubMed.
- T. Akasaka, S. Nagase, K. Kobayashi, M. Walchli, K. Yamamoto, H. Funasaka, M. Kako, T. Hoshino and T. Erata, Angew. Chem., Int. Ed. Engl., 1997, 36, 1643 CrossRef CAS.
- B. Q. Mercado, A. Jiang, H. Yang, Z. Wang, H. Jin, Z. Liu, M. M. Olmstead and A. L. Balch, Angew. Chem., Int. Ed. Engl., 2009, 48, 9114 CrossRef CAS PubMed.
- H. Yang, C. Lu, Z. Liu, H. Jin, Y. Che, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2008, 130, 17296 CrossRef CAS PubMed.
- C. M. Beavers, H. Jin, H. Yang, Z. Wang, X. Wang, H. Ge, Z. Liu, B. Q. Mercado, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2011, 133, 15338 CrossRef CAS PubMed.
- S. F. Yang and L. Dunsch, Angew. Chem., Int. Ed., 2006, 45, 1299 CrossRef CAS PubMed.
- T. Yang, X. Zhao and S. Nagase, Phys. Chem. Chem. Phys., 2011, 13, 5034 RSC.
- A. A. Popov and L. Dunsch, J. Am. Chem. Soc., 2007, 129, 11835 CrossRef CAS PubMed.
- R. Valencia, A. Rodríguez-Fortea and J. M. Poblet, J. Phys. Chem. A, 2008, 112, 4550 CrossRef CAS PubMed.
- H. Zheng, X. Zhao, W. W. Wang, J. S. Dang and S. Nagase, J. Phys. Chem. C, 2013, 117, 25195 CAS.
- X. Zhao, W. Y. Gao, T. Yang, J. J. Zheng, L. S. Li, L. He, R. J. Cao and S. Nagase, Inorg. Chem., 2012, 51, 2039 CrossRef CAS PubMed.
- Y. Guo, T. Yang, S. Nagase and X. Zhao, Inorg. Chem., 2014, 53, 2012 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. J. Rendell, C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.01, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- Z. Slanina, Int. Rev. Phys. Chem., 1987, 6, 251 CrossRef CAS.
- A. Nicklass, M. Dolg, H. Stoll and H. Preuss, J. Chem. Phys., 1995, 102, 8942 CrossRef CAS.
- R. S. Zhao, Y. J. Guo, P. Zhao, M. Ehara, S. Nagase and X. Zhao, J. Phys. Chem. C, 2016, 120, 1275–1283 CAS.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 CrossRef CAS.
- A. Dreuw and M. Headgordon, Chem. Rev., 2005, 105, 4009–4037 CrossRef CAS PubMed.
- C. H. Chen, K. B. Ghiassi, M. R. Ceron, M. A. G. Ayala, L. Echegoyen, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2015, 137, 10116 CrossRef CAS PubMed.
- Q. Deng and A. A. Popov, J. Am. Chem. Soc., 2014, 136, 4257 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra00717e |
| This journal is © The Royal Society of Chemistry 2017 |