Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Amino acid-based amphiphilic hydrogels: metal ion induced tuning of mechanical and thermal stability†
Shibaji Basaka,
Ishwar Singha,
Arindam Banerjeec and
Heinz-Bernhard Kraatz *ab
*ab
aDepartment of Physical and Environmental Sciences, University of Toronto Scarborough, 1265 Military Trail, Toronto, M1C 1A4, Canada
bDepartment of Chemistry, University of Toronto, 80 St George Street, Toronto, Ontario M5S 3H6, Canada. E-mail: bernie.kraatz@utoronto.ca
cDepartment of Biological Chemistry, Indian Association for the Cultivation of Science, Jadavpur, Kolkata – 700 032, India. E-mail: bcab@iacs.res.in
First published on 6th March 2017
Abstract
A phenylalanine based gelator was found to form a hydrogel in phosphate buffer solution. Addition of metal ions to this hydrogel dramatically increases the thermal stability and mechanical strength.
Supramolecular gels1 are gaining in importance, in part, due to their fascinating properties, and potential applications ranging from bio-mimetics for tissue and bone engineering,2a–c cell growth scaffolds,2d drug delivery2e,f to electronics.2g,h Guided by non-covalent cohesive forces, including hydrogen bonding, hydrophobic and π–π interactions, and metal–ligand interactions, 3D fibrous network structures forming a matrix in gel materials are formed.3 Non-covalent forces readily respond to external stimuli, which allows a modulation of the properties of the gel material. Examples of external stimuli include biological targets, pH, anions, small molecules chemicals, and redox behaviour and others.4 Biotechnological applications of supramolecular gels largely depend on their supramolecular properties, particularly thermal, pH and mechanical stabilities.5 Particular efforts have been made to improve the mechanical properties of gels, for example through the addition of vancomycin,6a glucono-δ-lactone6b and by using enzymes.6c In this context self-healing properties, that is the ability of gels to undergo a spontaneous sol–gel phase transition after application of a mechanical force that damaged the supramolecular gel network, are of interest and have been exploited for the generation of an injectable material loaded with drugs.7 Self-healing behaviour sometimes is achieved by the addition of external chemicals which is not possible in the native gel phase. For example, Davis and co-workers reported hydrogelation of guanosine and lithium borate where thioflavin T acts as stimuli for rapid gel formation and endows the gel with self-healing properties and enhanced mechanical strength.8 Gelation induced by metal ions recently gained enormous interest due to their fascinating properties and control over self-assembly by tuning metal–ligand coordination.9 For example, Banerjee and co-workers have reported a series of tyrosine based amphiphiles that trigger hydrogel formation in presence of Ni2+ ions and mechanical property is dependent on the length of alkyl chain.9f Lanthanide-based luminescent gels reported by the research groups of Gunnlaugsson and Holten-Andersen, provide control over luminescence and mechanical strength depending on the ratio of lanthanide ions.10 There is a significant body of work where metal co-ordinating ligands form self-supportable gels only when they come in contact with metal ion,11 i.e. where metal ion coordination is critical to gelation and no gel formation takes place in the absence of metal ions. Recently, our group have reported that a lipoic acid based diphenylalanine derivative forms metallo-hydrogel in presence of transition metal ions and the mechanical property is highly tuneable. We showed that the mechanical property is different in presence of different metal ions and that they follow the Irving–Williams series of stability of metal complexes.11a This illustrates that metal coordination to a gelator ligand allows control over the property exhibited by the gel. However, there is a high possibility that an existing hydrogel could respond to metal ions and self-assembly study of an existing hydrogel in presence of metal ions is rare in literature. In this context, Stupp and co-workers have studied mechanical stiffness of an amphiphilic peptide hydrogel as a function of ionic strength, pH and various metal ions.12 Interestingly, the mechanical strength of the gel is correlated with the Irving–Williams series, with Cu2+ displaying the highest mechanical stability. Therefore, affecting and tuning the mechanical properties of hydrogels by metal addition will have potential implications for the study of hydrogels as cell and tissue culture medium.13
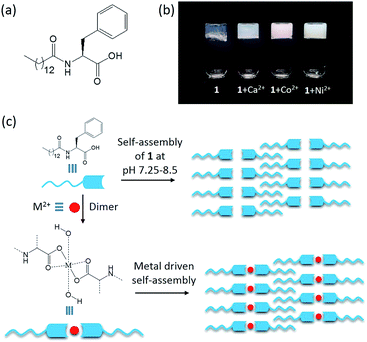
Herein, we report a phenylalanine-based amphiphile 1 (Fig. 1a), which forms a hydrogel in phosphate buffer. Remarkably, in the presence of Co2+ and Ni2+, the resulting hydrogel displays high thermal and mechanical stability, which is in line with complex stabilities as predicted by the Irving–Williams series.
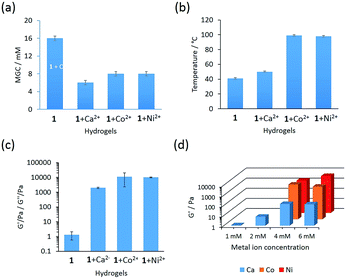
The phenylalanine based gelator 1 self-assembles to a self-supportable thermo-reversible hydrogel in phosphate buffer solution in a narrow pH range of 7.25–8.5 (Fig. 1b). At a pH below 7.25, gelator 1 has poor solubility and at pH above 8.5, it does not form a hydrogel. However, at pH 7.25–8.5, gelator 1 readily forms a solution by heating and after cooling to room temperature a self-supportable hydrogel is formed within 24 hours. Minimum gelation concentration (MGC) to form hydrogel was 16 mM. Hydrogels formed by gelator 1 display self-healing and undergo a sol–gel transition by applying and removal of mechanical force, respectively. The hydrogel undergo fully reversible sol–gel transitions even after repeated heating–cooling cycles, showing a high degree of thermo-reversibility. The gel melting temperature (Tgel) of the hydrogel is 41 °C at MGC. However, we were interested to check the interaction of metal ions such as Ca2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+ and Zn2+ with the hydrogel obtained from 1 in phosphate buffer (pH 7.25–8.5). Among these metal ions, addition of Ca2+, Co2+ and Ni2+ ions remarkably change the gelation property with respect to MGC, gelation time (hydrogel formation time) and thermal stability (Table S1†). This indicates a strong interaction between gelator 1 and metal ions (Ca2+, Co2+ and Ni2+) in aqueous medium. Metallo-hydrogels (Fig. 1b) were prepared by addition of the corresponding metal salt to a solution of gelator 1 in phosphate buffer. A precipitate was formed in each case after addition of metal salt solution to phosphate buffer solution of gelator 1 due to formation of metal hydroxides.9f Ultra-sonication for 1–2 min, readily dissolved this precipitate.14 Interestingly, the MGC for hydrogels obtained from gelator 1 in the presence of metal ions is significantly lower (Ca2+: 6 mM, Co2+: 8 mM, Ni2+: 8 mM, see Fig. 2a), suggesting that the metallo-hydrogels are more ordered. Metallo-gelation was tested at metal ion concentrations ranging from 1 to 6 mM while keeping the gelator concentration fixed at 8 mM. 1 + Ca2+ forms hydrogels for all conditions tested, while for Ni2+ and Co2+ concentrations below 4 mM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 gelator to metal ratio), did not result in hydrogelation but a viscous solution was formed instead. Looking to thermal stability, significant increases of Tgel were observed for 1 + Co2+ and 1 + Ni2+ hydrogels to double of that of the native hydrogel (Tgel: 1 + Co2+: 99 °C, 1 + Ni2+: 98 °C, see Fig. 2b). The Tgel is significantly dependent on the metal ion concentration. Tgel decreases with decrease in metal ion concentration (Table S2†). In addition, in the presence of these metal ions, the gelation time is significantly shortened (1 + Ca2+: 30 min; 1 + Co2+ and Ni2+ 4 h at MGC) compared to 24 h for the native gel.
0.5 gelator to metal ratio), did not result in hydrogelation but a viscous solution was formed instead. Looking to thermal stability, significant increases of Tgel were observed for 1 + Co2+ and 1 + Ni2+ hydrogels to double of that of the native hydrogel (Tgel: 1 + Co2+: 99 °C, 1 + Ni2+: 98 °C, see Fig. 2b). The Tgel is significantly dependent on the metal ion concentration. Tgel decreases with decrease in metal ion concentration (Table S2†). In addition, in the presence of these metal ions, the gelation time is significantly shortened (1 + Ca2+: 30 min; 1 + Co2+ and Ni2+ 4 h at MGC) compared to 24 h for the native gel.
Low MGC and high thermal stability of these metallo-hydrogels found for 1 + Co2+ and 1 + Ni2+ follow the Irving–Williams series, indicating that the effect is driven by complex formation.12,15 The self-assembly of native hydrogel (Fig. 1c) is driven by simple electrostatic interaction involving the carboxylic acid terminal in phosphate buffer. However, metal complex driven self-assembly brings extra stability to the metal containing hydrogels which reflects in high thermal stability. The properties of the metallo-hydrogels containing 1 + Mn2+, 1 + Fe2+ and 1 + Zn2+ are similar to those of the native hydrogel. However, 1 + Cu2+ does not form a hydrogel, presumably Jahn–Teller distortions disrupt or destabilize the supramolecular packing of gelator molecules, which shows the importance of metal coordination geometry on gel formation.
To investigate the mechanical property of the native hydrogel and metallo-hydrogels obtained from 1 + Ca2+, 1 + Co2+ and 1 + Ni2+ rheological studies were performed.16 Rheological studies using a frequency sweep from 0.1–100 rad s−1 were carried out to examine the mechanical strength of the native and metallo-hydrogels and monitor the storage (G′) and loss (G′′) moduli as a function of frequency (Fig. S4†). Both, G′ and G′′ were independent of frequency and G′ was always larger than G′′ (G′ > G′′). This property is inherent for visco-elastic materials indicating soft solid-like gel phase material that does not collapse over the entire frequency range tested. The elastic behaviour of these hydrogels could be expressed by tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ(G′′/G′) value, which indicates that elastic character decreases with an increase in tan
δ(G′′/G′) value, which indicates that elastic character decreases with an increase in tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ. Tan
δ. Tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ at 10 rad s−1 obtained from native hydrogel is 0.419. However, for metallo-hydrogels obtained from 1 + Ca2+, 1 + Co2+ and 1 + Ni2+ are 0.186, 0.089 and 0.077, respectively. The tan
δ at 10 rad s−1 obtained from native hydrogel is 0.419. However, for metallo-hydrogels obtained from 1 + Ca2+, 1 + Co2+ and 1 + Ni2+ are 0.186, 0.089 and 0.077, respectively. The tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ values follow an order as 1 > 1 + Ca2+ > 1 + Co2+ ∼ 1 + Ni2+ which indicates the 1 + Co2+ and 1 + Ni2+ have very high elastic character than Ca2+ and native hydrogel. The G′ value (as a function of strength measured at frequency 10 rad s−1) of the native hydrogel was very poor and found to be ∼1 Pa (Fig. 2c). However, addition of Ca2+, Co2+ and Ni2+ ions drastically changed the mechanical strength and G′ value increased to 1971 Pa, 11
δ values follow an order as 1 > 1 + Ca2+ > 1 + Co2+ ∼ 1 + Ni2+ which indicates the 1 + Co2+ and 1 + Ni2+ have very high elastic character than Ca2+ and native hydrogel. The G′ value (as a function of strength measured at frequency 10 rad s−1) of the native hydrogel was very poor and found to be ∼1 Pa (Fig. 2c). However, addition of Ca2+, Co2+ and Ni2+ ions drastically changed the mechanical strength and G′ value increased to 1971 Pa, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115 Pa and 9986 Pa respectively (all data was averaged over three consecutive runs on the same sample). The high mechanical strength obtained from 1 + Co2+ and 1 + Ni2+ in the frequency sweep rheology study again supports the stability indicated by the Irving–Williams series. The concentration dependent frequency sweep experiment was also performed for metallo-hydrogels from MGC to 16 mM. It was found that the mechanical strength of 1 + Ca2+ hydrogel decrease sharply with decrease in concentration, however, for 1 + Co2+ and 1 + Ni2+ it was small (Fig. S5†). Frequency sweep rheology studies were performed at various metal ion concentrations (1 mM to 6 mM) keeping the gelator concentration fixed at 8 mM. The strength of hydrogels remain unchanged at metal ion concentrations above 4 mM (Fig. 2d). However, at concentration below 4 mM, the strength of the hydrogel decreases with decreasing metal ion concentration.
115 Pa and 9986 Pa respectively (all data was averaged over three consecutive runs on the same sample). The high mechanical strength obtained from 1 + Co2+ and 1 + Ni2+ in the frequency sweep rheology study again supports the stability indicated by the Irving–Williams series. The concentration dependent frequency sweep experiment was also performed for metallo-hydrogels from MGC to 16 mM. It was found that the mechanical strength of 1 + Ca2+ hydrogel decrease sharply with decrease in concentration, however, for 1 + Co2+ and 1 + Ni2+ it was small (Fig. S5†). Frequency sweep rheology studies were performed at various metal ion concentrations (1 mM to 6 mM) keeping the gelator concentration fixed at 8 mM. The strength of hydrogels remain unchanged at metal ion concentrations above 4 mM (Fig. 2d). However, at concentration below 4 mM, the strength of the hydrogel decreases with decreasing metal ion concentration.
Metallo-hydrogels obtained from 1 + Ca2+, 1 + Co2+ and 1 + Ni2+ are self-healing. This property was studied by performing time dependent rheology studies applying an alternating shear force in the form of strain to demonstrate reversible healing of metallo-hydrogels (8 mM) at a constant angular frequency of 10 rad s−1 (Fig. S6†). Note that the mechanical strength is very low for the native hydrogel and it is very difficult to control such type of self-healing experiment. Metallo-hydrogels were cycled in gel–sol transition several times in a time sweep experiment by applying alternating oscillatory strain in an order 0.1% (120 s) → 100% (120 s) → 0.1% (240 s) → 100% (120 s) → 0.1% (240 s). In low strain step (0.1%) the G′ of metallo-hydrogels was higher than G′′ indicating immobilized gel phase whereas, at 100% oscillatory strain G′′ appeared higher than G′ indicating sol phase. Application of high strain (100%) immediately convert gel phase to sol phase and after removal of 100% oscillatory strain, metallo-hydrogels fully regained their original G′ value almost instantly indicating their quick healing property.
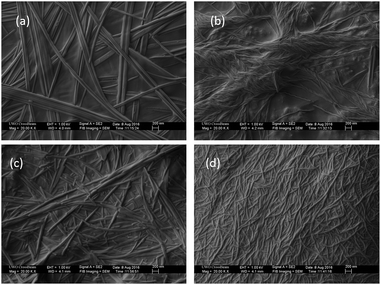
To gain insights into the morphology of the native hydrogel and metallo-hydrogels, FE-SEM (Fig. 3) and TEM (Fig. S7†) were carried out. FE-SEM image of the native hydrogel showed three dimensional network structure which is composed of several micro-meter long nano-fibers entangled to each-other (Fig. 3a). Average width of these nano-fibers is 60 nm. For 1 + Ca2+ hydrogel, fibers are long and 40 nm in width (Fig. 3b). Fibers obtained from 1 + Ca2+ hydrogel are helical, which is a different morphology compared to the native hydrogel. However, fibers obtained from 1 + Co2+ and 1 + Ni2+ are smaller in length and 65 nm and 40 nm in width respectively (Fig. 3c and d). This observations indicate that strength of hydrogels do not depend on length of nano-fibers. The high elastic nature and responsive mechanical property of 1 + Co2+ and 1 + Ni2+ metallo-hydrogels (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ value 0.089 and 0.077 respectively) makes them brittle in nature and may be responsible for shorter nano-fibers. The TEM images also indicate fibrous morphology for native hydrogel, 1 + Ca2+, 1 + Co2+ and 1 + Ni2+ metallo-hydrogels with 55 nm, 50 nm, 55 nm and 45 nm width respectively. Helicity appeared for 1 + Ca2+ fibers, presumably due to supramolecular chirality, is evident from our circular dichroism (CD) spectroscopy results (Fig. S8†). A positive signal at 220 nm appeared for the native hydrogel, whereas, a peak around 215 nm with a drastic increase in intensity appeared for the 1 + Ca2+ hydrogel which is consistent with the image obtained from FE-SEM. The 1 + Co2+ and 1 + Ni2+ hydrogels both produce a peak at 226 nm but with a negative CD signal for the 1 + Ni2+ hydrogel.
δ value 0.089 and 0.077 respectively) makes them brittle in nature and may be responsible for shorter nano-fibers. The TEM images also indicate fibrous morphology for native hydrogel, 1 + Ca2+, 1 + Co2+ and 1 + Ni2+ metallo-hydrogels with 55 nm, 50 nm, 55 nm and 45 nm width respectively. Helicity appeared for 1 + Ca2+ fibers, presumably due to supramolecular chirality, is evident from our circular dichroism (CD) spectroscopy results (Fig. S8†). A positive signal at 220 nm appeared for the native hydrogel, whereas, a peak around 215 nm with a drastic increase in intensity appeared for the 1 + Ca2+ hydrogel which is consistent with the image obtained from FE-SEM. The 1 + Co2+ and 1 + Ni2+ hydrogels both produce a peak at 226 nm but with a negative CD signal for the 1 + Ni2+ hydrogel.
To gain further structural insight into the nature of the metal–gelator interactions and supramolecular interactions within the hydrogels, FTIR studies were carried out using freeze-dried hydrogel/metallo-hydrogels (Fig. S9†). A strong signal at 1646 cm−1, corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, was observed for all hydrogels. Two other signals corresponding to N–H stretching and N–H bending frequencies appeared at 3325 cm−1 and 1537 cm−1, respectively. These characteristic peaks suggest the amide residue of the gelator forms hydrogen bonded structure for all hydrogels. A peak appeared at 1610 cm−1 for the native hydrogel and all metallo-hydrogels correspond to the carboxylate residue. This carboxylate residue is the most probable binding site for metal ions. Interestingly, an additional signal was observed for 1 + Ca2+, 1 + Co2+, and 1 + Ni2+ hydrogels. For both Ca2+ and Co2+, a signal was observed around 3530 cm−1, while for Ni2+ a peak was observed at 3417 cm−1. Importantly, for the native hydrogel this signal is absent and this suggests that the signal is associated with the presence of the metal. Give the region in the IR spectrum, we suggest that a coordinated aqua or hydroxo ligand is responsible for this signal. In order to gain more insight into the presence of this putative ligand, we carried out MALDI-TOF MS studies on the metallo-hydrogels (Fig. S10†). Importantly, 1 + Ca2+ and 1 + Co2+-metallo-hydrogels both display a m/z+ signal corresponding to [Ca(OH)212] and [Co(OH)212]+, respectively, which suggests an octahedral coordination environment around the metal centre (Fig. 1c).
O stretching vibration, was observed for all hydrogels. Two other signals corresponding to N–H stretching and N–H bending frequencies appeared at 3325 cm−1 and 1537 cm−1, respectively. These characteristic peaks suggest the amide residue of the gelator forms hydrogen bonded structure for all hydrogels. A peak appeared at 1610 cm−1 for the native hydrogel and all metallo-hydrogels correspond to the carboxylate residue. This carboxylate residue is the most probable binding site for metal ions. Interestingly, an additional signal was observed for 1 + Ca2+, 1 + Co2+, and 1 + Ni2+ hydrogels. For both Ca2+ and Co2+, a signal was observed around 3530 cm−1, while for Ni2+ a peak was observed at 3417 cm−1. Importantly, for the native hydrogel this signal is absent and this suggests that the signal is associated with the presence of the metal. Give the region in the IR spectrum, we suggest that a coordinated aqua or hydroxo ligand is responsible for this signal. In order to gain more insight into the presence of this putative ligand, we carried out MALDI-TOF MS studies on the metallo-hydrogels (Fig. S10†). Importantly, 1 + Ca2+ and 1 + Co2+-metallo-hydrogels both display a m/z+ signal corresponding to [Ca(OH)212] and [Co(OH)212]+, respectively, which suggests an octahedral coordination environment around the metal centre (Fig. 1c).
Powder X-ray Diffraction (XRD) studies were performed using freeze-dried samples of the native hydrogel and of metallo-hydrogels (Fig. S11†). For the native hydrogel composed of gelator 1, the small angle region shows two prominent signals at 2θ = 3.06° and 3.9°, corresponding to d spacing values of 28.7 Å and 22.5 Å. The later d spacing value matches well with the calculated molecular length of the gelator molecules 1. However, the former d spacing value is longer than the length of gelator molecule but less than the twice of this length. Small angle X-ray diffraction experiments by Hamley and Banerjee show that interdigitation of the hydrophobic alkyl chains in peptide and amino acid based amphiphilic conjugates occurs.17 Characteristic signals were observed at distances that are slightly smaller than twice the molecular length. This signal is often accompanied by a second peak that matches the molecular length of the conjugate. Our experimental observation for hydrogel 1 is in line with observations by Hamley and Banerjee. This suggests that the gelator molecules are presumably in a fully stretched conformation, forming an interdigitated packing structure very similar to amphiphilic molecules in hydrogel state (Fig. 1c). Furthermore, a signal at 2θ = 6.16°, corresponding to a d spacing of 14.3 Å, is almost half of the d spacing value of 28.7 Å which is also supportive of an interdigitated structure. This is contrasted by the XRD of the metallo-hydrogels, which all display sharp signals in the wide angle region, similar to those of the corresponding metal hydroxides.18 Additionally, a peak at 3.8° corresponds to d spacing value 22.9 Å appeared for 1 + Co2+ hydrogel that is also matched to molecular length of the gelator molecule, indicates after forming metal complex the gelator molecules remain in fully stretched state as indicated in Fig. 1c. This suggests that the effect of metal addition is essential to provide additional mechanical stability. Addition of metal ions causes dimerization, as is evident from our MALDI-TOF and FT-IR studies, while at the same time maintaining the interdigitation of the long alkyl chains (Fig. 1c). However, at small angle region no peaks were observed for other two metallo-hydrogels (1 + Ca2+ and 1 + Ni2+), probably due to suppression occurred for metal hydroxide.
In summary, a phenylalanine based gelator forms hydrogel in phosphate buffer solution and the effect of transition metal ions as an external stimuli has been described. Addition of transition metal ions (Ca2+, Co2+ and Ni2+) remarkably increases the thermal and mechanical stability. MALDI-TOF, FTIR and XRD studies suggest that octahedral coordination metal complexes are formed followed by hierarchical assembly into a modified supramolecular gel structure that involved interdigitation of the alkyl chains. Metal ion addition stiffens the gel by providing additional interactions involving the carboxyl group of gelator 1, which is reflected in an enhanced thermal and mechanical stability of these metallo-hydrogels. And we believe that it is this combination of interdigitation and metal coordination leading to further strong interactions between gelator molecules and metal ions that is responsible for the increase in mechanical stability of the gels.
Acknowledgements
We gratefully acknowledge funding from NSERC and from the University of Toronto Scarborough.Notes and references
- (a) X. Du, J. Zhou, J. Shi and B. Xu, Chem. Rev., 2015, 115, 13165–13307 CrossRef CAS PubMed; (b) D. J. Cornwell, O. J. Daubney and D. K. Smith, J. Am. Chem. Soc., 2015, 137, 15486–15492 CrossRef CAS PubMed; (c) A. R. Hirst, B. Escuder, J. F. Miravet and D. K. Smith, Angew. Chem., Int. Ed., 2008, 47, 8002–8018 Search PubMed; (d) V. Castelletto, R. J. Gouveia, C. J. Connon, I. W. Hamley, J. Seitsonen, J. Ruokolainen, E. Longo and G. Siligardi, Biomater. Sci., 2014, 2, 867–874 RSC; (e) I. W. Hamley, Angew. Chem., Int. Ed., 2007, 46, 8128–8147 CrossRef CAS PubMed; (f) E. R. Draper, T. O. McDonald and D. J. Adams, Chem. Commun., 2015, 51, 6595–6597 RSC; (g) J. Boekhoven, W. E. Hendriksen, G. J. M. Koper, R. Eelkema and J. H. van Esch, Science, 2015, 349, 1075–1079 CrossRef CAS PubMed; (h) S. Bhattacharjee and S. Bhattacharya, Chem. Commun., 2015, 51, 7019–7022 RSC; (i) M. Tena-Solsona, B. Escuder, J. F. Miravet, V. Casttelleto, I. W. Hamley and A. Dehsorkhi, Chem. Mater., 2015, 27, 3358–3365 CrossRef CAS.
- (a) J. D. Hartgerink, E. Beniash and S. I. Stupp, Science, 2001, 294, 1684–1688 CrossRef CAS PubMed; (b) L. C. Palmer, C. J. Newcomb, S. R. Kaltz, E. D. Spoerke and S. I. Stupp, Chem. Rev., 2008, 108, 4754–4783 CrossRef CAS PubMed; (c) J. L. Drury and D. J. Mooney, Biomaterials, 2003, 24, 4337–4351 CrossRef CAS PubMed; (d) V. Jayawarna, M. Ali, T. A. Jowitt, A. F. Miller, A. Saiani, J. E. Gough and R. V. Ulijn, Adv. Mater., 2006, 18, 611–614 CrossRef CAS; (e) G. Liang, Z. Yang, R. Zhang, L. Li, Y. Fan, Y. Kuang, Y. Gao, T. Wang, W. W. Lu and B. Xu, Langmuir, 2009, 25, 8419–8422 CrossRef CAS PubMed; (f) X. Li, J. Li, Y. Gao, Y. Kuang, J. Shi and B. Xu, J. Am. Chem. Soc., 2010, 132, 17707–17709 CrossRef CAS PubMed; (g) E. Krieg, E. Shirman, H. Weissman, E. Shimoni, S. G. Wolf, I. Pinkas and B. Rybtchinski, J. Am. Chem. Soc., 2009, 131, 14365–14373 CrossRef CAS PubMed; (h) S. S. Babu, S. Prasanthkumar and A. Ajayaghosh, Angew. Chem., Int. Ed., 2012, 51, 1766–1776 CrossRef CAS PubMed.
- (a) N. M. Sangeethaa and U. Maitra, Chem. Soc. Rev., 2005, 34, 821–836 RSC; (b) J. W. Steed, Chem. Commun., 2011, 47, 1379–1383 RSC.
- (a) R. V. Ulijn, N. Bibi, V. Jayawarna, P. D. Thornton, S. J. Todd, R. J. Mart, A. M. Smith and J. E. Gough, Mater. Today, 2007, 10, 40–48 CrossRef CAS; (b) R. J. Mart, R. D. Osborne, M. M. Stevens and R. V. Ulijn, Soft Matter, 2006, 2, 822–835 RSC; (c) S.-L. Zhou, S. Matsumoto, H.-D. Tian, H. Yamane, A. Ojida, S. Kiyonaka and I. Hamachi, Chem.–Eur. J., 2005, 11, 1130–1136 CrossRef CAS PubMed; (d) L. Meazza, J. A. Foster, K. Fucke, P. Metrangolo, G. Resnati and J. W. Steed, Nat. Chem., 2013, 5, 42–47 CrossRef CAS PubMed; (e) M. D. Segarra-Maset, V. J. Nebot, J. F. Miravet and B. Escuder, Chem. Soc. Rev., 2013, 42, 7086–7098 RSC; (f) B. Adhikari and H.-B. Kraatz, Chem. Commun., 2014, 50, 5551–5553 RSC; (g) R. Afrasiabi and H.-B. Kraatz, Chem.–Eur. J., 2015, 21, 7695–7700 CrossRef CAS PubMed; (h) W. Weng, J. B. Beck, A. M. Jamieson and S. J. Rowan, J. Am. Chem. Soc., 2006, 128, 11663–11672 CrossRef CAS PubMed.
- (a) E. F. Banwell, E. S. Abelardo, D. J. Adams, M. A. Birchall, A. Corrigan, A. M. Donald, M. Kirkland, L. C. Serpell, M. F. Butler and D. N. Woolfson, Nat. Mater., 2009, 8, 596–600 CrossRef CAS PubMed; (b) D. Chow, M. L. Nunalee, D. W. Lim, A. J. Simnick and A. Chilkoti, Mater. Sci. Eng., R, 2008, 62, 125–155 CrossRef PubMed; (c) C. Yana and D. J. Pochan, Chem. Soc. Rev., 2010, 39, 3528–3540 RSC.
- (a) Y. Zhang, Z. Yang, F. Yuan, H. Gu, P. Gao and B. Xu, J. Am. Chem. Soc., 2004, 126, 15028–15029 CrossRef CAS PubMed; (b) D. J. Adams, M. F. Butler, W. J. Frith, M. Kirkland, L. Mullen and P. Sanderson, Soft Matter, 2009, 5, 1856–1862 RSC; (c) K. Thornton, A. M. Smith, C. L. R. Merry and R. V. Ulijn, Biochem. Soc. Trans., 2009, 37, 660–664 CrossRef CAS PubMed.
- (a) A. Altunbas, S. J. Lee, S. A. Rajasekaran, J. P. Schneider and D. J. Pochan, Biomaterials, 2011, 32, 5906–5914 CrossRef CAS PubMed; (b) A. Baral, S. Roy, A. Dehsorkhi, I. W. Hamley, S. Mohapatra, S. Ghosh and A. Banerjee, Langmuir, 2014, 30, 929–936 CrossRef CAS PubMed.
- G. M. Peters, L. P. Skala and J. T. Davis, J. Am. Chem. Soc., 2016, 138, 134–139 CrossRef CAS PubMed.
- (a) M. Häring and D. D. Díaz, Chem. Commun., 2016, 52, 13068–13081 RSC; (b) P. J. Knerr, M. C. Branco, R. Nagarkar, D. J. Pochan and J. P. Schneider, J. Mater. Chem., 2012, 22, 1352–1357 RSC; (c) B. Adhikari, A. Shah and H.-B. Kraatz, J. Mater. Chem. B, 2014, 2, 4802–4810 RSC; (d) S. Saha, J. Bachl, T. Kundu, D. D. Díaz and R. Banerjee, Chem. Commun., 2014, 50, 3004–3006 RSC; (e) T. Feldner, M. Häring, S. Saha, J. Esquena, R. Banerjee and D. D. Díaz, Chem. Mater., 2016, 28, 3210–3217 CrossRef CAS; (f) S. Basak, J. Nanda and A. Banerjee, Chem. Commun., 2014, 50, 2356–2359 RSC; (g) J. Shi, Y. Gao, Y. Zhang, Y. Pan and B. Xu, Langmuir, 2011, 27, 14425–14431 CrossRef CAS PubMed.
- (a) M. Martínez-Calvo, O. Kotova, M. E. Möbius, A. P. Bell, T. McCabe, J. J. Boland and T. Gunnlaugsson, J. Am. Chem. Soc., 2015, 137, 1983–1992 CrossRef PubMed; (b) P. Chen, Q. Li, S. Grindy and N. Holten-Andersen, J. Am. Chem. Soc., 2015, 137, 11590–11593 CrossRef CAS PubMed.
- (a) S. Basak, I. Singh and H.-B. Kraatz, ChemistrySelect, 2017, 2, 451–457 CrossRef CAS; (b) M.-O. M. Piepenbrock, N. Clarke and J. W. Steed, Langmuir, 2009, 25, 8451–8456 CrossRef CAS PubMed; (c) H. Bunzen, Nonappa, E. Kalenius, S. Hietala and E. Kolehmainen, Chem.–Eur. J., 2013, 19, 12978–12981 CrossRef CAS PubMed; (d) Z. Džolić, M. Cametti, D. Milić and M. Žinić, Chem.–Eur. J., 2013, 19, 5411–5416 CrossRef PubMed; (e) W. J. Gee and S. R. Batten, Chem. Commun., 2012, 48, 4830–4832 RSC; (f) W. Edwards and D. K. Smith, Chem. Commun., 2012, 48, 2767–2769 RSC; (g) M.-O. M. Piepenbrock, N. Clarke and J. W. Steed, Soft Matter, 2010, 6, 3541–3547 RSC; (h) Q. Liu, Y. Wang, W. Li and L. Wu, Langmuir, 2007, 23, 8217–8223 CrossRef CAS PubMed; (i) S. A. Joshi and N. D. Kulkarni, Chem. Commun., 2009, 2341–2343 RSC.
- J. C. Stendahl, M. S. Rao, M. O. Guler and S. I. Stupp, Adv. Funct. Mater., 2006, 16, 499–508 CrossRef CAS.
- (a) E. F. Banwell, E. S. Abelardo, D. J. Adams, M. A. Birchall, A. Corrigan, A. M. Donald, M. Kirkland, L. C. Serpell, M. F. Butler and D. N. Woolfson, Nat. Mater., 2009, 8, 596–600 CrossRef CAS PubMed; (b) S. R. Caliari and J. A. Burdick, Nat. Methods, 2016, 13, 405–414 CrossRef CAS PubMed.
- (a) D. Bardelang, F. Camerel, J. C. Margeson, D. M. Leek, M. Schmutz, M. B. Zaman, K. Yu, D. V. Soldatov, R. Ziessel, C. I. Ratcliffe and J. A. Ripmeester, J. Am. Chem. Soc., 2008, 130, 3313–3315 CrossRef CAS PubMed; (b) K. Isozaki, Y. Haga, K. Ogata, T. Naota and H. Takaya, Dalton Trans., 2013, 42, 15953–15966 RSC.
- D. F. Shriver, P. Atkins and C. H. Langford, Inorganic Chemistry, W. H. Freeman and Co., New York, 2nd edn, 1997 Search PubMed.
- M.-O. M. Piepenbrock, G. O. Lloyd, N. Clarke and J. W. Steed, Chem. Rev., 2010, 110, 1960–2004 CrossRef CAS PubMed.
- (a) V. Castelletto, G. Cheng, C. Stain, C. J. Connon and I. W. Hamley, Langmuir, 2012, 28, 11599–11608 CrossRef CAS PubMed; (b) S. Basak, J. Nanda and A. Banerjee, J. Mater. Chem., 2012, 22, 11658–11664 RSC; (c) A. Baral, S. Basak, K. Basu, A. Dehsorkhi, I. W. Hamley and A. Banerjee, Soft Matter, 2015, 11, 4944–4951 RSC.
- (a) N. J. Amos, M. Widyawati, S. Kureti, D. Trimis, A. I. Minett, A. T. Harris and T. L. Church, J. Mater. Chem. A, 2014, 2, 4332–4339 RSC; (b) M. Wang, J. Ma, C. Chen, X. Zheng, Z. Dua and J. Xu, J. Mater. Chem., 2011, 21, 12609–12612 RSC; (c) S. Chen, J. Duan, Y. Tang and S. Z. Qiao, Chem.–Eur. J., 2013, 19, 7118–7124 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra01277b |
| This journal is © The Royal Society of Chemistry 2017 |