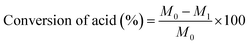Open Access Article
Open Access ArticleSynthesis of SO42−/TiO2–ZnAl2O4 composite solid acids as the esterification catalysts
An-Qi Wang,
Jun-Xia Wang *,
Hui Wang,
Ya-Nan Huang,
Ming-Liang Xu and
Xiu-Ling Wu*
*,
Hui Wang,
Ya-Nan Huang,
Ming-Liang Xu and
Xiu-Ling Wu*
Faculty of Materials Science and Chemistry, Engineering Research Center of Nano-Geomaterials of Ministry of Education, China University of Geosciences, Wuhan 430074, China. E-mail: wjx76@sina.com; xlwu@cug.edu.cn
First published on 2nd March 2017
Abstract
A series of novel sulfated ZnAl2O4–TiO2 (SO42−/ZnAl2O4–TiO2) composite solid acids were synthesized for the esterification of biomass derived acids. The properties were characterized by XRD, FT-IR, XPS, NH3-TPD, acid–base titration, TG and FE-SEM. The experimental results showed that the introduction of ZnAl2O4 spinel oxide played a key role in stabilizing the structure of the composite catalysts as well as the surface sulfate groups. The composition of SO42−/TiO2–ZnAl2O4 catalysts significantly influenced their catalytic properties and performance in esterification reactions. The optimal SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) catalyst with the largest number of acid sites showed superior conversion of 95.8% in oleic acid esterification and 98.5% conversion in acetic acid esterification. Moreover, the SO42−/ZnAl2O4–TiO2 (6
4) catalyst with the largest number of acid sites showed superior conversion of 95.8% in oleic acid esterification and 98.5% conversion in acetic acid esterification. Moreover, the SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) catalyst showed excellent reusability in these reactions, which was ascribed to its stable structure and good resistance of the surface sulfate groups. The efficient and reusable SO42−/ZnAl2O4–TiO2 composite solid acid coupled with spinel modification is expected to bring new opportunities in the design of favorable acid catalysts.
4) catalyst showed excellent reusability in these reactions, which was ascribed to its stable structure and good resistance of the surface sulfate groups. The efficient and reusable SO42−/ZnAl2O4–TiO2 composite solid acid coupled with spinel modification is expected to bring new opportunities in the design of favorable acid catalysts.
1. Introduction
Acid catalysts have wide applications in industrial reactions, such as esterification, alkylation, hydrogenation, etc.1 Among them, the esterification of carboxylic acids with alcohols has attracted growing importance due to the continual demand for renewable fuels, pharmaceutical intermediates, solvents, coatings, etc.1,2 In particular, esterification has gained much popularity in clean catalytic technologies for the conversion of biomass-derived chemicals into biofuels production.2 It was generally catalyzed by mineral acids because of their high activities and low prices. However, mineral acids accelerate the corrosion of equipment, and the residual acids are difficult to remove from the product stream. As a green alternative of the mineral acid, solid acid catalysts have important advantages of being non-corrosive, easy separation procedures, good reusability, etc.3Currently, a number of solid acids have been investigated in esterification, such as cation-exchange resins, clays, sulfated metal oxide (SO42−/MxOy), activated carbons, zeolites, MOFs.4–6 The SO42−/TiO2 catalyst, a representative SO42−/MxOy solid acid, is used extensively in esterification because of its outstanding performance of high catalytic activity, good selectivity, easy separation and so on.7 However, pure SO42−/TiO2 catalysts usually suffer from rapid deactivation and low life despite their high initial activities, which is mainly due to the loss of sulfur species and carbon deposition.8,9 Therefore, development of acid catalysts for liquid reaction is still a challenge from the view of not only high activity but also stability and life. To date, several MxOy oxides, including ZrO2, Al2O3, Fe3O4, etc. have been studied widely in the synthesis of SO42−/TiO2–MxOy composite solid acids because of the apparent improvement in catalytic activities and reusability.8–11 In our previous study, the ZnAl2O4 spinel-type oxide was used successfully to obtain the sulfated ZnAl2O4 solid acid which has the advantage of easy preparation, single crystal shape, stable structure and good performance in acetic esterification.12–14 It is therefore expected that using the ZnAl2O4 spinel-type oxide as MxOy to synthesize the SO42−/TiO2–MxOy composite may result in interesting properties and catalytic activities. In addition, it is generally accepted that metal oxides (MxOy) have their own superior catalytic crystallographic form.15,16 For example, titania was reported to have three crystallographic forms, including anatase, rutile and brookite. Among them, the anatase form of titania was regard as the most active form.15 Similarly, it was reported that spinel was the favorable form to perform catalytic activities in reactions for sulfated ZnAl2O4 oxides.12 Hence, it is necessary to maintain the superior crystallographic forms of TiO2 and ZnAl2O4 in the composite catalysts in this study.
To develop a catalyst with superior acid properties and stability, SO42−/TiO2–ZnAl2O4 solid acids were synthesized by compositing TiO2 and ZnAl2O4 oxides. The main aim was to maintain both the catalytic crystallographic forms of respective TiO2 and ZnAl2O4 components, which was a unique advantage compared to other SO42−/TiO2–MxOy composite solid acids. Moreover, this study modulated the acidic properties of SO42−/TiO2–ZnAl2O4 catalysts by adjusting the mass ratio of TiO2/ZnAl2O4 components. The obtained acid catalysts with different compositions were evaluated in the liquid-phase esterification of acetic acid with acetic acid with n-butanol and oleic acid with methanol. The esterification of oleic acid with methanol has attracted great interest in biodiesel production, as the oleic acid is a common type of free fatty acids (FFAs) existing in plant oils.17,18 The esterification of acetic acid, as a representative acid in initial bio-oils, can efficiently improve the stability and heating value of bio-oils.19,20 The product from the esterification of acetic acid with n-butanol is a common green chemical. In addition, it also discussed the roles of respective components present in the SO42−/ZnAl2O4–TiO2 composite catalyst. In the meantime, we assumed the relationships between catalyst composition, catalytic performances structure and acidic properties. To date, there are few studies on SO42−/ZnAl2O4–TiO2 solid acid catalysts, which are regarded as a referential study for the modification of SO42−/MxOy solid acid catalyst.
2. Experimental
2.1 Catalysts preparation
The ZnAl2O4–TiO2 was synthesized by a two-step sol–gel method. ZnAl2O4 powders were prepared according to the procedure of the sol–gel method mentioned in a former study.12 The Al(NO3)3·9H2O and Zn(NO3)2·6H2O with a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were dissolved in ethanol with magnetic stirring. Moreover, 5 wt% (based on total mass of metal nitrate) of polyethylene glycol was added to the mixture solution with magnetic stirring for 4 h at room temperature. The solution was evaporated with further stirring for 2 h in a water bath at 60 °C to obtain the sol. The sol was dried and ground. The obtained powder was calcined at 600 °C for 5 h in air to obtain the spinel ZnAl2O4. The Ti-contained sol–gel hydrolysis was formed by adding Ti(OC4H9)4 (titanium butoxide) dropwise into a mixture solution, which contained water, ethanol and acetic acid. The volume ratio of the titanium butoxide, water, ethanol and acetic acid in the mixed solution was 1
1 were dissolved in ethanol with magnetic stirring. Moreover, 5 wt% (based on total mass of metal nitrate) of polyethylene glycol was added to the mixture solution with magnetic stirring for 4 h at room temperature. The solution was evaporated with further stirring for 2 h in a water bath at 60 °C to obtain the sol. The sol was dried and ground. The obtained powder was calcined at 600 °C for 5 h in air to obtain the spinel ZnAl2O4. The Ti-contained sol–gel hydrolysis was formed by adding Ti(OC4H9)4 (titanium butoxide) dropwise into a mixture solution, which contained water, ethanol and acetic acid. The volume ratio of the titanium butoxide, water, ethanol and acetic acid in the mixed solution was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.76
0.76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Thereafter, a calculated amount of ZnAl2O4 powder was added to the mixed solution to stir for 4 h and then evaporated with stirring for 1 h in a water bath at 65 °C. The obtained sol was dried overnight at 100 °C and ground into fine powders. The obtained powders were sulfated by impregnating with a 0.75 mol L−1 (NH4)2SO4 solution for 4 h. Subsequently, it was dried at 80 °C for 4 h and ground. The resulting powders were calcined at 500 °C for 3 h in air to obtain the SO42−/ZnAl2O4–TiO2 catalysts. The SO42−/ZnAl2O4–TiO2 catalysts with different mass ratios of ZnAl2O4 to TiO2, including 1
1. Thereafter, a calculated amount of ZnAl2O4 powder was added to the mixed solution to stir for 4 h and then evaporated with stirring for 1 h in a water bath at 65 °C. The obtained sol was dried overnight at 100 °C and ground into fine powders. The obtained powders were sulfated by impregnating with a 0.75 mol L−1 (NH4)2SO4 solution for 4 h. Subsequently, it was dried at 80 °C for 4 h and ground. The resulting powders were calcined at 500 °C for 3 h in air to obtain the SO42−/ZnAl2O4–TiO2 catalysts. The SO42−/ZnAl2O4–TiO2 catalysts with different mass ratios of ZnAl2O4 to TiO2, including 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 8
0, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 6
2, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 4
4, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 2
6, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and 0
8 and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, were denoted as SO42−/ZnAl2O4, SO42−/ZnAl2O4–TiO2 (8
1, were denoted as SO42−/ZnAl2O4, SO42−/ZnAl2O4–TiO2 (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), SO42−/ZnAl2O4–TiO2 (6
2), SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), SO42−/ZnAl2O4–TiO2 (4
4), SO42−/ZnAl2O4–TiO2 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), SO42−/ZnAl2O4–TiO2 (2
6), SO42−/ZnAl2O4–TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) and SO42−/TiO2, respectively. The reused mixed oxide catalysts after three repeated cycles were denoted as SO42−/ZnAl2O4-used, SO42−/ZnAl2O4–TiO2 (6
8) and SO42−/TiO2, respectively. The reused mixed oxide catalysts after three repeated cycles were denoted as SO42−/ZnAl2O4-used, SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4)-used, and SO42−/TiO2-used.
4)-used, and SO42−/TiO2-used.
2.2 Catalysts characterization
Wide angle X-ray diffraction (XRD) was conducted on a Bruker AXS D8-Focus X Diffractometer using CuKα radiation at 40 kV and 40 mA. The data were collected by varying the 2θ from 10° to 70°; the Fourier transform infrared spectroscopy (FT-IR) spectra of the catalysts were recorded in the range of 400–4000 cm−1 by a Nicolet 6700 IR spectrometer, and the catalyst was pretreated by drying for 4 h at 60 °C and subjected to KBr pellet technique. The morphology of the catalysts was examined by Field Emission Scanning Electron Microscopy (FE-SEM) on a SU 8010 electron microscope. The dried samples were coated with a thin film of gold. X-ray Photoelectron Spectroscopy (XPS) was performed on a VG Multilab 2000. A NH3 Temperature Programmed Desorption (NH3-TPD) experiment was carried out using a Micromeritics AutoChem II 2920 quipped with a TCD detector and the sample was heated from room temperature to 400 °C at a rate of 10 °C min−1 and further treated in a helium flow (50 mL min−1). The samples were then exposed to NH3 (50 mL min−1) at room temperature for 1 h, followed by helium to remove the gas-phase and physically adsorbed NH3. Measurements were carried out in helium at a heating rate of 10 °C min−1. Thermogravimetric analysis (TG) was performed on a STA449F3 thermo analyzer from room temperature to 800 °C with a ramp rate of 10 °C min under 30 mL min−1 flow of a nitrogen oxygen mixture. The acid site density of the catalysts was determined using ion-exchange titration. Moreover, 200 mg of the catalyst was added to 50 mL of a 0.1 mol L−1 NaCl solution and stirred for 24 h at room temperature. The catalyst was separated by filtration. The filtrate with two drops of a phenolphthalein solution was titrated against 0.01 mol L−1 NaOH solution to neutrality. The total acid densities of the catalysts were estimated by calculating the amount of NaOH consumed.21,222.3 Catalytic activity test
The esterification of acetic acid with n-butanol was carried out in a three-necked flask equipped with a magnetic stirrer, a thermometer and a reflux condenser tube. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio of acetic acid to n-butanol and 7.26 wt% catalyst, based on the weight of acetic acid, were added to a three-necked flask. The reaction was carried out under the atmospheric pressure, and the temperature was controlled in the range of 112–115 °C for 2 h. The formed water was removed continuously by becoming an azeotrope with n-butanol to drive the equilibria to completion.
3 molar ratio of acetic acid to n-butanol and 7.26 wt% catalyst, based on the weight of acetic acid, were added to a three-necked flask. The reaction was carried out under the atmospheric pressure, and the temperature was controlled in the range of 112–115 °C for 2 h. The formed water was removed continuously by becoming an azeotrope with n-butanol to drive the equilibria to completion.
The esterification of oleic acid with methanol was carried out in 50 mL autoclave immersed in an oil bath. The reactors were initially loaded with the reaction mixture consisting of a 5 wt% (based on the oleic acid) of catalysts and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 molar ratio of oleic acid and methanol. The autoclave was sealed and immersed in the oil bath at 100 °C for 2 h.
10 molar ratio of oleic acid and methanol. The autoclave was sealed and immersed in the oil bath at 100 °C for 2 h.
The conversion of acids can be calculated by titration according to the report of Pires et al.23 and the method of GB1668-81 using the following equation:
The used SO42−/ZnAl2O4, SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and SO42−/TiO2 catalysts were recovered by filtering and drying, after completing each reaction.
4) and SO42−/TiO2 catalysts were recovered by filtering and drying, after completing each reaction.
3. Results and discussion
3.1 Characterization of the catalysts
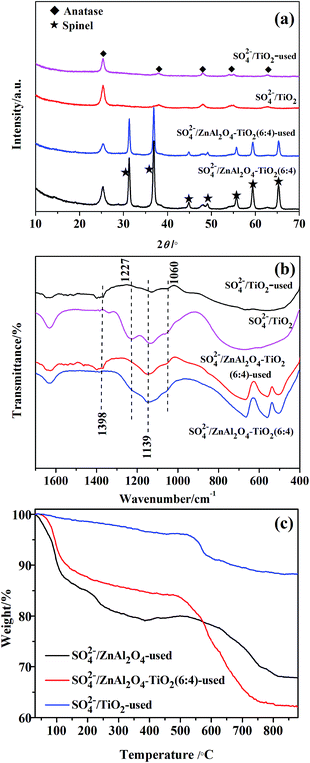
Fig. 1 shows the XRD patterns of SO42−/ZnAl2O4, SO42−/TiO2 and SO42−/ZnAl2O4–TiO2 catalysts. The characteristic diffraction peaks of ZnAl2O4 spinel (JCPDS no. 05-0669) at 2θ values of 31.3°, 36.9°, 44.9°, 49.1°, 55.7°, 59.4° and 65.3° were present in the SO42−/ZnAl2O4 and SO42−/ZnAl2O4–TiO2 catalysts. The other peaks, located at 25.3°, 37.8°, 48.1°, 54.6° and 62.8°, were all assigned to the anatase phase (JCPDS no. 65-5714), which could be observed in SO42−/TiO2 and SO42−/ZnAl2O4–TiO2. The diffraction intensity for the respective phases was changed by varying the mass ratio of the two components. Moreover, there was no detectable impurity. This indicated that SO42−/ZnAl2O4–TiO2 catalysts only contained the simple spinel phase and anatase phase which were the catalytic crystallographic forms of the two component oxides.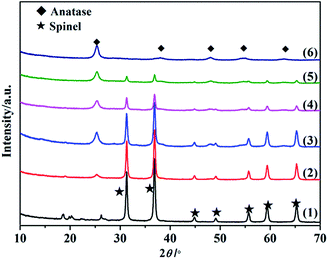 | ||
Fig. 1 XRD patterns of (1) SO42−/ZnAl2O4, (2) SO42−/ZnAl2O4–TiO2 (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), (3) SO42−/ZnAl2O4–TiO2 (6 2), (3) SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), (4) SO42−/ZnAl2O4–TiO2 (4 4), (4) SO42−/ZnAl2O4–TiO2 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), (5) SO42−/ZnAl2O4–TiO2 (2 6), (5) SO42−/ZnAl2O4–TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) and (6) SO42−/TiO2 catalysts. 8) and (6) SO42−/TiO2 catalysts. | ||
The surface sulfate groups are responsible for inducing acidity in the catalyst. It has been assumed that the sulfate group is bonded covalently to the oxide.24 All the catalysts shown in Fig. 2 exhibited a broad peak between 900 and 1400 cm−1, which was assigned to sulfate groups bonded covalently to the surface metal oxides (Scheme 1a and b).25,26 The sulfate group belonged to C2v symmetry can be bonded to surface metals by two oxygen atoms forming bridge (Scheme 1a) or chelate structures (Scheme 1b). The broad peaks of all catalysts could be divided into three peaks at 1227, 1139 and 1060 cm−1. Peaks at 1139 and 1060 cm−1 were ascribed to asymmetric and symmetric stretching of S–O bonds, respectively, and the others at 1398 and 1227 cm−1 were due to asymmetric and symmetric stretching of the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.26,27 The peaks at 1227 cm−1 indicated that bonded sulfate groups were formed with the chelate structure (Scheme 1b).28 In addition, the peaks that belonged to Al–O, Zn–O and Al–O vibrations for ZnAl2O4 spinel were observed at 669, 561 and 505 cm−1 in all SO42−/ZnAl2O4–TiO2 catalysts.29 The existence of a spinel phase in mixed oxides catalysts was consistent with the results of XRD. The broad band in the range of 400–800 cm−1 occurred in SO42−/TiO2 was regarded as the stretching vibration of Ti–O–Ti. The broad band was not detected clearly in SO42−/ZnAl2O4–TiO2, which might be due the band being covered by the intense peaks of Zn–O and Al–O vibrations.30 In addition, peaks at 1621 and 3400 cm−1 were associated with the bending mode and stretching mode of the OH group of water molecules, respectively.27
O.26,27 The peaks at 1227 cm−1 indicated that bonded sulfate groups were formed with the chelate structure (Scheme 1b).28 In addition, the peaks that belonged to Al–O, Zn–O and Al–O vibrations for ZnAl2O4 spinel were observed at 669, 561 and 505 cm−1 in all SO42−/ZnAl2O4–TiO2 catalysts.29 The existence of a spinel phase in mixed oxides catalysts was consistent with the results of XRD. The broad band in the range of 400–800 cm−1 occurred in SO42−/TiO2 was regarded as the stretching vibration of Ti–O–Ti. The broad band was not detected clearly in SO42−/ZnAl2O4–TiO2, which might be due the band being covered by the intense peaks of Zn–O and Al–O vibrations.30 In addition, peaks at 1621 and 3400 cm−1 were associated with the bending mode and stretching mode of the OH group of water molecules, respectively.27
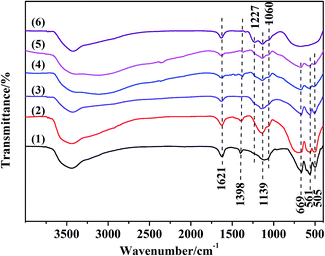 | ||
Fig. 2 FT-IR spectra of (1) SO42−/ZnAl2O4, (2) SO42−/ZnAl2O4–TiO2 (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), (3) SO42−/ZnAl2O4–TiO2 (6 2), (3) SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), (4) SO42−/ZnAl2O4–TiO2 (4 4), (4) SO42−/ZnAl2O4–TiO2 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), (5) SO42−/ZnAl2O4–TiO2 (2 6), (5) SO42−/ZnAl2O4–TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) and (6) SO42−/TiO2 catalysts. 8) and (6) SO42−/TiO2 catalysts. | ||
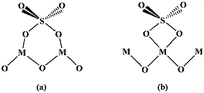 | ||
| Scheme 1 Structure of bonded sulfate group with C2v bridge (a) and bonded sulfate ion with C2v chelate (b). | ||
In the XPS spectra for the SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), the peaks for Zn 2p, Al 2p, Ti 2p, O 1s and S 2p were presented in Fig. 3. The spectra centered at 169.4 eV, corresponding to S 2p binding energy, was typical of the S6+ high oxidation state. This high oxidation state is essential evidence of formation of bidentate sulfate group, indicating that catalyst possessed acid sites.30 In addition, the O 1s spectra in Fig. 3c can be decomposed into three peaks at 530.4 eV, 531.4 eV and 532.3 eV, which were assigned to lattice oxygen of metal oxide, bridged oxygen of surface hydroxyl groups and the oxygen of sulfate group, respectively.31 For the oxidation state of the metal elements, the peaks at 1045.8 eV and 1022.7 eV shown in Fig. 3d belonged to Zn 2p1/2 and Zn 2p3/2 of Zn2+ ion.32 Fig. 3e shows the Al 2p peak of the catalyst at 75.2 eV, which was assigned to the Al3+ ion.33 The binding energy of Ti 2p3/2, shown in Fig. 3f, was 459.1 eV and that of Ti 2p1/2 was 464.5 eV, revealing that Ti was in the 4+ oxidation state.34
4), the peaks for Zn 2p, Al 2p, Ti 2p, O 1s and S 2p were presented in Fig. 3. The spectra centered at 169.4 eV, corresponding to S 2p binding energy, was typical of the S6+ high oxidation state. This high oxidation state is essential evidence of formation of bidentate sulfate group, indicating that catalyst possessed acid sites.30 In addition, the O 1s spectra in Fig. 3c can be decomposed into three peaks at 530.4 eV, 531.4 eV and 532.3 eV, which were assigned to lattice oxygen of metal oxide, bridged oxygen of surface hydroxyl groups and the oxygen of sulfate group, respectively.31 For the oxidation state of the metal elements, the peaks at 1045.8 eV and 1022.7 eV shown in Fig. 3d belonged to Zn 2p1/2 and Zn 2p3/2 of Zn2+ ion.32 Fig. 3e shows the Al 2p peak of the catalyst at 75.2 eV, which was assigned to the Al3+ ion.33 The binding energy of Ti 2p3/2, shown in Fig. 3f, was 459.1 eV and that of Ti 2p1/2 was 464.5 eV, revealing that Ti was in the 4+ oxidation state.34
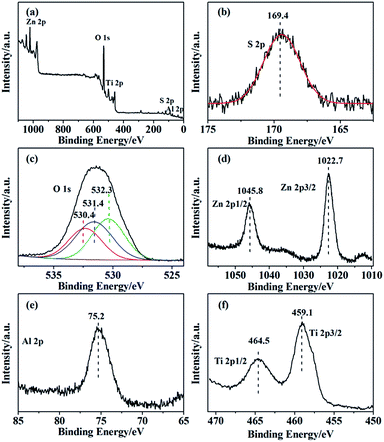 | ||
Fig. 3 XPS spectra of the (a) SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) catalysts and the high-resolution photoelectron spectra of (b) Zn 2p, (c) Al 2p, (d) S 2p, (e) O 1s and (f) Ti 2p. 4) catalysts and the high-resolution photoelectron spectra of (b) Zn 2p, (c) Al 2p, (d) S 2p, (e) O 1s and (f) Ti 2p. | ||
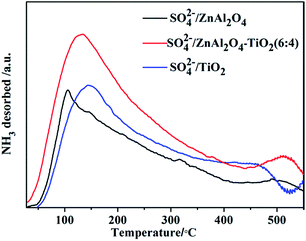
NH3-TPD was used to estimate the amount and strength of acid sites formed on the surface of the catalysts. All the SO42−/ZnAl2O4, SO42−/ZnAl2O4–TiO2 and SO42−/TiO2 catalysts contain a certain amount of acid sites, indicating both TiO2 and ZnAl2O4 acted as active components to form the acid sites on the surface by sulfation. The broadened peaks, shown in Fig. 4, were divided into peaks assigned to weak acid sites (below 200 °C), middle strength acid sites (200–400 °C) and strong acid sites (above 400 °C).35 These profiles showed that the catalysts contained various amounts of acid sites and different acid strengths. Compared to SO42−/ZnAl2O4 and SO42−/TiO2, the SO42−/ZnAl2O4–TiO2 contained the largest number of acid sites with a clear increase in weak and middle strength acid sites. This may be explained that the synergistic interaction of two active components improved the generation of acid sites, which was commonly observed in the modified SO42−/TiO2–MxOy composite catalysts.35,36 Moreover, the acid site density of the catalysts with different compositions was studied by the acid–base titration, as shown in Table 2. The mass ratio of ZnAl2O4 and TiO2 had a strong effect on the acid site density of the catalysts. With increasing TiO2 component, the acid site density was improved gradually. SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) showed a higher density of acid sites than SO42−/ZnAl2O4 and SO42−/TiO2, which is in agreement with NH3-TPD. However, excess TiO2 had a negative influence in acid site density. This may be due to the accumulation of TiO2 on the outer surface of ZnAl2O4, which can be seen in other composite catalysts.37 It was suggested that the acidic properties of SO42−/ZnAl2O4–TiO2 can be tuned by the mass ratio of the ZnAl2O4 and TiO2 components and the acidic property was maximized with the optimal ratio of 6
4) showed a higher density of acid sites than SO42−/ZnAl2O4 and SO42−/TiO2, which is in agreement with NH3-TPD. However, excess TiO2 had a negative influence in acid site density. This may be due to the accumulation of TiO2 on the outer surface of ZnAl2O4, which can be seen in other composite catalysts.37 It was suggested that the acidic properties of SO42−/ZnAl2O4–TiO2 can be tuned by the mass ratio of the ZnAl2O4 and TiO2 components and the acidic property was maximized with the optimal ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.
4.
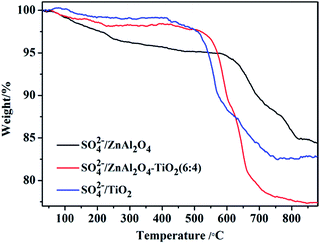
TG analysis of the SO42−/ZnAl2O4, SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and SO42−/TiO2 catalysts was carried out, as shown in Fig. 5. The initial weight loss (around 100–200 °C) on the TG profiles was assigned to the desorption of physically adsorbed water.37 Further increasing the temperature from 200 °C to 550 °C led to a gradual weight loss due to the removal of surface hydroxyl groups.38 The third weight loss that started at about 550 °C was associated with the decomposition of surface sulfate groups, which was a typical feature in the TG profiles of SO42−/MxOy.38 Based on the TG profiles, the weight percentages of the sulfate groups eliminated from SO42−/ZnAl2O4, SO42−/ZnAl2O4–TiO2 (6
4) and SO42−/TiO2 catalysts was carried out, as shown in Fig. 5. The initial weight loss (around 100–200 °C) on the TG profiles was assigned to the desorption of physically adsorbed water.37 Further increasing the temperature from 200 °C to 550 °C led to a gradual weight loss due to the removal of surface hydroxyl groups.38 The third weight loss that started at about 550 °C was associated with the decomposition of surface sulfate groups, which was a typical feature in the TG profiles of SO42−/MxOy.38 Based on the TG profiles, the weight percentages of the sulfate groups eliminated from SO42−/ZnAl2O4, SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and SO42−/TiO2 were estimated to be 13.1%, 20.2% and 16.5%, respectively, as shown in Table 1. This also proved that both TiO2 and ZnAl2O4 acted as active components to generate surface sulfate groups after sulfation. Moreover, more the weight losses, more the sulfate groups that exist.13,38 Thus, it was revealed that the SO42−/ZnAl2O4–TiO2 (6
4) and SO42−/TiO2 were estimated to be 13.1%, 20.2% and 16.5%, respectively, as shown in Table 1. This also proved that both TiO2 and ZnAl2O4 acted as active components to generate surface sulfate groups after sulfation. Moreover, more the weight losses, more the sulfate groups that exist.13,38 Thus, it was revealed that the SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) catalyst contained more sulfate groups over their pure components, which may be due to the cooperation of ZnAl2O4 and TiO2. This result was consistent with that of NH3-TPD. In addition, the decomposition temperatures of sulfate groups were 660, 610 and 550 °C for SO42−/ZnAl2O4, SO42−/ZnAl2O4–TiO2 (6
4) catalyst contained more sulfate groups over their pure components, which may be due to the cooperation of ZnAl2O4 and TiO2. This result was consistent with that of NH3-TPD. In addition, the decomposition temperatures of sulfate groups were 660, 610 and 550 °C for SO42−/ZnAl2O4, SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and SO42−/TiO2 respectively, indicating that the sulfate groups on ZnAl2O4-based catalysts showed better thermal stability than that of SO42−/TiO2 catalysts. This suggests that the ZnAl2O4 component might help stabilize surface sulfate groups by enhancing its thermal stability.
4) and SO42−/TiO2 respectively, indicating that the sulfate groups on ZnAl2O4-based catalysts showed better thermal stability than that of SO42−/TiO2 catalysts. This suggests that the ZnAl2O4 component might help stabilize surface sulfate groups by enhancing its thermal stability.
Fig. 6 shows FE-SEM images of SO42−/ZnAl2O4, SO42−/ZnAl2O4–TiO2 and SO42−/TiO2 catalysts. The SO42−/ZnAl2O4 catalyst, shown in Fig. 6a, had a near spherical particle appearance. However, Fig. 6b revealed plate-like morphologies of pure SO42−/TiO2. As shown in Fig. 6c, the morphology of SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) showed that both ZnAl2O4 and TiO2 kept up their unique morphologies. Moreover, spherical ZnAl2O4 particles were dispersed on or crossed through plate-like TiO2 bulk. This further confirmed that the two components were composited in SO42−/ZnAl2O4–TiO2 (6
4) showed that both ZnAl2O4 and TiO2 kept up their unique morphologies. Moreover, spherical ZnAl2O4 particles were dispersed on or crossed through plate-like TiO2 bulk. This further confirmed that the two components were composited in SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) catalyst.
4) catalyst.
 | ||
Fig. 6 FE-SEM micrographs of (a)SO42−/ZnAl2O4, (b) SO42−/TiO2 and (c) SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) catalysts. 4) catalysts. | ||
On the basis of the abovementioned characterization, the possible structures are shown in Fig. 7. The XPS corroborating FT-IR analysis gave the possible chelate structure of sulfate groups bonded on the surface metal oxides of catalysts. FE-SEM showed that spherical ZnAl2O4 particles were dispersed on or crossed through plate-like TiO2 particles, and the M–O–Ti linkages were expected on the interface, resulting from the cooperation of two components, which may have improved the acidic properties of the composite catalysts.
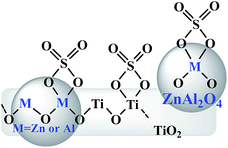 | ||
| Fig. 7 Possible structures of the sulfate groups formed on the surface of SO42−/ZnAl2O4–TiO2 catalysts. | ||
3.2 Catalytic performance
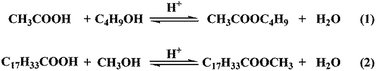
The esterification of acetic acid with n-butanol and oleic acid with methanol (Scheme 2) were conducted over SO42−/ZnAl2O4, SO42−/TiO2 and SO42−/ZnAl2O4–TiO2 catalysts. The effects of the different mass ratios of ZnAl2O4 to TiO2 on the catalytic activities of SO42−/ZnAl2O4–TiO2 are shown in Fig. 8. Increasing the mass ratio of TiO2 could significantly enhance the catalytic activities of the SO42−/ZnAl2O4–TiO2 catalysts for both reactions. When the mass ratio of ZnAl2O4 to TiO2 was 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the catalytic activities were maximized for both reactions, with 98.5% conversion for the esterification of acetic acid with n-butanol and 95.8% conversion for oleic acid esterification. The improvement in catalyst activities may be due to the increase in the number of acid sites, which had been characterized in the NH3-TPD, acid–base titration and TG analysis. By further increasing the mass ratio of the TiO2 component, the conversion of composite catalysts decreased, which was attributed to the reduction of the number of acid sites. The composition of catalyst clearly influenced the catalytic activities of the SO42−/TiO2–ZnAl2O4 composite in these reactions by adjusting their acidic properties. Furthermore, the reactivity of the active sites was investigated in terms of the turnover frequency (TOF), shown in Table 2. The results similarly demonstrated that the active sites present on SO42−/ZnAl2O4–TiO2 (6
4, the catalytic activities were maximized for both reactions, with 98.5% conversion for the esterification of acetic acid with n-butanol and 95.8% conversion for oleic acid esterification. The improvement in catalyst activities may be due to the increase in the number of acid sites, which had been characterized in the NH3-TPD, acid–base titration and TG analysis. By further increasing the mass ratio of the TiO2 component, the conversion of composite catalysts decreased, which was attributed to the reduction of the number of acid sites. The composition of catalyst clearly influenced the catalytic activities of the SO42−/TiO2–ZnAl2O4 composite in these reactions by adjusting their acidic properties. Furthermore, the reactivity of the active sites was investigated in terms of the turnover frequency (TOF), shown in Table 2. The results similarly demonstrated that the active sites present on SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) showed a comparable rate per active site to that of SO42−/TiO2 and much greater efficiency than that of SO42−/ZnAl2O4. This indicates that both ZnAl2O4 and TiO2 can produce active sites to catalyze the esterification after sulfation. Moreover, the TiO2 component acted as the main catalytic promotor in the composite to generate superior acid sites. The obtained SO42−/ZnAl2O4–TiO2 (6
4) showed a comparable rate per active site to that of SO42−/TiO2 and much greater efficiency than that of SO42−/ZnAl2O4. This indicates that both ZnAl2O4 and TiO2 can produce active sites to catalyze the esterification after sulfation. Moreover, the TiO2 component acted as the main catalytic promotor in the composite to generate superior acid sites. The obtained SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was suggested to be an efficient catalyst for these two types of esterifications.
4) was suggested to be an efficient catalyst for these two types of esterifications.
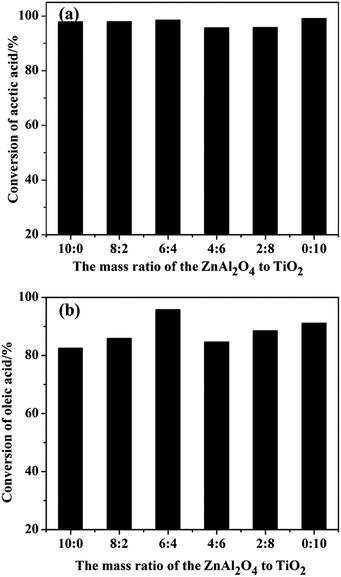 | ||
| Fig. 8 Esterification of (a) acetic acid with n-butanol and (b) oleic acid with methanol over varied catalysts. | ||
| Catalysts | Acid sites (mmol g−1) | Conv. (%) | TOFa (min−1) | ||
|---|---|---|---|---|---|
| Acetic acid (20 min) | Oleic acid (30 min) | Acetic acid | Oleic acid | ||
| a TOF = Macid/Msite, where Macid and Msite are mole of the initial acid consumed in the reaction per unit time (min) and mole of active sites, respectively. Msite is obtained from the result of acid–base titration for each catalyst. | |||||
| SO42−/ZnAl2O4 | 1.84 | 45.3 | 50.2 | 2.81 | 0.57 |
SO42−/ZnAl2O4–TiO2 (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
2.21 | 55.8 | 55.2 | 2.88 | 0.52 |
SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
2.31 | 63.8 | 75.8 | 3.15 | 0.68 |
SO42−/ZnAl2O4–TiO2 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
1.62 | 40.2 | 52.6 | 2.83 | 0.67 |
SO42−/ZnAl2O4–TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) 8) |
1.69 | 36.6 | 63.1 | 2.47 | 0.78 |
| SO42−/TiO2 | 2.16 | 69.8 | 70.9 | 3.69 | 0.68 |
It is generally accepted that different esterification reactions have their preferential acid sites in terms of acidic strength.39,40 In this study, the amounts of acid sites with different acidic strengths were calculated based on the NH3-TPD profiles. Fig. 9 shows that the SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) catalyst showed different acidic properties from SO42−/ZnAl2O4 and SO42−/TiO2 catalysts, which may indicate their different catalytic activities. It was found that the trend of acetic acid conversion corresponded to the number of strong acid sites. On the other hand, the change in catalytic activities in oleic acid esterification increased in line with middle strength acid sites or weak acid sites. These two reactions may have their own favorable acid strength of acid sites. The relationship between acidic properties and catalytic activities gave a referential study for the design of catalysts for these reactions.
4) catalyst showed different acidic properties from SO42−/ZnAl2O4 and SO42−/TiO2 catalysts, which may indicate their different catalytic activities. It was found that the trend of acetic acid conversion corresponded to the number of strong acid sites. On the other hand, the change in catalytic activities in oleic acid esterification increased in line with middle strength acid sites or weak acid sites. These two reactions may have their own favorable acid strength of acid sites. The relationship between acidic properties and catalytic activities gave a referential study for the design of catalysts for these reactions.
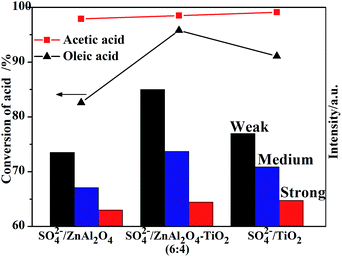 | ||
Fig. 9 Conversion of different acids and intensity of acidic amount as a function of SO42−/ZnAl2O4, SO42−/TiO2 and SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) catalysts with different acidic strengths. 4) catalysts with different acidic strengths. | ||
In order to develop a green and reusable catalyst, the capability of the catalyst to be recovered and reused should be considered seriously in the study of SO42−/MxOy solid acids. Thus, SO42−/ZnAl2O4, SO42−/TiO2 and optimal SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) solid acid catalysts were recycled to study the stability of catalytic activity. As shown in Fig. 10, spinel-based catalysts outperformed SO42−/TiO2 catalyst in terms of the reusability for both reactions. This advantage was evidently manifested in the SO42−/ZnAl2O4–TiO2 (6
4) solid acid catalysts were recycled to study the stability of catalytic activity. As shown in Fig. 10, spinel-based catalysts outperformed SO42−/TiO2 catalyst in terms of the reusability for both reactions. This advantage was evidently manifested in the SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) catalysts. Fig. 10a shows that SO42−/ZnAl2O4–TiO2 (6
4) catalysts. Fig. 10a shows that SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) can maintain the conversion of acetic acid above 85% in the fifth cycle, while SO42−/TiO2 showed a clear deactivation (∼80%) after two repeated cycles. The catalytic activities for the esterification of oleic acid with methanol was ∼40% and ∼8% lower in reused SO42−/TiO2 and SO42−/ZnAl2O4–TiO2 (6
4) can maintain the conversion of acetic acid above 85% in the fifth cycle, while SO42−/TiO2 showed a clear deactivation (∼80%) after two repeated cycles. The catalytic activities for the esterification of oleic acid with methanol was ∼40% and ∼8% lower in reused SO42−/TiO2 and SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), respectively, in the second cycle (Fig. 10b). These results show that the SO42−/ZnAl2O4–TiO2 (6
4), respectively, in the second cycle (Fig. 10b). These results show that the SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) catalyst as a reusable catalyst can be recycled several times.
4) catalyst as a reusable catalyst can be recycled several times.
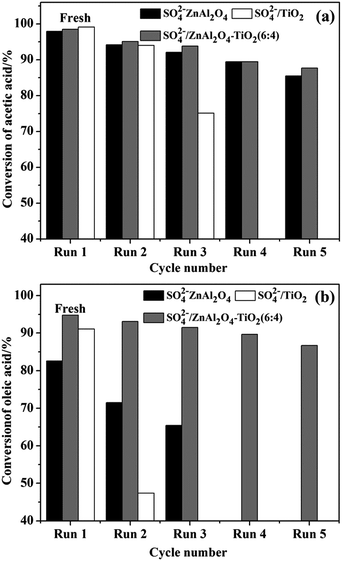 | ||
Fig. 10 Reusability of SO42−/ZnAl2O4, SO42−/TiO2 and SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) catalysts for esterification of (a) acetic acid with n-butanol and (b) oleic acid with methanol. 4) catalysts for esterification of (a) acetic acid with n-butanol and (b) oleic acid with methanol. | ||
To study the stability and the reason for the deactivation, the used catalysts after two repeated cycles were characterized by XRD, FT-IR and TG, as shown in Fig. 11. According to FT-IR and XRD analysis, no evident change in the structure of the used SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) catalyst was observed by comparing to the fresh catalyst. This indicated that SO42−/ZnAl2O4–TiO2 (6
4) catalyst was observed by comparing to the fresh catalyst. This indicated that SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) had good structural stability after recycling. The IR spectra, shown in Fig. 11b, also gave broadened peaks of surface sulfate group in the range of 900–1400 cm−1. The peaks of sulfate groups almost disappeared in the IR spectra of used SO42−/TiO2, while they were kept relatively better in the spectra of used SO42−/ZnAl2O4–TiO2. SO42−/ZnAl2O4–TiO2 had superior resistance to the loss of sulfur species, which might interpret its better reusability. The TG curves in Fig. 11c were also performed to estimate the amount of residual sulfate groups on the surface of the used catalysts. As shown in Table 1, the weight percentages of surface sulfate groups on the used SO42−/ZnAl2O4, SO42−/ZnAl2O4–TiO2 (6
4) had good structural stability after recycling. The IR spectra, shown in Fig. 11b, also gave broadened peaks of surface sulfate group in the range of 900–1400 cm−1. The peaks of sulfate groups almost disappeared in the IR spectra of used SO42−/TiO2, while they were kept relatively better in the spectra of used SO42−/ZnAl2O4–TiO2. SO42−/ZnAl2O4–TiO2 had superior resistance to the loss of sulfur species, which might interpret its better reusability. The TG curves in Fig. 11c were also performed to estimate the amount of residual sulfate groups on the surface of the used catalysts. As shown in Table 1, the weight percentages of surface sulfate groups on the used SO42−/ZnAl2O4, SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and SO42−/TiO2 were estimated to be 10.9%, ∼16.2% and ∼7.8%, respectively. ZnAl2O4-based catalysts showed relatively minimal sulfur loss particularly in the SO42−/ZnAl2O4–TiO2 (6
4) and SO42−/TiO2 were estimated to be 10.9%, ∼16.2% and ∼7.8%, respectively. ZnAl2O4-based catalysts showed relatively minimal sulfur loss particularly in the SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) catalysts. Accordingly, it was suggested that the ZnAl2O4 component played a significant role in stabilizing sulfate groups on the surface of the SO42−/ZnAl2O4–TiO2 catalyst and enhance its resistance of sulfur loss, which was also approved in IR analysis. Based on IR and TG analysis, a decrease in the intensity of peaks and the number of sulfate groups was observed in all used catalysts. This indicated that the catalysts inevitably suffered from the loss of sulfur species. The loss of surface sulfate groups is one of the main reasons for the deactivation of these catalysts in recycling reaction, which is common in other sulfated catalysts.40–42
4) catalysts. Accordingly, it was suggested that the ZnAl2O4 component played a significant role in stabilizing sulfate groups on the surface of the SO42−/ZnAl2O4–TiO2 catalyst and enhance its resistance of sulfur loss, which was also approved in IR analysis. Based on IR and TG analysis, a decrease in the intensity of peaks and the number of sulfate groups was observed in all used catalysts. This indicated that the catalysts inevitably suffered from the loss of sulfur species. The loss of surface sulfate groups is one of the main reasons for the deactivation of these catalysts in recycling reaction, which is common in other sulfated catalysts.40–42
Table 3 lists these two types of esterifications over various catalysts, such as modified SO42−/TiO2, MCM-41, MOFs, and H2SO4, and compares the reported catalysts with catalysts obtained in this study. The results showed that the high conversion in the esterification of acetic acid n-butanol over SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was comparable to that of modified SO42−/TiO2 and Al–MCM-41 mesoporous catalysts presented in the literature, whereas the relatively milder reaction condition was applied in this study.9,43,44 For the esterification of oleic acid with methanol, the SO42−/ZnAl2O4–TiO2 (6
4) was comparable to that of modified SO42−/TiO2 and Al–MCM-41 mesoporous catalysts presented in the literature, whereas the relatively milder reaction condition was applied in this study.9,43,44 For the esterification of oleic acid with methanol, the SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) catalyst with a smaller catalyst amount and shorter reaction time exhibited a higher catalytic activity (95.8%) than [TMEDAPS][HSO4] and MOF-808-2.5SO4.6,45 Although a homogeneous acid, i.e., H2SO4, showed high conversion (91%) in low molar ratio of alcohol to acid and at low temperature,46 the use of eco-friendly heterogeneous catalysts was more advantageous due to the easy separation and re-use of the catalyst. This suggested that the SO42−/ZnAl2O4–TiO2 (6
4) catalyst with a smaller catalyst amount and shorter reaction time exhibited a higher catalytic activity (95.8%) than [TMEDAPS][HSO4] and MOF-808-2.5SO4.6,45 Although a homogeneous acid, i.e., H2SO4, showed high conversion (91%) in low molar ratio of alcohol to acid and at low temperature,46 the use of eco-friendly heterogeneous catalysts was more advantageous due to the easy separation and re-use of the catalyst. This suggested that the SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) is more or less superior compared to the reported catalysts in terms of the high acid conversion with short reaction times and mild conditions, easy recoverability and high recyclability.
4) is more or less superior compared to the reported catalysts in terms of the high acid conversion with short reaction times and mild conditions, easy recoverability and high recyclability.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) catalyst with other reported solid acid catalysts
4) catalyst with other reported solid acid catalysts
| Catalyst | Reaction conditions | Conv./% | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| Amounta/wt% | Acid | Alcohol | Molar ratiob | Temp./°C | Time/h | |||
a Based on the acid.b The molar ratio of acid to alcohol.c Present study.d n([TMEDAPS][HSO4])![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(Methanol). n(Methanol). |
||||||||
| SO42−/TiO2–Zr–La | 14.3 | Acetic acid | n-Butanol | 1.56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98–112 | 0.5 | 85 | 9 |
| SO42−–Ce0.02/TiO2 | 12 | Acetic acid | n-Butanol | 3/1 | 145 | 0.75 | 97.1 | 43 |
| Al–MCM-41 (Si/Al = 25) | 1.67 | Acetic acid | n-Butanol | 1/2 | 125 | 6 | 87.3 | 44 |
SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
7.3 | Acetic acid | n-Butanol | 3/1 | 115 | 2 | 98.5 | —c |
| MOF-808-2.5SO4 | 20 | Oleic acid | Methanol | 70/1 | 65 | 6 | 80 | 6 |
| H2SO4 | — | Oleic acid | Methanol | 3/1 | 80 | 6 | 91 | 46 |
| [TMEDAPS][HSO4] | 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8d 1.8d |
Oleic acid | Methanol | 1.8/1 | 70 | 6 | 95 | 45 |
SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
5 | Oleic acid | Methanol | 10/1 | 100 | 2 | 95.8 | —c |
4. Conclusions
This study synthesized a series of novel SO42−/ZnAl2O4–TiO2 composite catalysts with single spinel and anatase phases and applied these catalysts in the esterification of biomass derived acids. In the composite catalyst, both ZnAl2O4 and TiO2 oxides acted as catalytic components, and generated acid sites by sulfation. The catalytic properties and performance of composite catalysts can be tuned by adjusting the mass ratio of ZnAl2O4/TiO2. The optimal SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) exhibited excellent conversion of 95.8% and 98.5% in oleic acid and acetic acid esterification, respectively, which was attributed to the larger number of acid sites due to the cooperation of ZnAl2O4 and TiO2 oxides. In addition, the ZnAl2O4 component played a key role in stabilizing the structural stability as well as the surface sulfate groups. The advantage of superior reusability accordingly showed in the SO42−/ZnAl2O4–TiO2 (6
4) exhibited excellent conversion of 95.8% and 98.5% in oleic acid and acetic acid esterification, respectively, which was attributed to the larger number of acid sites due to the cooperation of ZnAl2O4 and TiO2 oxides. In addition, the ZnAl2O4 component played a key role in stabilizing the structural stability as well as the surface sulfate groups. The advantage of superior reusability accordingly showed in the SO42−/ZnAl2O4–TiO2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) catalyst with high conversion above 85% up to 5 cycles for both esterification reactions. Correlating the acidic properties and activities, it was assumed that the amount of acid sites varied in acid strength on the different catalysts so that these catalysts performed differently for these two reactions. The esterification of acetic acid and n-butanol may require strong acid sites, while middle strength or weak acid sites were favorable to the esterification of oleic acid with methanol.
4) catalyst with high conversion above 85% up to 5 cycles for both esterification reactions. Correlating the acidic properties and activities, it was assumed that the amount of acid sites varied in acid strength on the different catalysts so that these catalysts performed differently for these two reactions. The esterification of acetic acid and n-butanol may require strong acid sites, while middle strength or weak acid sites were favorable to the esterification of oleic acid with methanol.
This study suggested that the ZnAl2O4 spinel oxide can be used as a novel component to modify the SO42−/MxOy catalyst. The spinel-type component in composites plays a role in tuning the catalytic properties and stabilizing the structure and surface sulfate groups.
Acknowledgements
This study has been financially supported by the National Natural Science Foundation of China (NSFC No. 21203170) and the National College Students' Innovative Training Program (NCSITP No. 201610491017 and 201610491033).Notes and references
- P. A. Alaba, Y. M. Sanib and W. M. A. W. Daud, RSC Adv., 2016, 6, 78351–78368 RSC.
- J. A. Melero, J. Iglesias and G. Morales, Green Chem., 2009, 54, 1285–1308 RSC.
- A. F. Lee, J. A. Bennett, J. C. Manayil and K. Wilson, Chem. Soc. Rev., 2014, 43, 7887–7916 RSC.
- K. Saravanan, B. Tyagi, R. S. Shukla and H. C. Bajaj, Fuel, 2016, 165, 298–305 CrossRef CAS.
- L. S. Do Nascimento, L. M. Z. Tito, R. S. Angélica, C. E. F. Da Costa, J. R. Zamian and G. N. Da Rocha Filho, Appl. Catal., B, 2011, 101, 495–503 CrossRef CAS.
- J. Jiang, F. Gándara, Y. B. Zhang, K. Na, O. M. Yaghi and W. G. Klemperer, J. Am. Chem. Soc., 2014, 136, 12844–12847 CrossRef CAS PubMed.
- K. Arata, Adv. Catal., 1990, 37, 165–211 CAS.
- Y. Li, X. D. Zhang, L. Sun, J. Zhang and H. P. Xu, Appl. Energy, 2010, 87, 156–159 CrossRef CAS.
- W. P. Shi, Catal. Lett., 2010, 143, 732–738 CrossRef.
- M. Yang, Y. Men, S. L. Li and G. W. Chen, Appl. Catal., A, 2012, 433–434, 26–34 CrossRef CAS.
- Q. Lu, X. N. Ye, Z. B. Zhang, C. Q. Dong and Y. Zhang, Bioresour. Technol., 2014, 171, 10–15 CrossRef CAS PubMed.
- A. Q. Wang, X. L. Wu, J. X. Wang, H. Pan, X. Y. Tian and Y. L. Xing, RSC Adv., 2015, 5, 19652–19658 RSC.
- J. X. Wang, A. Q. Wang, Y. L. Xing, Z. X. Zhu, X. L. Wu, Y. Q. Wang and L. X. Yang, RSC Adv., 2015, 5, 105908–105916 RSC.
- J. X. Wang, H. Pan, A. Q. Wang, X. Y. Tian, X. L. Wu and Y. Q. Wang, Catal. Commun., 2015, 62, 29–33 CrossRef CAS.
- K. R. Sunajadevi and S. Sugunan, Catal. Commun., 2004, 5, 575–581 CrossRef CAS.
- C. Morterra, G. Cerrato, F. Pinna and M. Signoretto, J. Catal., 1995, 157, 109–123 CrossRef CAS.
- A. Patel and N. Narkhede, Energy Fuels, 2012, 26, 6025–6032 CrossRef CAS.
- G. Corro, F. Bañuelos, E. Vidal and S. Cebada, Fuel, 2014, 115, 625–628 CrossRef CAS.
- J. C. Manayil, C. V. M. Inocencio, A. F. Lee and K. Wilson, Green Chem., 2016, 18, 1387–1394 RSC.
- R. Hilten, J. Weber and J. R. Kastner, Energy Fuels, 2016, 30, 9480–9489 CrossRef CAS.
- E. Tabrizian and A. Amoozadeh, RSC Adv., 2016, 6, 96606–96615 RSC.
- Y. Wang, D. Wang, M. H. Tan, B. Jiang, J. T. Zheng, N. Tsubaki and M. B. Wu, ACS Appl. Mater. Interfaces, 2015, 7, 26767–26775 CAS.
- L. H. O. Pires, A. N. De Oliveira, O. V. Monteiro Jr, R. S. Angélica, C. E. F. Da Costa, J. R. Zamian, L. A. S. Do Nascimento and G. N. Rocha Filho, Appl. Catal., B, 2014, 160–161, 122–128 CrossRef CAS.
- G. D. Yadav and R. V. Sharma, J. Catal., 2014, 311, 121–128 CrossRef CAS.
- C. C. Huang, C. J. Yang, P. J. Gao, N. C. Wang, C. L. Chen and J. S. Chang, Green Chem., 2015, 17, 3609–3620 RSC.
- F. Haase and J. Sauer, J. Am. Chem. Soc., 1998, 120, 13503–13512 CrossRef CAS.
- Z. L. Li, R. Wnetrzak, W. Kwapinski and J. J. Leahy, ACS Appl. Mater. Interfaces, 2012, 4, 4499–4505 CAS.
- K. Saravanan, B. Tyagi, R. S. Shukla and H. C. Bajaj, Appl. Catal., B, 2015, 172–173, 108–115 CrossRef CAS.
- S. Farhadi and S. Panahandehjoo, Appl. Catal., A, 2010, 382, 293–302 CrossRef CAS.
- D. Ma, Y. J. Xin, M. C. Gao and J. Wu, Appl. Catal., B, 2014, 147, 49–57 CrossRef CAS.
- H. Y. Zhao, S. Bennici, J. Y. Shen and A. Auroux, J. Catal., 2010, 272, 176–189 CrossRef CAS.
- L. J. Liu, G. L. Zhang, L. Wang, T. Huang and L. Qin, Ind. Eng. Chem. Res., 2011, 50, 7219–7227 CrossRef CAS.
- X. L. Duan, D. R. Yuan and F. P. Yu, Inorg. Chem., 2011, 50, 5460–5467 CrossRef CAS PubMed.
- L. C. Peng, L. Lin, H. Li and Q. L. Yang, Appl. Energy, 2011, 88, 4590–4596 CrossRef CAS.
- B. M. Reddy, B. Chowdhury and P. G. Smirniotis, Appl. Catal., A, 2001, 211, 19–30 CrossRef CAS.
- M. E. Manríquez, T. López, R. Gómez and J. Navarrete, J. Mol. Catal. A: Chem., 2004, 220, 229–237 CrossRef.
- T. Wu, J. W. Wan and X. B. Ma, Chin. J. Catal., 2015, 36, 425–431 CrossRef CAS.
- T. Witoon, T. Permsirivanich, N. Kanjanasoontorn, C. Akkaraphataworn, A. Seubsai, K. Faungnawakij, C. Warakulwit, M. Chareonpanich and J. Limtrakul, Catal. Sci. Technol., 2015, 5, 2347–2357 CAS.
- K. Ngaosuwan, J. G. Goodwin Jr and P. Prasertdham, Renewable Energy, 2016, 86, 262–269 CrossRef CAS.
- J. Zhu, P. C. Wang and M. Lu, Catal. Sci. Technol., 2015, 5, 3383–3393 CAS.
- L. Zhang, W. X. Zou, K. L. Ma, Y. Cao, Y. Xiong, S. G. Wu, C. J. Tang, F. Gao and L. Dong, J. Phys. Chem. C, 2014, 119, 1155–1163 Search PubMed.
- K. Suwannakarn, E. Lotero, J. G. Goodwin Jr and C. Q. Lu, J. Catal., 2008, 255, 279–286 CrossRef CAS.
- G. Xiao, J. F. Zhou, X. Huang, X. P. Liao and B. Shi, RSC Adv., 2014, 4, 4010–4019 RSC.
- B. R. Jermy and A. Pandurangan, Appl. Catal., A, 2005, 288, 25–33 CrossRef CAS.
- D. Fang, J. M. Yang and C. M. Jiao, ACS Catal., 2011, 1, 42–47 CrossRef CAS.
- J. Y. Park, Z. M. Wang, D. K. Kim and J. S. Lee, Renewable Energy, 2010, 35, 614–618 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |