DOI:
10.1039/C7RA01294B
(Paper)
RSC Adv., 2017,
7, 12318-12321
Both degradation and AOX accumulation are significantly enhanced in UV/peroxymonosulfate/4-chlorophenol/Cl− system: two sides of the same coin?†
Received
31st January 2017
, Accepted 14th February 2017
First published on 21st February 2017
Abstract
The presence of external chloride can lead to a 47-fold increment in degradation rates of 4-chlorophenol than those in the absence of chloride in UV/peroxymonosulfate process. The other side of the same coin is an undesirable accumulation and increase in absorbable organic halogen (AOX) was observed in the presence of chloride, with formation of some more toxic tetrachlorinated byproducts.
Introduction
Recently sulfate radical-based Advanced Oxidation Processes (AOPs) are attracting much attention in degrading recalcitrant organic pollutants due to its strong oxidizing capacity (E = 2.5–3.1 V versus NHE) and longer half-time (30–40 ps) of sulfate radical (SO4˙−).1 Peroxymonosulfate (PMS) is known as an efficient precursor of SO4˙− production, with assistance of transition metals,2–5 heat,6,7 base,8,9 ultrasound,10 carbon catalysts,11,12 microwave13 and ultraviolet (UV).14–16 Upon the cleavage of the peroxo bond, PMS photolyzed by UV was suggested to produce SO4˙− and ˙OH in a benign and economic way.17 UV/PMS process can decompose organic pollutants either by direct photolysis or indirectly by derived SO4˙− and ˙OH. For example, Sharma et al.15 reported about 96.7% of 0.22 mmol L−1 bisphenol (BPA) was efficiently degraded while 0.66 mmol L−1 PMS was applied in a UVC/PMS process. Our recent investigation also indicates that complete decomposition of 4-chloro-2-nitrophenol (4C2NP) with UVA/PMS was feasible within 120 min.16
Although PMS-based processes are known as promising AOPs for the degradation of various organics, their reaction efficiency would be greatly constrained by the presence of chloride, a common inorganic anion in industrial wastewater.18–22 Chloride, from external (saline wastewater20) or internal (released from dechlorination of chlorinated compounds18,21) sources, may scavenge SO4˙− or ˙OH to form less reactive chlorine radical (Cl˙/Cl2˙−) or react with PMS to generate Cl2/HClO. In the past, effects of external or internal chloride were often separately investigated. A dual effect (inhibitory and then accelerating) of external chloride was observed in dye degradation with Co/PMS process.5,23 Our more recent work demonstrates that even the released chloride from trichlorophenol also can be activated to generate polychlorinated byproducts in PMS-based system.18 In fact, dechlorination and re-chlorination should simultaneously occur when degrading chlorinated compounds in the presence of chloride. Currently few studies concern the combined effects of external and internal chloride in degradation of chlorophenols in UV/PMS process where direct photodechlorination may happen.24 To assess the overall outcome of these competing processes, absorbable organic halogen (AOX) is applied as a measure of extent of chlorination.21 For a simple dechlorination process, AOX value should decline gradually. Increase in AOX value implies accumulation of more chlorinated compounds.
Thus, the aim of this work is to examine effects of external chloride on chlorophenol degradation and byproducts generation, and evaluate the overall balance between chlorination and dechlorination reflected by AOX. 4-Chlorophenol (4-CP) was selected because both its dechlorination and chlorination are comparatively easy and its degradation mechanism has been well documented.24,25 The results may provide useful reference for UV/PMS application in the presence of chloride.
Experimental section
Chemicals
4-CP (>98.0%) were purchased from TCI (Shanghai, China). Oxone® ([2KHSO5·KHSO4·K2SO4] salt, 95%) and methanol (HPLC grade) were obtained from Sigma-Aldrich. NaCl, NaNO2, NaNO3 and Na2SO3 were at least analytical grade and used without further purification. Barnstead UltraPure water (18.2 MΩ cm) was used for all experiments. Stock solutions of all chemicals were always freshly prepared. Prior to each experiment, certain aliquots were added to the reactor vessel to obtain the specific concentrations.
Procedure and analysis
The photodegradation of 4-CP was performed in an XPA-7 type photochemical reactor (Xujiang Electromechanical Plant, Nanjing, China). A 100 W medium-pressure mercury vapor lamp (λmax = 365 nm) was used as the light source in a water cooled borosilicate glass immersion well. All experiments were performed at room temperature in 50 mL continuously stirred quartz tubes. Samples were withdrawn from the solution for analysis at specific time intervals and quenched with methanol (for HPLC analysis), NaNO2 (for GC-MS analysis) immediately. All experiments were performed in duplicate, with error bars in figures representing one standard deviation.
The concentration of 4-CP was measured by an Agilent 1100 high-performance liquid chromatography (HPLC) instrument (UV-vis detector) with GL Inertsil ODS-SP column (4.6 mm × 250 mm, 5 μm) maintained at 30 °C. The mobile phase was methanol/water (65/35 (v/v)), with a flow rate of 1.0 mL min−1. The detector wavelength was set at 272 nm. Gas chromatography-mass spectrometry (GC-MS, Agilent 7890A-5975C, USA) analysis was conducted to identify the intermediate products formed during 4-CP degradation process. The detailed procedure has been described in our previous work.18 AOX determination was performed by instrumental analysis (multi X 2500, Jena, Germany) after enrichment on activated carbon (European Standard EN 1485 H14, 1996). Samples were pretreated by APU2 (Automatic Preparation Unit), an automatic adsorption system for sample preparation. The detection range of AOX analysis was from 1 μg to 100 μg (expressed as the absolute content of chloride).
Results and discussion
Degradation kinetics
In agreement with literature,24 photolyzed 4-CP is unstable and about 80 μmol L−1 of 4-CP was removed under UVA irradiation (data not shown). However, as shown in Fig. 1, its degradation was largely enhanced in UV/PMS system. Nearly 90% of 4-CP disappeared within 120 min under the given condition. Fig. 1 illustrates 4-CP degradation with chloride concentration increasing from 1 to 300 mmol L−1. Similar to our recent reports in other PMS-based systems,5,23 a dual effect of external chloride was observed. At low level of chloride (<5 mmol L−1), degradation rates of 4-CP declined very slightly. A further increase of chloride content to 10 mmol L−1 resulted in a slight increase in degradation rates. As chloride concentration continued to increase from 30 to 300 mmol L−1, degradation efficiency of 4-CP was enhanced significantly. By fitting curves with pseudo-first-order kinetics, reaction rate constants (k, min−1) were obtained and plotted as a function of chloride level. There was an exponential increase in k with an increase in external chloride dosage, with a 47-fold increment in pseudo-first-order rate constant at [Cl−] = 300 mmol L−1 as compared to the UV/PMS system.
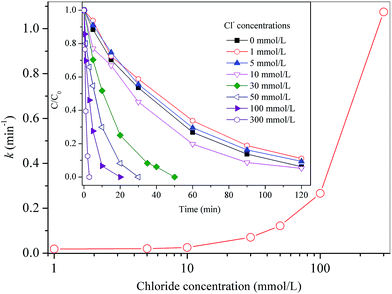 |
| | Fig. 1 Pseudo-first-order rate constants (k, min−1) versus external chloride concentration in the UV/PMS system. Inset: degradation kinetics of 4-CP in the UV/PMS system at different level of chloride. Conditions: [4-CP]0 = 0.2 mmol L−1; [PMS]0 = 10 mmol L−1; pH = 3.0. | |
AOX accumulation
Apparently, UV/PMS/Cl− process is supposed to be a favourable technology in efficiently degrading 4-CP as compared to original UV/PMS system. However, complete degradation of target pollutant does not necessarily mean all organochlorines have been removed. To quantify the total chlorinated byproducts, AOX values were measured with reaction time (Fig. 2). The initial AOX value at t = 0 min represented the contribution of 4-CP alone. In the absence of chloride, AOX content gradually decreased due to the (photo)dechlorination. However, as 100 mmol L−1 of chloride was added, AOX values did not drop, but unexpectedly increased. In the investigated time scale (180 min), AOX values in UV/PMS/Cl− solution increased 2.5 times from the initial AOX level originated from 4-CP itself. Since the measured AOX reflected the level of total chlorinated compounds including residual 4-CP and newly generated organochlorines, the AOX increase implies that external chloride has been transformed to organochlorines and re-chlorination was an overwhelming process against dechlorination in UV/PMS/Cl− system.
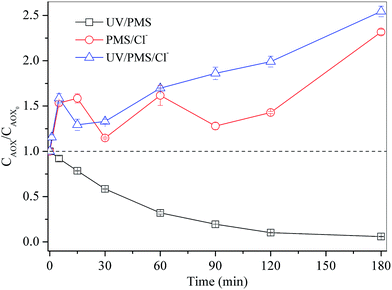 |
| | Fig. 2 AOX levels as a function of reaction time in UV/PMS, PMS/Cl−, and UV/PMS/Cl− processes. Conditions: [4-CP]0 = 0.2 mmol L−1; [PMS]0 = 10 mmol L−1; [Cl−] = 100 mmol L−1; pH = 3.0. | |
Chlorinated byproducts identification
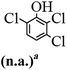
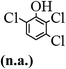
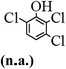
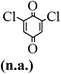
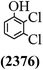
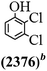
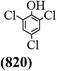
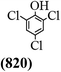
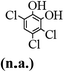
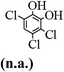
The main chlorinated intermediates during 4-CP degradation were identified by GC-MS (Table 1, see GC-MS spectra in Fig. S1–S7†). Only 2,3,6-trichlorophenol was identified in UV/PMS system at the early stage of reaction (15 min). In contrast, in PMS/Cl− system, 5 chlorinated compounds were detected, including 2,3,6-trichlorophenol, 2,6-dichloro-[1,4]benzoquinone, 2,3-dichlorophenol, 2,4,6-trichlorophenol, 3,4,6-trichloro-benzene-1,2-diol. Another two tetrachlorophenols, 2,3,4,6-tetrachlorophenol and 2,3,5,6-tetrachloro-benzene-1,4-diol, were identified in UV/PMS/Cl− system. It should be emphasized that chlorine atom numbers in all identified chlorinated compounds were higher than the original 4-CP. Again, it indicates de novo formation of organochlorines dominated even 4-CP has been completely removed within 20 min (Fig. 1). Although acute toxicities of generated 2,3-dichlorophenol and 2,4,6-trichlorophenol were lower than 4-CP, two tetrachlorinated compounds show much greater toxicity in UV/PMS/Cl− system. Quantification of all chlorinated compounds is not reported in this communication, but will be conducted while all chemicals are purchased or synthesized in the laboratory.
Table 1 Main identified chlorinated byproducts with GC-MS and their reported acute toxicity (in parenthesis) in different systems
| System |
UV/PMS |
PMS/Cl− |
UV/PMS/Cl− |
| n.a. = not available. Rat LD50 oral (mg kg−1), data from MSDS for each chemical. LD50 oral for 4-CP is 670 mg kg−1. |
| Identified product structure |
 |
 |
 |
| |
 |
 |
| |
 |
 |
| |
 |
 |
| |
 |
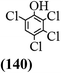 |
| |
|
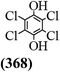 |
Mechanism discussion
There are two major reaction pathways in UV/PMS/Cl− system.5,23 The scission of the peroxo bond in PMS structure under UV irradiation leads to generation of sulfate and hydroxyl radicals (eqn (1)). Both chloride and 4-CP can be oxidized by these strongly oxidizing radicals, leading to formation of chlorine radicals (eqn (2)–(9)) and photodechlorinaton of 4-CP. Rates of 4-CP degradation may be decelerated in the presence of chloride (<5 mmol L−1), because the resulting chlorine radicals are less oxidizing (E0(Cl˙/Cl−) = 2.41 V vs. NHE; E0(Cl2˙−/2Cl−) = 2.0 V vs. NHE).26,27 Meanwhile, chloride can be directly oxidized by PMS to produce reactive chlorine species (RCS, Cl2/HClO, eqn (10) and (11)).28 RCS is able to further decompose 4-CP (eqn (12)) and therefore, 4-CP degradation was significantly enhanced while excess of external chloride was added (Fig. 1).
Dechlorination and re-chlorination may occur simultaneously in UV/PMS/4-CP/Cl− solution. It is known that C–Cl bond cleavage is the first step of 4-CP photoreaction.24 Hydroquinone and other hydroxylated compounds after dechlorination are the main products, however, which are expected to be readily re-chlorinated. The released chlorine atom or chloride ion, as an internal chloride, can be added to benzene ring after reaction with PMS or sulfate radical. This can explain the occurrence of 2,3,6-trichlorophenol in UV/PMS system. Chlorine radicals and RCS are excellent chlorination agents and lead to formation of polychlorinated compounds, such as 2,3,4,6-tetrachloro-phenol and 2,3,5,6-tetrachloro-benzene-1,4-diol. Transformation of external chloride to organochlorine accounts for the increased AOX values observed in PMS/Cl− and UV/PMS/Cl− systems in Fig. 2.
| | |
HSO5− + hν → SO4˙− + ˙OH
| (1) |
| | |
ClOH˙− + H+ → Cl˙ + H2O
| (3) |
| | |
SO4˙− + Cl− ↔ Cl˙ + SO42−
| (4) |
| | |
Cl2˙− + Cl2˙− → Cl2 + 2Cl−
| (7) |
| | |
Cl2˙− + H2O ↔ ClOH˙− + H+ + Cl−
| (8) |
| | |
ClOH˙− + H+ ↔ Cl˙ + H2O
| (9) |
| | |
HSO5− + Cl− → SO42− + HOCl
| (10) |
| | |
HSO5− + 2Cl− + H+ → SO42− + H2O + Cl2
| (11) |
| | |
R–H + HOCl → R–Cl + H2O
| (12) |
Conclusions
Effects of external chloride ion on degradation of 4-CP by UV/PMS process were investigated in this study. A dual effect of chloride (i.e. inhibitory and accelerating effect) on chlorophenol degradation kinetics was observed. Chloride ions with high concentrations (>30 mmol L−1) can greatly increase the degradation efficacy of 4-CP. AOX values declined gradually with the degradation of 4-CP in UV/PMS system, whereas AOX accumulation with the concentration of added chloride ion was observed in the presence of external chloride. Based on GC/MS data, several polychlorinated products, namely 2,3,6-trichlorophenol, 2,6-dichloro-[1,4]benzoquinone, 2,3-dichlorophenol, 2,4,6-trichlorophenol, 3,4,6-trichloro-benzene-1,2-diol, 2,3,4,6-tetrachloro-phenol and 2,3,5,6-tetrachloro-benzene-1,4-diol. Therefore, UV/PMS AOPs is not recommended in highly saline wastewater treatment, as even this kind of AOPs is able to rapidly degrade target organic pollutants. A novel nanocarbon/PMS process seems promising because it can achieve a fast organic mineralization in the presence of chloride ions due to a moderate oxidative potential of the nonradical pathway (compared with hydroxyl and sulfate radicals).29
Acknowledgements
This work was supported by the National Science Foundation of China (NSFC) (No. 21377023 and 21677031), National Key Research and Development Program of China (2016YFC0400501/2016YFC0400509), Shanghai Pujiang Program and DHU Distinguished Young Professor Program. The authors also appreciate the financial support from the Fundamental Research funds for Central Universities Central (15D311312).
Notes and references
- F. Ghanbari and M. Moradi, Chem. Eng. J., 2017, 310, 41 CrossRef CAS.
- G. P. Anipsitakis and D. D. Dionysiou, Appl. Catal., B, 2004, 54, 155 CrossRef CAS.
- P. Nfodzo and H. Choi, Chem. Eng. J., 2011, 174, 629 CrossRef CAS.
- Y. Huang, Z. Wang, C. Fang, W. Liu, X. Lou and J. Liu, RSC Adv., 2016, 6, 70271 RSC.
- R. Yuan, S. N. Ramjaun, Z. Wang and J. Liu, J. Hazard. Mater., 2011, 196, 173 CrossRef CAS PubMed.
- M. G. Antoniou, A. A. de la Cruz and D. D. Dionysiou, Appl. Catal., B, 2010, 96, 290 CrossRef CAS.
- S. Yang, P. Wang, X. Yang, L. Shan, W. Zhang, X. Shao and R. Niu, J. Hazard. Mater., 2010, 179, 552 CrossRef CAS PubMed.
- C. Qi, X. Liu, J. Ma, C. Lin, X. Li and H. Zhang, Chemosphere, 2016, 151, 280 CrossRef CAS PubMed.
- X. Lou, C. Fang, Z. Geng, Y. Jin, D. Xiao, Z. Wang, J. Liu and Y. Guo, Chemosphere, 2017, 173, 529 CrossRef CAS PubMed.
- J. Liu, J. Zhou, Z. Ding, Z. Zhao, X. Xu and Z. Fang, Ultrason. Sonochem., 2017, 34, 953 CrossRef CAS PubMed.
- X. Duan, H. Sun, Z. Ao, L. Zhou, G. Wang and S. Wang, Carbon, 2016, 107, 371 CrossRef CAS.
- S. Yang, T. Xiao, J. Zhang, Y. Chen and L. Li, Sep. Purif. Technol., 2015, 143, 19 CrossRef CAS.
- C. Qi, X. Liu, C. Lin, H. Zhang, X. Li and J. Ma, Chem. Eng. J., 2017, 315, 201 CrossRef CAS.
- T. Olmez-Hanci, C. Imren, I. Kabdaslı, O. Tünay and I. Arslan-Alaton, Photochem. Photobiol. Sci., 2011, 10, 408 CAS.
- J. Sharma, I. M. Mishra, D. D. Dionysiou and V. Kumar, Chem. Eng. J., 2015, 276, 193 CrossRef CAS.
- J. Zhou, J. H. Xiao, C. L. Fang, D. X. Xiao, Y. G. Guo, X. Y. Lou, Z. H. Wang and J. S. Liu, China Environ. Sci., 2016, 36, 66 Search PubMed.
- Y. Guan, J. Ma, X. C. Li, J. Fang and L. Chen, Environ. Sci. Technol., 2011, 45, 9308 CrossRef CAS PubMed.
- L. Xu, R. X. Yuan, Y. G. Guo, D. X. Xiao, Y. Cao, Z. H. Wang and J. S. Liu, Chem. Eng. J., 2013, 217, 169 CrossRef CAS.
- J. Zhou, J. H. Xiao, D. X. Xiao, Y. G. Guo, C. L. Fang, X. Y. Lou, Z. H. Wang and J. S. Liu, Chemosphere, 2015, 134, 446 CrossRef CAS PubMed.
- X. Y. Lou, Y. G. Guo, D. X. Xiao, Z. H. Wang and J. S. Liu, Environ. Sci. Pollut. Res., 2013, 20, 6317 CrossRef CAS PubMed.
- C. L. Fang, D. X. Xiao, W. Q. Liu, X. Y. Lou, J. Zhou, Z. H. Wang and J. S. Liu, Chemosphere, 2016, 144, 2415 CrossRef CAS PubMed.
- Y. Yang, J. J. Pignatello, J. Ma and W. A. Mitch, Environ. Sci. Technol., 2014, 48, 2344 CrossRef CAS PubMed.
- Z. Wang, R. Yuan, Y. Guo, L. Xu and J. Liu, J. Hazard. Mater., 2011, 190, 1083 CrossRef CAS PubMed.
- M. Czaplicka, J. Hazard. Mater., 2006, 134, 45 CrossRef CAS PubMed.
- F. J. Benitez, J. Beltran-Heredia, J. L. Acero and F. J. Rubio, Chemosphere, 2000, 41, 1271 CrossRef CAS PubMed.
- R. E. Huie, C. L. Clifton and P. Neta, Int. J. Radiat. Appl. Instrum., Part C, 1991, 38, 477 CAS.
- S. Steenken, in Free Radicals: Chemistry, Pathology and Medicine, ed. C. Rice-Evans and T. Dormandy, Richelieu Press, London, 1988, p. 51 Search PubMed.
- G. Lente, J. Kalmár, Z. Baranyai, A. Kun, I. Kék, D. Bajusz, M. Takács, L. Veres and I. Fábián, Inorg. Chem., 2009, 48, 1763–1773 CrossRef CAS PubMed.
- X. Duan, Z. Ao, L. Zhou, H. Sun, G. Wang and S. Wang, Appl. Catal., B, 2016, 188, 98 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: GC-MS spectra (Fig. S1–S7). See DOI: 10.1039/c7ra01294b |
|
| This journal is © The Royal Society of Chemistry 2017 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *ab,
Min Fenga,
Changling Fanga,
Ying Huanga,
Luoyan Aia,
Fei Yanga,
Ying Xuea,
Wenqian Liua and
Jianshe Liua
*ab,
Min Fenga,
Changling Fanga,
Ying Huanga,
Luoyan Aia,
Fei Yanga,
Ying Xuea,
Wenqian Liua and
Jianshe Liua














