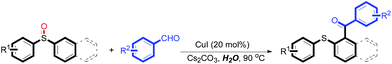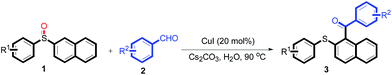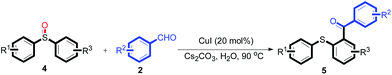Open Access Article
Open Access ArticleCopper-catalyzed C–C direct cross-coupling: an efficient approach to phenyl-2-(phenylthiophenyl)-methanones†
Qi Zhang,
Wei Wang,
Chong Gao,
Rong-Rong Cai and
Run-Sheng Xu *
*
Department of Biology and Environment, Jiyang College of Zhejiang A&F University, Hangzhou 311800, Zhejiang, China. E-mail: 20140041@zafu.edu.cn
First published on 6th April 2017
Abstract
An efficient copper-catalyzed C–C bond direct cross-coupling reaction has been developed. Starting from diaryl sulfoxides and aromatic aldehydes, versatile phenyl-2-(phenylthiophenyl)-methanones were efficiently synthesized with high tolerance of functional groups under moderate conditions in water. This new methodology provides an economical and environmentally friendly approach toward C(sp2)–C(sp2) bond formation.
Transition-metal catalyzed direct cross-coupling reactions enable efficient preparation of multifunctional complex molecules, playing an essential role in the development of natural products, pharmaceuticals and functional materials.1–5 Over the past few decades, nitrogen6 and oxygen7 group directed C–C bond coupling reactions via C–H bond activation have made great progress. Sulfur groups have a high electron density and are easy to modify, and have a non-maximal advantage in C–C bond direct cross-coupling.8 However, the application of sulfur group-directed C–C cross-coupling reactions remains a formidable challenge.9 One major problem is that regarding the competition between the sulfur atom and the reaction site, the coordination of the sulfur atom with the transition metal is very stable.10 Namely, the transition metals are more inclined to lose their catalytic activity. Therefore, the development of a more efficient strategy of sulfur directed C–H functionalization is still highly desirable.
Modern green synthetic chemistry is now well-established in the design and realization of organic synthesis, because it is seriously concerned with the environment and sustainable development.11 Among the challenges currently facing synthetic chemists is the development of efficient and environmentally friendly chemical processes for the synthesis of complex molecules, especially required in the area of drug discovery and natural product synthesis.12 Here, we report a practical method for the environmentally friendly and highly selective Cu-catalyzed C–C bond direct cross-coupling of diaryl sulfoxides with aromatic aldehydes. Versatile phenyl-2-(phenylthiophenyl)-methanones were efficiently synthesized with high tolerance of functional groups under moderate conditions in water. This new methodology provides an efficient and economical approach toward C(sp2)–C(sp2) formation.
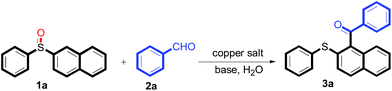
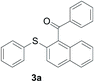
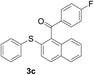
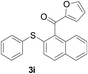
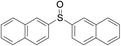
The reaction conditions were screened based on a model reaction of 2-benzenesulfinyl-naphthalene 1a with benzaldehyde 2a in water (Table 1). Initially, the experimental results demonstrated that Cu(I) salts have higher catalytic activities than Cu(II) salts (entries 1–3). Besides, those results demonstrated that the reaction temperature is an important parameter whereby the desired product was formed in 66% yield at 80 °C (entry 8) and 73% yield at 100 °C (entry 9). Meanwhile, we observed that this reaction also proceeds smoothly in organic solvent, such as CH3CN (entry 11). However, the product yield was lower than that in water. Finally, the desired product 3a was formed in 82% yield under the optimum reaction conditions employing CuI (20 mol%) and Cs2CO3 (2 equiv.) at 90 °C (entry 10).
| Entry | Copper salt | Base | 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a |
3ab [%] |
|---|---|---|---|---|
| a Unless otherwise noted, reaction conditions were 1a (0.3 mmol), 2a (0.3 mmol), copper salt (20 mol%), Cs2CO3 (2 equiv.), H2O (4 mL), 90 °C for 10 h.b Isolated yield.c At 80 °C.d At 100 °C.e In CH3CN. | ||||
| 1 | Cu(OAc)2 | Cs2CO3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16 |
| 2 | CuSO4 | Cs2CO3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
45 |
| 3 | CuBr2 | Cs2CO3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
nr |
| 4 | CuBr | Cs2CO3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
54 |
| 5 | CuI | Cs2CO3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
79 |
| 6 | CuI | Na2CO3 | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
nr |
| 7 | CuI | K3PO4 | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
43 |
| 8 | CuI | Cs2CO3 | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
66c |
| 9 | CuI | Cs2CO3 | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
73d |
| 10 | CuI | Cs2CO3 | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
82 |
| 11 | CuI | Cs2CO3 | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
71e |
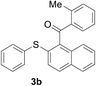
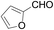
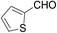
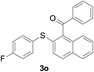
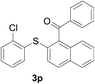
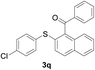
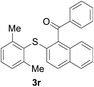
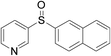
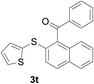
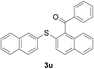
With the optimized conditions in hand, the reaction scope was next investigated. A wide array of 2-benzenesulfinyl-naphthalenes 1 and aromatic aldehydes 2 gave the desired products in good to excellent yields (Table 2). It was found that both the electron-donating and electron-withdrawing 2-benzenesulfinyl-naphthalenes 1 reacted smoothly with aromatic aldehydes 2 to give the desired compounds. 2-Benzenesulfinyl-naphthalenes 1 bearing electron-donating groups showed better activity than 1 bearing electron-withdrawing groups. Aromatic aldehydes 2 bearing electron-withdrawing groups showed better activity than 2 bearing electron-donating groups. Heteroatom aromatic aldehydes also gave the desired product (entries 9 and 10).
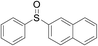
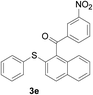
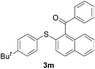
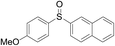
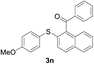
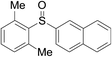
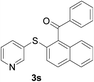
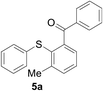
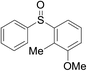
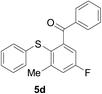
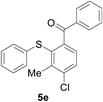
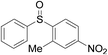
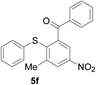
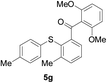
Furthermore, we turned our attention to other diaryl sulfoxides (Table 3). To our delight, diphenyl sulfoxides also smoothly gave the desired products in 79–85% yield. Diphenyl sulfoxides 4 bearing electron-donating groups (entry 6, 85% nitro group) showed better activity than those bearing electron-withdrawing groups (entry 2, 78% MeO group).
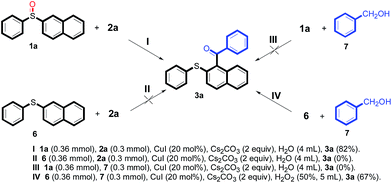
To obtain the preliminary details of the reaction mechanism, some additional reactions were done (Scheme 1). At first, the model reaction (Scheme 1I) was conducted in three other parallel reactions (Scheme 1II, III and IV). However, the results showed that only 2-phenylsulfanylnaphthalene 6 reacted with phenylmethanol 7 promoted by hydrogen peroxide under our standard conditions, successfully obtaining the target product 3a in 67% yield (Scheme 2IV), indicating that diaryl sulfoxide was the necessary substrate for this reaction.
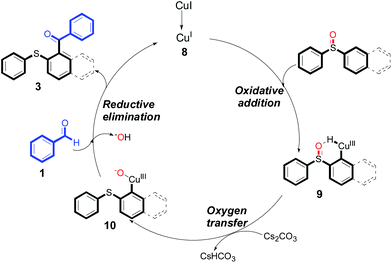
Many precedents from the literature have proved that sulfoxides are good directing groups13 and oxidants14 in C–H bond functionalization reactions. Based on the above results, a reaction mechanism was proposed (Scheme 2). At the beginning of the reaction, an intermediate 9 was formed from CuI with diaryl sulfoxides by oxidative addition step.15 There was a six-membered ring in the intermediate 9 which was very favorable for the intramolecular oxidation reaction, namely the oxygen transfer step. Next, intermediate 9 produced intermediate 10 by intramolecular oxygen transfer.16 Finally, intermediate 10 reacted with aromatic aldehyde 1 to furnish the desired product 3 and meanwhile concomitantly generated Cu(I), which re-entered the catalytic cycle.
Conclusions
In summary, an efficient copper-catalyzed C–C bond direct cross coupling of diaryl sulfoxides with aromatic aldehydes has been developed. Employing this methodology, various phenyl-2-(phenylthiophenyl)-methanone derivatives were efficiently synthesized under mild conditions in water. This new methodology provides an economical and environmentally friendly approach toward C(sp2)–C(sp2) formation. Finally, a plausible reaction mechanism of the Cu(I)/Cu(III) catalytical cycle was proposed.Notes
General procedure for preparation of 3 and 5
A mixture of 2-benzenesulfinyl-naphthalene 1a (90.7 mg, 0.36 mmol), benzaldehyde 2a (69 mg, 0.3 mmol), CuI (38.0 mg, 20 mol%) and Cs2CO3 (326.5 mg, 2 equiv.) in H2O (4 mL) was stirred in N2 at 90 °C for 10 h. After completion of the reaction, the mixture was quenched with saturated salt water (10 mL), and then the solution was extracted with ethyl acetate (3 × 10 mL). The organic layers were combined and dried by sodium sulfate. The pure product phenyl-(2-phenylsulfanylnaphthalen-1-yl)-methanone 3a (83.7 mg, 82% yield) was obtained by flash column chromatography on silica gel, washed with 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cyclohexane/ethyl acetate.
1 cyclohexane/ethyl acetate.
Acknowledgements
This work was supported by the Natural Science Foundation of Zhejiang Province (No. LQ15B020004).References
- For C–C direct cross-coupling, see: (a) M. Giannerini, M. Fañanás-Mastral and B. L. Feringa, Nat. Chem., 2013, 5, 667–672 CrossRef CAS PubMed; (b) R. Rossi, F. Bellina, M. Lessi and C. Manzini, Adv. Synth. Catal., 2014, 356, 17–117 CrossRef CAS; (c) L. Zhao, H. Zhan, J. Liao, J. Huang, Q. Chen, H. Qiu and H. Cao, Catal. Commun., 2014, 56, 65–67 CrossRef CAS; (d) Z. Zuo, D. T. Ahneman, L. Chu, J. A. Terrett, A. G. Doyle and D. W. MacMillan, Science, 2014, 345, 437–440 CrossRef CAS PubMed; (e) J. D. Denis, C. C. Scully, C. F. Lee and A. K. Yudin, Org. Lett., 2014, 16, 1338–1341 CrossRef PubMed; (f) N. Mukherjee, D. Kundu and B. C. Ranu, Chem. Commun., 2014, 5, 15784–15787 RSC; (g) Z. C. Cao, D. G. Yu, R. Y. Zhu, J. B. Wei and Z. J. Shi, Chem. Commun., 2015, 51, 2683–2686 RSC; (h) J. He, R. Takise, H. Fu and J. Q. Yu, J. Am. Chem. Soc., 2015, 137, 4618–4621 CrossRef CAS PubMed; (i) X. Zhang and D. W. MacMillan, J. Am. Chem. Soc., 2016, 138, 13862–13865 CrossRef CAS PubMed; (j) B. J. Shields and A. G. Doyle, J. Am. Chem. Soc., 2016, 138, 12719–12722 CrossRef CAS PubMed.
- For C–N direct cross-coupling, see: (a) J. Ke, C. He, H. Liu, M. Li and A. W. Lei, Chem. Commun., 2013, 49, 7549–7551 RSC; (b) R. W. Evans, J. R. Zbieg, S. Zhu, W. Li and D. W. MacMillan, J. Am. Chem. Soc., 2013, 135, 16074–16077 CrossRef CAS PubMed; (c) L. L. Zhou, S. Tang, X. T. Qi, C. T. Lin, K. Liu, C. Liu, Y. Lan and A. W. Lei, Org. Lett., 2014, 16, 3404–3407 CrossRef CAS PubMed; (d) X. H. Wei, G. W. Wang and S. D. Yang, Chem. Commun., 2015, 51, 832–835 RSC; (e) H. Yu, C. A. Dannenberg, Z. Li and C. Bolm, Chem.–Asian J., 2016, 11, 54–57 CrossRef CAS PubMed; (f) F. F. Duan, S. Q. Song and R. S. Xu, Chem. Commun., 2017, 53, 2737–2739 RSC.
- For C–O direct cross-coupling, see: (a) Z. Chen, H. Zeng, S. A. Girard, F. Wang, N. Chen and C. J. Li, Angew. Chem., Int. Ed., 2015, 54, 14487–14491 CrossRef CAS PubMed; (b) A. M. Whittaker and V. M. Dong, Angew. Chem., 2015, 127, 1328–1331 CrossRef; (c) A. P. Jadhav, D. Ray, V. U. Rao and R. P. Singh, Eur. J. Org. Chem., 2016, 14, 2369–2382 CrossRef.
- For C–S direct cross-coupling, see: (a) Y. Xu, X. Tang, W. Hu, W. Wu and H. Jiang, Green Chem., 2014, 16, 3720–3723 RSC; (b) P. F. Wang, X. Q. Wang, J. J. Dai, Y. S. Feng and H. J. Xu, Org. Lett., 2014, 16, 4586–4589 CrossRef CAS PubMed; (c) G. Zhang, L. Zhang, H. Yi, Y. Luo, X. Qi, C. H. Tung and A. Lei, Chem. Commun., 2016, 52, 10407–10410 RSC.
- For C–P direct cross-coupling, see: (a) D. Julienne, O. Delacroix and A. C. Gaumont, C. R. Chim., 2010, 13, 1099–1103 CrossRef CAS; (b) T. Miao and L. Wang, Adv. Synth. Catal., 2014, 356, 967–971 CrossRef CAS.
- (a) L. Grigorjeva and O. Daugulis, Angew. Chem., 2014, 126, 10373–10376 CrossRef; (b) M. Bera, A. Modak, T. Patra, A. Maji and D. Maiti, Org. Lett., 2014, 16, 5760–5763 CrossRef CAS PubMed; (c) W. Han, G. Zhang, G. Li and H. Huang, Org. Lett., 2014, 16, 3532–3535 CrossRef CAS PubMed; (d) Z.-W. Zhang, A. Lin and J. Yang, J. Org. Chem., 2014, 79, 7041–7050 CrossRef CAS PubMed; (e) H. Kinuta, M. Tobisu and N. Chatani, J. Am. Chem. Soc., 2015, 137, 1593–1600 CrossRef CAS PubMed.
- (a) B. Liu, H. Z. Jiang and B. F. Shi, J. Org. Chem., 2014, 79, 1521–1526 CrossRef CAS PubMed; (b) A. Seoane, N. Casanova, N. Quiñones, J. L. Mascareñas and M. Gulías, J. Am. Chem. Soc., 2014, 136, 7607–7610 CrossRef CAS PubMed; (c) S. Kujawa, D. Best, D. J. Burns and H. W. Lam, Chem.–Eur. J., 2014, 20, 8599–8602 CrossRef CAS PubMed; (d) F. L. Zhang, K. Hong, T. J. Li, H. Park and J. Q. Yu, Science, 2016, 351, 252–256 CrossRef CAS PubMed.
- (a) A. Wilsily, F. Tramutola, N. A. Owston and G. C. Fu, J. Am. Chem. Soc., 2012, 134, 5794–5797 CrossRef CAS PubMed; (b) M. V. Pham, B. Ye and N. Cramer, Angew. Chem., 2012, 124, 10762–10766 CrossRef.
- (a) I. Kato, M. Higashimoto and O. Tamura, J. Org. Chem., 2003, 68, 7983–7989 CrossRef CAS PubMed; (b) S. E. Mann, A. E. Aliev and G. J. Tizzard, Organometallics, 2011, 30, 1772–1775 CrossRef CAS; (c) D. Wang, X. Yu and W. Yao, Chem.–Eur. J., 2016, 22, 5543–5546 CrossRef CAS PubMed; (d) C. Luo, S. Niu and G. Zhou, Chem. Commun., 2016, 52, 12143–12146 RSC.
- (a) A. Garca-Rubia, R. G. Arrays and J. C. Carretero, Angew. Chem., Int. Ed., 2009, 48, 6511–6515 CrossRef PubMed; (b) P. Villuendas and E. P. Urriolabeitia, Org. Lett., 2015, 17, 3178–3181 CrossRef CAS PubMed; (c) H. Fei and S. M. Cohen, J. Am. Chem. Soc., 2015, 137, 2191–2194 CrossRef CAS PubMed; (d) C. Lin, D. Li and B. Wang, Org. Lett., 2015, 17, 1328–1331 CrossRef CAS PubMed.
- (a) M. Irimia-Vladu, Chem. Soc. Rev., 2014, 43, 588–610 RSC; (b) A. A. Ryan and M. O. Senge, Photochem. Photobiol. Sci., 2015, 14, 638–660 RSC; (c) F. Roschangar, R. A. Sheldon and C. H. Senanayake, Green Chem., 2015, 17, 752–768 RSC; (d) M. Sutter, E. D. Silva, N. Duguet, Y. Raoul, E. Métay and M. Lemaire, Chem. Rev., 2015, 115, 8609–8651 CrossRef CAS PubMed.
- (a) A. Farrán, C. Cai, M. Sandoval, Y. Xu, J. Liu, M. J. Hernáiz and R. J. Linhardt, Chem. Rev., 2015, 115, 6811–6853 CrossRef PubMed; (b) P. RekhaáBoruah and A. AzizáAli, Chem. Commun., 2015, 51, 11489–11492 RSC.
- For C–H bond functionalization reactions directed by sulfoxides, see: (a) B. Wang, Y. Liu, C. Lin, Y. Xu, Z. Liu and Y. Zhang, Org. Lett., 2014, 16, 4574–4577 CrossRef CAS PubMed; (b) S. E. Ammann, G. T. Rice and M. C. White, J. Am. Chem. Soc., 2014, 136, 10834–10837 CrossRef CAS PubMed; (c) Z. Chen, B. Wang, J. Zhang, W. Yu, Z. Liu and Y. Zhang, Org. Chem. Front., 2015, 2, 1107–1295 RSC; (d) Q. Cai, D. K. Li, R. R. Zhou, S. Y. Zhuang, J. T. Ma, Y. D. Wu and A. X. Wu, J. Org. Chem., 2016, 81, 8104–8111 CrossRef CAS PubMed; (e) M. Y. Chang, Y. H. Huang and H. S. Wang, Tetrahedron, 2016, 72, 3022–3031 CrossRef CAS; (f) J. A. Fernández-Salas, A. J. Eberhart and D. J. Procter, J. Am. Chem. Soc., 2016, 138, 790–793 CrossRef PubMed.
- For sulfoxides as oxidant in C–H bond functionalization reactions, see: (a) T. J. Osberger and M. C. White, J. Am. Chem. Soc., 2014, 136, 11176–11181 CrossRef CAS PubMed; (b) D. L. Priebbenow and C. Bolm, Org. Lett., 2014, 16, 1650–1652 CrossRef CAS PubMed; (c) M. Lin, Z. Wang, H. Fang, L. Liu, H. Yin, C. H. Yan and X. Fu, RSC Adv., 2016, 6, 10861–10864 RSC; (d) T. Yanagi, S. Otsuka, Y. Kasuga, K. Fujimoto, K. Murakami, K. Nogi and A. Osuka, J. Am. Chem. Soc., 2016, 138, 14582–14585 CrossRef CAS PubMed.
- (a) D. Y. Lee, I. J. Kim and C. H. Jun, Angew. Chem., Int. Ed., 2002, 41, 3031–3033 CrossRef CAS; (b) D. Y. Lee, B. S. Hong, E. G. Cho, H. Lee and C. H. Jun, J. Am. Chem. Soc., 2003, 125, 6372–6373 CrossRef CAS PubMed.
- (a) K. Nobushige, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2014, 16, 1188–1191 CrossRef CAS PubMed; (b) Y. F. Liang, K. Wu, S. Song, X. Li, X. Huang and N. Jiao, Org. Lett., 2015, 17, 876–879 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra01403a |
| This journal is © The Royal Society of Chemistry 2017 |