Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of fluoro-polycarbonates containing aliphatic/aromatic segments on the characteristics of thermo-optic waveguide devices†
Zhenzhen Caia,
Qixuan Yua,
Yang Zhengc,
Xiaoyu Shia,
Xuesong Wanga,
Zhanchen Cui *ab,
Zuosen Shi*a,
Changming Chenc and
Daming Zhangc
*ab,
Zuosen Shi*a,
Changming Chenc and
Daming Zhangc
aState Key Laboratory of Supramolecular Structure and Materials, Jilin University, Changchun 130012, People's Republic of China. E-mail: cuizc@jlu.edu.cn; shizs@jlu.edu.cn
bDepartment of Chemistry and Pharmacy, Zhuhai College of Jilin University, Zhuhai 519041, People's Republic of China
cState Key Lab on Integrated Opto-electronics, Jilin University Region, Changchun 130012, People's Republic of China
First published on 31st March 2017
Abstract
Fluorinated polycarbonates containing dodecyl groups (AF-Ali-PC EPs), biphenyl groups (AF-Ar-PC EPs), and cross-linkable epoxy groups were synthesized to investigate the effects of the flexibility of monomers on the properties of thermo-optic waveguide devices. Transparent polymer films were prepared via photocuring of a solution (FPRs) containing AF-Ali-PC EPs or AF-Ar-PC EPs, a photo initiator, and cyclopentanone. The optical films have low surface energies and roughnesses, high thermal stabilities, and tunable refractive indices. Straight optical waveguide and MZI thermo-optic waveguide switch arrays were fabricated using Ali-PR 1 and Ar-PR 1. The propagation losses of the channel waveguides, measured by the cutback method, were 0.21 dB cm−1 and 0.19 dB cm−1 at the monitoring light of 1550 nm for the Ali-PR 1 and Ar-PR 1, respectively. The rise and fall times of the thermo-optic switch upon application of a 200 Hz square-wave voltage were 1.822 ms and 1.364 ms, respectively, for Ali-PRs and 2.994 ms and 2.301 ms, respectively, for Ar-PRs at the square-wave voltage of 110 Hz. The insertion loss and extinction ratio were measured to be 9.2 dB and 12.0 dB for the aliphatic segment-containing photoresists and 8.9 dB and 12.3 dB for the aromatic segment-containing photoresists, respectively. The applied electric powers were 15.0 mW and 20.0 mW, which were also the switching powers. In contrast with our previous work, fluoro-polycarbonates containing aliphatic segments were more suitable for optical waveguide devices than those containing aromatic segments.
Introduction
Optical switches, which are implemented to establish reconfigurable connections between multiple sources and multiple destinations, are essential building blocks for both long-haul optical communications and short-reach photonic networks-on-chips.1 Nowadays, optical switches and their related arrays are widely used in optical cross connects (OXCs), optical add-drop multiplexers (OADMs), optical network monitoring, and on-chip optical interconnections.2 It is desirable for these switches to have large optical bandwidths, small physical dimensions, and good performance.3 To realize these properties, various device structures such as Mach–Zehnder interferometer (MZI) switches, multimode interference (MMI) switches, and directional coupler (DC) switches have been employed.4The thermo-optic MZI is a basic device in planar light-wave circuits. In optical communication systems, it is widely used in optical modulators or switches because of its low optical loss, polarization independence, and long-term stability.5 Polymer materials have been intensively investigated due to their potential applications in optical waveguides because of their excellent characteristics4 such as low optical loss, high extinction ratio, small cubage, easy integration, high thermal and environmental stability, and flexible refractive index controllability.6 Various organic polymers including polyimides, acrylic polymers, polycarbonates, polystyrenes, polysiloxanes, hyperbranched polymers, perfluorinated methacrylates, and bis-phenol-A novolac resin (SU-8) have been tested as optical waveguide materials.7
In general, glass and silicon wafers are selected as substrates for the fabrication of optical waveguides. However, Si–O or Si–OH groups on the surfaces of the substrates result in highly polar surfaces; moreover, it is difficult to obtain good adhesion with polymers. To solve this problem, polar functional groups, such as carbonate groups, can be introduced to enhance the adhesion of the polymer layer to the substrates.7g Apart from this, the properties such as transparency, broad temperature resistance, inherent toughness, and high index of refraction of polycarbonates have increased their popularity in the optical waveguide field.8 However, polycarbonates have relatively high absorption losses at optical communication wavelengths (0.6 dB cm−1 at 1550 nm), resulting from the absorption of C–H bonds.7f Replacing C–H bonds with C–F bonds in polymers can reduce the intrinsic optical loss at the key telecommunication wavelengths of 1310 and 1550 nm.10 Fluorine-containing polymers exhibit good thermal and chemical stability and decreased intermolecular attractive forces (which results in better solubility) compared to hydrocarbon polymers.7g,10a,11 The refractive indices of the polymers that we have synthesized9 (AF-Z-PC EPs) can be adjusted by controlling the content of bisphenol Z. For AF-Z-PC EPs containing epoxy groups, we utilized a simple method to fabricate the waveguide devices. Encouraged by these results, we designed another experiment for further exploration.
The crystallinity of a polymer depends on its chain flexibility. Bisphenol-A polycarbonate (BAPC) presents poor crystallization because of its rigidity.12 In this study, a series of fluoro-polycarbonates containing aliphatic segments (AF-Ali-PC EPs) and biphenyl segments (AF-Ar-PC EPs) were synthesized with the intention of investigating the effects of the flexibility of the monomers on the characteristics of thermo-optic waveguide devices. AF-Ali-PC EPs and AF-Ar-PC EPs were mixed with cyclopentanone and a photoinitiator, respectively, to prepare a series of negative-type fluorinated photoresists (Ali-PRs and Ar-PRs, respectively). Through direct UV-writing, the photoresists could be fabricated into straight optical waveguide and MZI thermo-optic waveguide switch arrays. The propagation losses of the channel waveguides, measured by the cutback method, were 0.21 dB cm−1 and 0.19 dB cm−1 at the monitoring light of 1550 nm for the Ali-PR 1 and Ar-PR 1, respectively. The rise and fall times of the thermo-optic switch upon application of a 200 Hz square-wave voltage were 1.822 ms and 1.364 ms, respectively, for Ali-PRs and 2.994 ms and 2.301 ms, respectively, for Ar-PRs at the square-wave voltage of 110 Hz. The insertion loss and extinction ratio were measured to be 9.2 dB and 12.0 dB, respectively, for the aliphatic segment-containing photoresists and 8.9 dB and 12.3 dB, respectively, for the aromatic segment-containing photoresists. The applied electric power was 15.0 mW and 20.0 mW.
Experimental
Materials
4,4′-(Hexafluoroisopropylidene) diphenol (98%), 4,4′-dihydroxydiphenyl (97%), and triphosgene (99%) were purchased from Aladdin Industrial Co. and used as received. Dodecanedioic acid (99%) (Sigma-Aldrich), thionyl chloride (99.5%) (Fuchen Chemical Co., Ltd), and triphenylsulfonium hexafluorophosphate (Sigma-Aldrich) were used as received without further purification. Epoxy chloropropane, pyridine, and dichloromethane were purchased from Beijing Chemical Co., Ltd and were used after purification.Measurements
IR spectrwerea were obtained via an AVATAR 360 spectrophotometer using KBr pellets. UV-vis-NIR spectra were acquired using a SHIMADZU UV-3600 spectrometer. 1H NMR, 13C NMR, and 19F NMR spectra were obtained via a Bruker AVANCE NMR spectrometer, operated at 500, 125, or 300 MHz, respectively. 1H, 13C, and 19F chemical shifts have been reported as values (ppm) relative to CDCl3 (7.24). The molecular weights of the polymers were determined relative to polystyrene standards using gel permeation chromatography (GPC) in THF at room temperature. Thermal analyses were conducted via a NETZSCH 4 differential scanning calorimeter (DSC) and a Perkin-Elmer TGA 7 thermogravimetric analyzer (TGA) at the heating rate of 10 °C min−1 under a nitrogen atmosphere. Cross-section images of the specimens were obtained via a scanning electron microscope (JEOL FESEM 6700F). An M-2000VI ellipsometer was used to determine the refractive indices of the films. The surface morphologies of the films were investigated under ambient conditions using a Digital Instruments Nanoscope IIIa instrument.Synthesis of the polymers
AF-Ali-PC OH 1: 6FBPA (13.44 g, 40 mmol), pyridine (6.0 mL), and triphosgene (3.71 g, 12.5 mmol). AF-Ali-PC OH 1 was obtained as a white powder (11.59 g, 65%). GPC: Mn = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 892 g mol−1; Mw/Mn = 1.20. IR (KBr, cm−1): γ(C–F) = 1177–1239, γ(aromatic) = 1607 and 1509, γ(CO–O–Ar) = 1777, γ(–CH2–) = 1712 and 732. 1H NMR (500 MHz, CDCl3): δ (ppm): 7.51–7.50 (t, 11H, ArH), 7.44–7.43 (t, 1H, ArH), 7.37–7.36 (t, 11H, ArH), 7.16–7.13 (t, 1H, ArH), 2.62–2.59 (m, 1H, –CH2), 1.82–1.75 (m, 1H, –CH2), 1.44–1.36 (m, 3H, –CH2). 13C NMR (125 MHz, CDCl3), δ (ppm): 171.93, 151.22, 151.17, 131.69, 131.28, 123.94, 121.53, 120.77, 120.52, 115.16, 64.00, 34.37, 29.39, 29.23, 29.07, 24.86. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −63.87 (6F, –CF3).
892 g mol−1; Mw/Mn = 1.20. IR (KBr, cm−1): γ(C–F) = 1177–1239, γ(aromatic) = 1607 and 1509, γ(CO–O–Ar) = 1777, γ(–CH2–) = 1712 and 732. 1H NMR (500 MHz, CDCl3): δ (ppm): 7.51–7.50 (t, 11H, ArH), 7.44–7.43 (t, 1H, ArH), 7.37–7.36 (t, 11H, ArH), 7.16–7.13 (t, 1H, ArH), 2.62–2.59 (m, 1H, –CH2), 1.82–1.75 (m, 1H, –CH2), 1.44–1.36 (m, 3H, –CH2). 13C NMR (125 MHz, CDCl3), δ (ppm): 171.93, 151.22, 151.17, 131.69, 131.28, 123.94, 121.53, 120.77, 120.52, 115.16, 64.00, 34.37, 29.39, 29.23, 29.07, 24.86. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −63.87 (6F, –CF3).
AF-Ali-PC OH 2: 6FBPA (7.392 g, 22 mmol), pyridine (3.6 mL), and triphosgene (2.227 g, 7.5 mmol). AF-Ali-PC OH 2 was obtained as a white powder (7.94 g, 68%). GPC: Mn = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 541 g mol−1; Mw/Mn = 1.31. IR (KBr, cm−1): γ(C–F) = 1174–1242, γ(aromatic) = 1605 and 1508, γ(CO–O–Ar) = 1777, γ(–CH2–) = 1709 and 736. 1H NMR (500 MHz, CDCl3), δ (ppm): 7.51–7.50 (t, 8H, ArH), 7.44–7.43 (t, 1H, ArH), 7.37–7.35 (t, 8H, ArH), 7.16–7.14 (t, 1H, ArH), 2.62–2.59 (m, 1H, –CH2), 1.81–1.75 (m, 1H, –CH2), 1.44–1.36 (m, 3H, –CH2). 13C NMR (125 MHz, CDCl3), δ (ppm): 172.01, 151.22, 131.69, 131.39, 131.28, 122.17, 121.48, 120.77, 120.52, 115.18, 64.00, 34.37, 29.39, 29.23, 29.07, 24.86. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −63.87 (6F, –CF3).
541 g mol−1; Mw/Mn = 1.31. IR (KBr, cm−1): γ(C–F) = 1174–1242, γ(aromatic) = 1605 and 1508, γ(CO–O–Ar) = 1777, γ(–CH2–) = 1709 and 736. 1H NMR (500 MHz, CDCl3), δ (ppm): 7.51–7.50 (t, 8H, ArH), 7.44–7.43 (t, 1H, ArH), 7.37–7.35 (t, 8H, ArH), 7.16–7.14 (t, 1H, ArH), 2.62–2.59 (m, 1H, –CH2), 1.81–1.75 (m, 1H, –CH2), 1.44–1.36 (m, 3H, –CH2). 13C NMR (125 MHz, CDCl3), δ (ppm): 172.01, 151.22, 131.69, 131.39, 131.28, 122.17, 121.48, 120.77, 120.52, 115.18, 64.00, 34.37, 29.39, 29.23, 29.07, 24.86. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −63.87 (6F, –CF3).
AF-Ali-PC OH 3: 6FBPA (3.36 g, 10 mmol), pyridine (2.0 mL), triphosgene (1.238 g, 4.17 mmol). AF-Ali-PC OH 3 was obtained as a white powder (5.04 g, 66%). GPC: Mn = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 568 g mol−1; Mw/Mn = 1.35. IR (KBr, cm−1): γ(C–F) = 1163–1239, γ(aromatic) = 1612 and 1515, γ(CO–O–Ar) = 1784, γ(–CH2) = 1715 and 736. 1H NMR (500 MHz, CDCl3), δ (ppm): 7.51–7.50 (t, 5H, ArH), 7.44–7.43 (t, 1H, ArH), 7.37–7.36 (t, 5H, ArH), 7.16–7.14 (t, 1H, ArH), 2.62–2.59 (m, 1H, –CH2), 1.81–1.75 (m, 1H, –CH2), 1.44–1.36 (m, 3H, –CH2). 13C NMR (125 MHz, CDCl3), δ (ppm): 171.94, 151.22, 151.16, 131.70, 131.40, 130.33, 123.95, 121.54, 120.78, 115.15, 64.00, 34.37, 29.39, 29.24, 29.08, 24.86. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −63.87 (6F, –CF3).
568 g mol−1; Mw/Mn = 1.35. IR (KBr, cm−1): γ(C–F) = 1163–1239, γ(aromatic) = 1612 and 1515, γ(CO–O–Ar) = 1784, γ(–CH2) = 1715 and 736. 1H NMR (500 MHz, CDCl3), δ (ppm): 7.51–7.50 (t, 5H, ArH), 7.44–7.43 (t, 1H, ArH), 7.37–7.36 (t, 5H, ArH), 7.16–7.14 (t, 1H, ArH), 2.62–2.59 (m, 1H, –CH2), 1.81–1.75 (m, 1H, –CH2), 1.44–1.36 (m, 3H, –CH2). 13C NMR (125 MHz, CDCl3), δ (ppm): 171.94, 151.22, 151.16, 131.70, 131.40, 130.33, 123.95, 121.54, 120.78, 115.15, 64.00, 34.37, 29.39, 29.24, 29.08, 24.86. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −63.87 (6F, –CF3).
AF-Ar-PC OH 1: 6FBPA (16.13 g, 48 mmol), pyridine (7.3 mL), and triphosgene (4.46 g, 15 mmol). AF-Ar-PC OH 1 was obtained as a white powder (11.22 g, 61%). GPC: Mn = 9894 g mol−1; Mw/Mn = 1.32. IR (KBr, cm−1): γ(C–F) = 1173–1211, γ(aromatic) = 1609 and 1512, γ(CO–O–Ar) = 1782. 1H NMR (500 MHz, CDCl3): δ (ppm): 7.66–7.64 (t, 3H, ArH), 7.51–7.49 (t, 17H, ArH), 7.40–7.35 (m, 20H, ArH), 7.27 (s, 1H, ArH), 6.82 (s, 1H, ArH). 13C NMR (125 MHz, CDCl3), δ (ppm): 156.20, 151.33, 151.22, 150.44, 138.50, 131.69, 131.27, 128.38, 123.95, 121.26, 120.76, 120.52, 115.17, 64.01. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −63.86 (6F, –CF3).
AF-Ar-PC OH 2: 6FBPA (12.10 g, 36 mmol), pyridine (5.65 mL), and triphosgene (3.47 g, 11.7 mmol). AF-Ar-PC OH 2 was obtained as a white powder (8.33 g, 59%). GPC: Mn = 8057 g mol−1; Mw/Mn = 1.29. IR (KBr, cm−1): γ(C–F) = 1176–1215, γ(aromatic) = 1612 and 1512, γ(CO–O–Ar) = 1776. 1H NMR (500 MHz, CDCl3): δ (ppm): 7.66–7.64 (t, 3H, ArH), 7.51–7.50 (t, 12H, ArH), 7.40–7.36 (m, 15H, ArH), 7.27 (s, 1H, ArH), 6.81 (s, 1H, ArH). 13C NMR (125 MHz, CDCl3), δ (ppm): 156.25, 151.34, 151.22, 150.46, 138.50, 131.69, 131.29, 128.38, 123.94, 121.33, 121.26, 120.76, 115.17, 63.97. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −63.87 (6F, –CF3).
AF-Ar-PC OH 3: 6FBPA (8.06 g, 24 mmol), pyridine (4.02 mL), triphosgene (2.48 g, 8.33 mmol). AF-Ar-PC OH 3 was obtained as a white powder (5.50 g, 55%). GPC: Mn = 7900 g mol−1; Mw/Mn = 1.34. IR (KBr, cm−1): γ(C–F) = 1173–1211, γ(aromatic) = 1612 and 1512, γ(CO–O–Ar) = 1782. 1H NMR (500 MHz, CDCl3): δ (ppm): 7.66–7.64 (t, 4H, ArH), 7.51–7.50 (t, 10H, ArH), 7.40–7.36 (m, 14H, ArH), 7.27 (s, 1H, ArH), 6.80 (s, 1H, ArH). 13C NMR (125 MHz, CDCl3), δ (ppm): 156.22, 151.34, 151.23, 150.47, 138.51, 131.69, 131.28, 128.38, 123.95, 121.36, 120.83, 120.77, 115.16, 64.01. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −63.86 (6F, –CF3).
AF-Ali-PC EP 1:AF-Ali-PC OH 1 (11.59 g, 0.97 mmol), epoxy chloropropane (40 g, 0.432 mol), and sodium hydroxide (0.2 g, 5 mmol). PEC-EP-1 was obtained as a light yellow viscous liquid (11.03 g, 96%). GPC: Mn = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 001 g mol−1; Mw/Mn = 1.19. IR (KBr, cm−1): γ(C–F) = 1170–1251, γ(aromatic) = 1612 and 1516, γ(CO–O–Ar) = 1750, γ(–CH2) = 1715 and 736, γ(epoxy group) = 826. 1H NMR (500 MHz, CDCl3), δ (ppm): 7.34–7.32 (t, 34H, ArH), 6.94–6.92 (t, 34H, ArH), 4.30–4.29 (m, 1H, –CH2), 4.00–3.96 (m, 1H, –CH2), 3.40–3.37 (m, 1H, –CH), 2.95–2.93 (m, 1H, –CH2), 2.79–2.78 (m, 1H, –CH2), 2.41–2.38 (m, 2H, –CH2), 1.70–1.64 (m, 2H, –CH2), 1.32–1.29 (m, 6H, –CH2). 13C NMR (125 MHz, CDCl3), δ (ppm): 171.93, 158.65, 158.15, 153.64, 131.59, 131.51, 126.46, 125.96, 124.35, 114.15, 68.80, 63.55, 50.01, 44.64, 34.17, 29.34, 29.17, 29.01, 24.88. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −64.16 (6F, –CF3).
001 g mol−1; Mw/Mn = 1.19. IR (KBr, cm−1): γ(C–F) = 1170–1251, γ(aromatic) = 1612 and 1516, γ(CO–O–Ar) = 1750, γ(–CH2) = 1715 and 736, γ(epoxy group) = 826. 1H NMR (500 MHz, CDCl3), δ (ppm): 7.34–7.32 (t, 34H, ArH), 6.94–6.92 (t, 34H, ArH), 4.30–4.29 (m, 1H, –CH2), 4.00–3.96 (m, 1H, –CH2), 3.40–3.37 (m, 1H, –CH), 2.95–2.93 (m, 1H, –CH2), 2.79–2.78 (m, 1H, –CH2), 2.41–2.38 (m, 2H, –CH2), 1.70–1.64 (m, 2H, –CH2), 1.32–1.29 (m, 6H, –CH2). 13C NMR (125 MHz, CDCl3), δ (ppm): 171.93, 158.65, 158.15, 153.64, 131.59, 131.51, 126.46, 125.96, 124.35, 114.15, 68.80, 63.55, 50.01, 44.64, 34.17, 29.34, 29.17, 29.01, 24.88. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −64.16 (6F, –CF3).
AF-Ali-PC EP 2:AF-Ali-PC OH 2 (7.94 g, 0.69 mmol), epoxy chloropropane (27 g, 0.276 mol), and sodium hydroxide (0.138 g, 3.45 mmol). PEC-EP-2 was obtained as a light yellow viscous liquid (7.78 g, 96.8%). GPC: Mn = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 655 g mol−1; Mw/Mn = 1.29. IR (KBr, cm−1): γ(C–F) = 1170–1256, γ(aromatic) = 1605 and 1516, γ(CO–O–Ar) = 1753, γ(–CH2) = 736, γ(epoxy group) = 826. 1H NMR (500 MHz, CDCl3), δ (ppm): 7.34–7.32 (t, 20H, ArH), 6.94–6.92 (t, 20H, ArH), 4.29–4.28 (m, 1H, –CH2), 3.99–3.96 (m, 1H, –CH2), 3.39 (s, 1H, –CH), 2.94 (s, 1H, –CH2), 2.79 (s, 1H, –CH2), 2.39 (s, 2H, –CH2), 1.67–1.66 (m, 2H, –CH2), 1.29 (s, 6H, –CH2). 13C NMR (125 MHz, CDCl3), δ (ppm): 173.03, 158.65, 158.40, 158.15, 153.64, 131.51, 127.78, 125.96, 124.35, 114.15, 68.49, 63.55, 50.01, 44.63, 34.17, 29.34, 29.17, 29.01, 24.87. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −64.16 (6F, –CF3).
655 g mol−1; Mw/Mn = 1.29. IR (KBr, cm−1): γ(C–F) = 1170–1256, γ(aromatic) = 1605 and 1516, γ(CO–O–Ar) = 1753, γ(–CH2) = 736, γ(epoxy group) = 826. 1H NMR (500 MHz, CDCl3), δ (ppm): 7.34–7.32 (t, 20H, ArH), 6.94–6.92 (t, 20H, ArH), 4.29–4.28 (m, 1H, –CH2), 3.99–3.96 (m, 1H, –CH2), 3.39 (s, 1H, –CH), 2.94 (s, 1H, –CH2), 2.79 (s, 1H, –CH2), 2.39 (s, 2H, –CH2), 1.67–1.66 (m, 2H, –CH2), 1.29 (s, 6H, –CH2). 13C NMR (125 MHz, CDCl3), δ (ppm): 173.03, 158.65, 158.40, 158.15, 153.64, 131.51, 127.78, 125.96, 124.35, 114.15, 68.49, 63.55, 50.01, 44.63, 34.17, 29.34, 29.17, 29.01, 24.87. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −64.16 (6F, –CF3).
AF-Ali-PC EP 3:AF-Ali-PC OH 3 (5.04 g, 0.43 mmol), epoxy chloropropane (16 g, 0.172 mol), and sodium hydroxide (0.086 g, 2.15 mmol). PEC-EP-3 was obtained as a light yellow viscous liquid (4.88 g, 97.1%). GPC: Mn = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 683 g mol−1; Mw/Mn = 1.34. IR (KBr, cm−1): γ(C–F) = 1170–1246, γ(aromatic) = 1612 and 1516, γ(CO–O–Ar) = 1743, γ(–CH2) = 743, γ(epoxy group) = 826. 1H NMR (500 MHz, CDCl3), δ (ppm): 7.34–7.32 (t, 12H, ArH), 6.94–6.92 (t, 12H, ArH), 4.30–4.27 (m, 1H, –CH2), 4.00–3.97 (m, 1H, –CH2), 3.40–3.37 (m, 1H, –CH), 2.95–2.93 (m, 1H, –CH2), 2.80–2.78 (m, 1H, –CH2), 2.41–2.37 (m, 2H, –CH2), 1.68–1.66 (m, 2H, –CH2), 1.31–1.29 (m, 6H, –CH2). 13C NMR (125 MHz, CDCl3), δ (ppm): 173.03, 158.64, 158.36, 158.14, 153.60, 131.51, 126.22, 125.97, 124.35, 114.14, 68.79, 63.55, 50.01, 44.65, 34.17, 29.34, 29.17, 29.01, 24.87. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −64.17 (6F, –CF3).
683 g mol−1; Mw/Mn = 1.34. IR (KBr, cm−1): γ(C–F) = 1170–1246, γ(aromatic) = 1612 and 1516, γ(CO–O–Ar) = 1743, γ(–CH2) = 743, γ(epoxy group) = 826. 1H NMR (500 MHz, CDCl3), δ (ppm): 7.34–7.32 (t, 12H, ArH), 6.94–6.92 (t, 12H, ArH), 4.30–4.27 (m, 1H, –CH2), 4.00–3.97 (m, 1H, –CH2), 3.40–3.37 (m, 1H, –CH), 2.95–2.93 (m, 1H, –CH2), 2.80–2.78 (m, 1H, –CH2), 2.41–2.37 (m, 2H, –CH2), 1.68–1.66 (m, 2H, –CH2), 1.31–1.29 (m, 6H, –CH2). 13C NMR (125 MHz, CDCl3), δ (ppm): 173.03, 158.64, 158.36, 158.14, 153.60, 131.51, 126.22, 125.97, 124.35, 114.14, 68.79, 63.55, 50.01, 44.65, 34.17, 29.34, 29.17, 29.01, 24.87. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −64.17 (6F, –CF3).
AF-Ar-PC EP 1:AF-Ar-PC OH 1 (11.22 g, 1.13 mmol), epoxy chloropropane (46 g, 0.503 mol), and sodium hydroxide (0.23 g, 5.8 mmol). AF-Ar-PC EP 1 was obtained as a light yellow viscous liquid (10.76 g, 97%). GPC: Mn = 9923 g mol−1; Mw/Mn = 1.30. IR (KBr, cm−1): γ(C–F) = 1176–1254, γ(aromatic) = 1609 and 1516, γ(CO–O–Ar) = 1754, γ(epoxy group) = 829. 1H NMR (500 MHz, CDCl3): δ (ppm): 7.50–7.49 (t, 3H, ArH), 7.34–7.32 (t, 20H, ArH), 7.01–6.99 (t, 3H, ArH), 6.93–6.92 (t, 20H, ArH), 4.26–4.23 (m, 2H, –CH2), 3.94–3.90 (m, 2H, –CH2), 3.28–3.24 (m, 2H, –CH), 2.92–2.90 (m, 2H, –CH2), 2.71–2.70 (m, 2H, –CH2). 13C NMR (125 MHz, CDCl3), δ (ppm): 158.64, 158.15, 157.68, 153.64, 133.84, 131.58, 131.51, 127.79, 126.39, 125.95, 124.35, 114.94, 114.15, 68.78, 63.64, 50.03, 44.66. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −64.15 (6F, –CF3).
AF-Ar-PC EP 2:AF-Ar-PC OH 2 (8.33 g, 1.03 mmol), epoxy chloropropane (43 g, 0.459 mol), and sodium hydroxide (0.21 g, 5.31 mmol). AF-Ar-PC EP 2 was obtained as a light yellow viscous liquid (7.98 g, 97.2%). GPC: Mn = 8101 g mol−1; Mw/Mn = 1.28. IR (KBr, cm−1): γ(C–F) = 1176–1254, γ(aromatic) = 1612 and 1516, γ(CO–O–Ar) = 1759, γ(epoxy group) = 829. 1H NMR (500 MHz, CDCl3): δ (ppm): 7.50–7.49 (t, 9H, ArH), 7.34–7.33 (t, 43H, ArH), 7.01–6.99 (t, 9H, ArH), 6.94–6.92 (t, 43H, ArH), 4.25–4.23 (m, 2H, –CH2), 3.95–3.92 (m, 2H, –CH2), 3.27–3.24 (m, 2H, –CH), 2.93–2.89 (m, 2H, –CH2), 2.71–2.69 (m, 2H, –CH2). 13C NMR (125 MHz, CDCl3), δ (ppm): 158.66, 158.17, 157.70, 153.64, 133.66, 133.83, 131.58, 131.51, 127.78, 125.94, 124.37, 114.96, 114.17, 114.11, 68.79, 63.56, 50.04, 44.62. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −64.16 (6F, –CF3).
AF-Ar-PC EP 3:AF-Ar-PC OH 3 (5.50 g, 0.696 mmol), epoxy chloropropane (29 g, 0.310 mol), and sodium hydroxide (0.144 g, 3.59 mmol). AF-Ar-PC EP 3 was obtained as a light yellow viscous liquid (5.23 g, 96.5%). GPC: Mn = 7954 g mol−1; Mw/Mn = 1.32. IR (KBr, cm−1): γ(C–F) = 1176–1254, γ(aromatic) = 1612 and 1516, γ(CO–O–Ar) = 1755, γ(epoxy group) = 829. 1H NMR (500 MHz, CDCl3): δ (ppm): 7.50–7.49 (t, 11H, ArH), 7.34–7.32 (t, 27H, ArH), 7.01–6.99 (t, 11H, ArH), 6.94–6.92 (t, 27H, ArH), 4.27–4.25 (m, 2H, –CH2), 3.94–3.91 (m, 2H, –CH2), 3.28–3.23 (m, 2H, –CH), 2.95–2.94 (m, 2H, –CH2), 2.72–2.69 (m, 2H, –CH2). 13C NMR (125 MHz, CDCl3), δ (ppm): 158.64, 158.14, 157.69, 153.63, 133.89, 131.60, 131.51, 127.80, 125.98, 125.48, 124.34, 114.95, 114.15, 68.79, 63.75, 50.01, 44.74. 19F NMR (470 MHz, CDCl3, CFCl3), δ (ppm): −64.16 (6F, –CF3).
Preparation of the polymer films and their exposure to UV light
To determine the optical properties and fabricate the optical waveguide, photoresist solutions were prepared by dissolving synthesized AF-Ali-PC EPs or AF-Ar-PC EPs and initiator in cyclopentanone to form 30 to 60 wt% solutions (FPRs (Ali-PR 1–3 and Ar-PR 1–3)). FPRs were filtered using a 0.2 μm PTFE membrane to remove large particulates and spin-coated onto Si/SiO2 wafer substrates (500 r/s 9 s and 1000 r/s 30 s). The spin-coated films were dried on a hot plate for 30 min (60 °C) and then in a vacuum oven for 12 h to remove the residual solvent. Then, the films were irradiated with UV light for 6 min and post-baked at 120 °C for 30 min to afford highly transparent films.Fabrication of the straight waveguide
To study the applications of Ali-PRs and Ar-PRs in optical waveguides, a straight waveguide was fabricated according to a previously reported method.14 To determine the propagation loss of the FPRs, a straight waveguide structure was fabricated on a Si/SiO2 substrate via direct UV-writing, as shown in Fig. 1(a). After spinning the core solution (FPRs) onto a Si/SiO2 wafer at 1000 r/s for 30 s, the coated layers (3–4 μm in thickness) were dried at 110 °C for 90 min to remove the residual solvent. Then, the films were exposed to UV light for 6 min through a contact chromium mask and post-baked at 120 °C for 30 min. After this, the non-cross-linked FPRs were removed by development in propylene glycol methyl ether acetate (PGMEA) for 5 s at room temperature, followed by rinsing the wafer in isopropanol. For the upper cladding layer, PMMA was selected. PMMA was spin-coated onto the core layer and baked at 125 °C for 1 h to form the cladding film.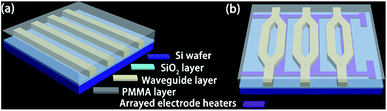 | ||
| Fig. 1 Schematic of waveguide devices: (a) channel waveguide and (b) MZI thermo-optic waveguide switch arrays. | ||
Fabrication of the MZI thermo-optic waveguide switch arrays
The MZI thermo-optic waveguide switch array device was fabricated, as described in Fig. 1(b). First, the gold stripes were patterned by photolithography and wet etching. Second, as described in the “fabrication of the straight waveguide” section in this article, the FPRs were spin-coated onto the gold electrode to form the core layer. Then, a PMMA thin film was formed as the upper cladding layer and was then baked at 125 °C for 1 h.Results and discussion
Synthesis of the polymers
Epoxy-terminated fluoro-polycarbonates containing aliphatic segments (AF-Ali-PC EPs) and epoxy-terminated fluoro-polycarbonates containing aromatic segments (AF-Ar-PC EPs) were synthesized according to Scheme 1 and 2, respectively. The purpose of the functionalization was to incorporate functional groups, such as aliphatic segments, and aromatic segments, into fluoro-polycarbonates chain bearing epoxy units as cross-linking sites.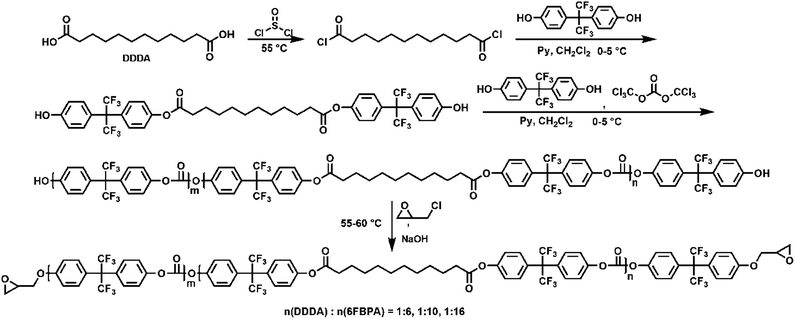 | ||
| Scheme 1 The preparation of epoxy-terminated fluoro-polycarbonates containing aliphatic segments (AF-Ali-PC EPs). | ||
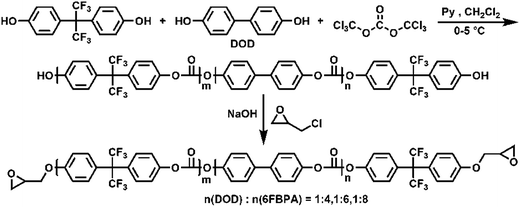 | ||
| Scheme 2 The preparation of epoxy-terminated fluoro-polycarbonates containing aromatic segments (AF-Ar-PC EPs). | ||
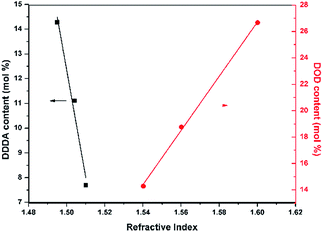
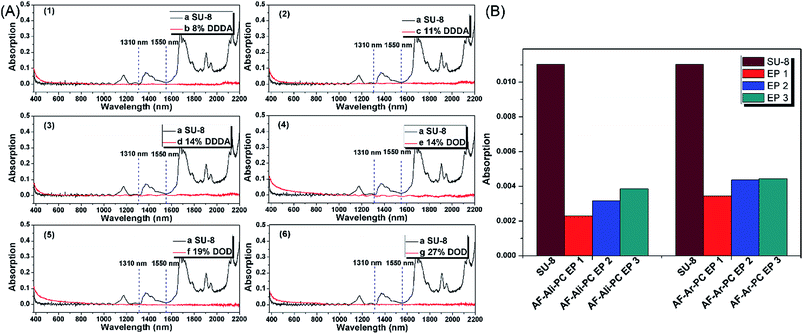
To acquire AF-Ali-PC EPs, we first synthesized hydroxyl-terminated fluoro-polycarbonates containing aliphatic segments (AF-Ali-PC OHs). For the purpose of obtaining AF-Ali-PC OHs, we controlled the molar feed ratio of (6FBPA + DDDA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTC at 18
BTC at 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The structure of AF-Ali-PC OHs was determined by 1H NMR, 13C, and DEPT 135 NMR spectroscopy. In the 1H NMR spectra (Fig. S1†), the signals of dodecyl in AF-Ali-PC OHs were observed at 2.62–2.59, 1.82–1.75, and 1.44–1.36 ppm. In the 13C and DEPT 135 NMR spectra (Fig. S2†), the characteristic peaks of dodecyl in AF-Ali-PC OHs were observed at 34.37, 29.39, 29.24, 29.08, and 24.86 ppm; the –CF3 peaks were observed at 127.37, 125.10, 122.80, and 120.81 ppm; and the –C
5. The structure of AF-Ali-PC OHs was determined by 1H NMR, 13C, and DEPT 135 NMR spectroscopy. In the 1H NMR spectra (Fig. S1†), the signals of dodecyl in AF-Ali-PC OHs were observed at 2.62–2.59, 1.82–1.75, and 1.44–1.36 ppm. In the 13C and DEPT 135 NMR spectra (Fig. S2†), the characteristic peaks of dodecyl in AF-Ali-PC OHs were observed at 34.37, 29.39, 29.24, 29.08, and 24.86 ppm; the –CF3 peaks were observed at 127.37, 125.10, 122.80, and 120.81 ppm; and the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak was observed at 151.2 ppm. The compositions of the copolymers (AF-Ali-PC OHs), calculated from their 1H NMR spectra, were 6FBPA/DDDA = 12
O peak was observed at 151.2 ppm. The compositions of the copolymers (AF-Ali-PC OHs), calculated from their 1H NMR spectra, were 6FBPA/DDDA = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8
1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 6
1, and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 according to the feed ratios. In the next step, we synthesized AF-Ali-PC EPs with the AF-Ali-PC OHs and epoxy chloropropane. In the 1H NMR spectra (Fig. S1†), the signals of the epoxy group in AF-Ali-PC EPs were observed at 4.30–4.27, 3.97–4.00, 3.37–3.40, 2.93–2.95, and 2.78–2.80 ppm; in the 13C and DEPT 135 NMR spectra (Fig. S2†), the characteristic peaks of the epoxy group were observed at 68.79, 50.01, and 44.65 ppm. These results suggest that the DDDA content of the fluoro-polycarbonates containing aliphatic segments could be readily controlled by adjusting the (6FBPA + DDDA)
1 according to the feed ratios. In the next step, we synthesized AF-Ali-PC EPs with the AF-Ali-PC OHs and epoxy chloropropane. In the 1H NMR spectra (Fig. S1†), the signals of the epoxy group in AF-Ali-PC EPs were observed at 4.30–4.27, 3.97–4.00, 3.37–3.40, 2.93–2.95, and 2.78–2.80 ppm; in the 13C and DEPT 135 NMR spectra (Fig. S2†), the characteristic peaks of the epoxy group were observed at 68.79, 50.01, and 44.65 ppm. These results suggest that the DDDA content of the fluoro-polycarbonates containing aliphatic segments could be readily controlled by adjusting the (6FBPA + DDDA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTC feed ratios.
BTC feed ratios.
Epoxy-terminated fluoro-polycarbonates containing aromatic segments (AF-Ar-PC EPs) were synthesized according to Scheme 2. The intermediate hydroxyl-terminated fluoro-polycarbonates containing aromatic segments (AF-Ar-PC OHs) were synthesized by controlling the molar feed ratio of DOD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTC at 18
BTC at 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; the structures were determined by 1H NMR, 13C, and DEPT 135 NMR spectroscopy. In the 1H NMR spectra (Fig. S7†), the signals of diphenyl in AF-Ar-PC OHs were observed at 7.66–7.64 and 7.40–7.36 ppm. In the 13C and DEPT 135 NMR spectra (Fig. S8†), the characteristic peaks of diphenyl in AF-Ar-PC OHs were observed at 121.26 and 131.27 ppm; the –CF3 peaks were observed at 127.36, 125.09, 122.81, and 120.80 ppm; and the –C
5; the structures were determined by 1H NMR, 13C, and DEPT 135 NMR spectroscopy. In the 1H NMR spectra (Fig. S7†), the signals of diphenyl in AF-Ar-PC OHs were observed at 7.66–7.64 and 7.40–7.36 ppm. In the 13C and DEPT 135 NMR spectra (Fig. S8†), the characteristic peaks of diphenyl in AF-Ar-PC OHs were observed at 121.26 and 131.27 ppm; the –CF3 peaks were observed at 127.36, 125.09, 122.81, and 120.80 ppm; and the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak was observed at 151.22 ppm. The compositions of the copolymers (AF-Ar-PC OHs), calculated from their 1H NMR spectra, were 6FBPA/DOD = 6
O peak was observed at 151.22 ppm. The compositions of the copolymers (AF-Ar-PC OHs), calculated from their 1H NMR spectra, were 6FBPA/DOD = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4.33
1, 4.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2.75
1, and 2.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 according to the feed ratios. The end products AF-Ali-PC EPs were synthesized using AF-Ali-PC OHs and epoxy chloropropane. In the 1H NMR spectra (Fig. S7†), the signals of the epoxy groups in AF-Ar-PC EPs were observed at 4.26–4.23, 3.94–3.90, 3.28–3.24, 2.92–2.90, and 2.718–2.70 ppm; in the 13C and DEPT 135 NMR spectra (Fig. S8†), the characteristic peaks of the epoxy group were observed at 68.79, 50.04, and 44.62 ppm. These results suggest that the DOD content of the fluoro-polycarbonates containing aromatic segments could be readily controlled by adjusting the (6FBPA + DOD)
1 according to the feed ratios. The end products AF-Ali-PC EPs were synthesized using AF-Ali-PC OHs and epoxy chloropropane. In the 1H NMR spectra (Fig. S7†), the signals of the epoxy groups in AF-Ar-PC EPs were observed at 4.26–4.23, 3.94–3.90, 3.28–3.24, 2.92–2.90, and 2.718–2.70 ppm; in the 13C and DEPT 135 NMR spectra (Fig. S8†), the characteristic peaks of the epoxy group were observed at 68.79, 50.04, and 44.62 ppm. These results suggest that the DOD content of the fluoro-polycarbonates containing aromatic segments could be readily controlled by adjusting the (6FBPA + DOD)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTC feed ratio.
BTC feed ratio.
The properties of the cross-linking films
| Polymer | Molar feed ratio 6FBPA to DDDA/DOD | Actual molar ratio 6FBPA to DDDA/DOD | Tga (°C) | Tdb (°C) | Tdc (°C) | Gel fractiond (%) |
|---|---|---|---|---|---|---|
| a Determined at the heating rate of 10 °C min−1 under nitrogen by DSC.b Defined as the weight loss of the non-cross-linked polymers at 5 wt%, which was determined at the heating rate of 10 °C min−1 under nitrogen by TGA.c Defined as the weight loss of the cross-linked polymers at 5 wt%, which was determined at the heating rate of 10 °C min−1 under nitrogen by TGA.d Calculated from the weight ratio of the cross-linked polymers from chloroform: initial weight. | ||||||
| AF-Ali-PC EP 1 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
22.8 | 268 | 305 | 99.98 |
| AF-Ali-PC EP 2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
22.0 | 260 | 313 | 99.96 |
| AF-Ali-PC EP 3 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
21.8 | 272 | 306 | 99.97 |
| AF-Ar-PC EP 1 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7.3 | 274 | 299 | 99.95 |
| AF-Ar-PC EP 2 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8.4 | 277 | 303 | 99.97 |
| AF-Ar-PC EP 3 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
13.5 | 263 | 308 | 99.96 |
The thermal properties of the FPRs were evaluated by TGA and DSC measurements (Fig. S5, S6, S11 and S12†). The thermal decomposition behaviors and glass transition temperatures were measured under nitrogen, and the relevant results are summarized in Table 1. Weight losses of less than 5% were observed up to 260 °C before cross-linking and up to 305 °C after cross-linking for Ali-PRs, whereas those for Ar-PRs were observed up to 263 °C before cross-linking and up to 299 °C after cross-linking. The Tgs of the non-cross-linked FPRs were observed in the range of 7.3–22.8 °C. Since the flexibility of the polymer chains decreased through UV light curing, no Tg was observed after cross-linking. These results indicate that all the FPRs have excellent thermal stabilities, which are sufficient for practical applications in optical waveguides.
Straight optical waveguide and propagation loss measurements
Typical near-field patterns of the straight waveguide at the wavelength of 1550 nm are shown in Fig. 5(A) and (B) for Ali-PR 1 and Ar-PR 1, respectively. The propagation losses at 1550 nm, which were obtained from the slope of the optical insertion loss versus the waveguide length curves by the cut-back method, were 0.21 dB cm−1 and 0.19 dB cm−1.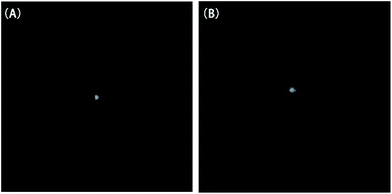 | ||
| Fig. 5 Near-field patterns of the straight waveguide at 1550 nm (A) for Ali-PR 1 and (B) for Ar-PR 1. | ||
MZI thermo-optic waveguide switch arrays and thermo-optic characterization
Fig. 6(A) and (B) show the results of the thermo-optic switching response. The rise and fall times of the thermo-optic switch upon application of a 200 Hz square-wave voltage were 1.822 ms and 1.364 ms, respectively, for Ali-PRs and 2.994 ms and 2.301 ms, respectively, for Ar-PRs at the square-wave voltage of 110 Hz. The insertion loss and extinction ratio were measured to be 9.2 dB and 12.0 dB for the aliphatic segment-containing photoresists and 8.9 dB and 12.3 dB for the aromatic segment-containing waveguides, respectively. The applied electric powers were 15.0 mW and 20.0 mW, which were also the switching powers. Combined with our previous work9 (AF-Z-PC EPs: insertion loss 9.0 dB, rise and fall times 1.546 ms and 1.226 ms (200 Hz), switching power 15.5 mW, and extinction ratio 13.0 dB), we can predict that the fluoro-polycarbonates containing aliphatic segments are more suitable for thermo-optic waveguide switches than those containing aromatic chains.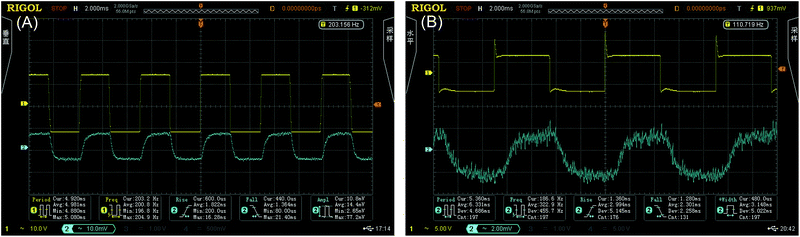 | ||
| Fig. 6 Thermo-optic switch responses obtained by applying a square-wave voltage at the frequencies of 200 Hz for Ali-PR 1 (A) and at the 110 Hz for Ar-PR 1 (B). | ||
Conclusions
In conclusion, to investigate the effects of the flexibility of the polymer chain on the characteristics of thermo-optic waveguide devices, a series of fluoro-polycarbonates were designed and successfully prepared in this study. As reported in our previous work, photocuring of solutions (Ali-PRs and Ar-PRs) containing AF-Ali-PC EPs or AF-Ar-PC EPs, a photoinitiator, and cyclopentanone yielded optical films with low surface energies and roughnesses, high thermal stabilities, and tunable refractive indices. Straight optical waveguide and MZI thermo-optic waveguide switch arrays were fabricated using Ali-PR 1 and Ar-PR 1. The propagation losses of the channel waveguide measured by the cutback method were 0.21 dB cm−1 and 0.19 dB cm−1 at 1550 nm. For Ali-PRs, the rise and fall times of the thermo-optic switch upon application of a 200 Hz square-wave voltage were 1.822 ms and 1.364 ms, respectively. The insertion loss and extinction ratio were measured to be 9.2 dB and 12.0 dB, respectively. The applied electric power was 15.0 mW. For Ar-PRs, the rise and fall times of the thermo-optic switch upon application of a 110 Hz square-wave voltage were 2.994 ms and 2.301 ms, respectively. The insertion loss and extinction ratio were measured to be 8.9 dB and 12.3 dB, respectively. The applied electric power was 20.0 mW. Compared AF-Ali-PC EPs with AF-Ar-PC EPs, fluoro-polycarbonates containing aliphatic segments were more suitable for use in optical waveguide devices than those containing aromatic segments; thus, they may be more attractive for improving stabilities and realizing cross-connectors for applications in large-scale PICs such as OXC and OADM systems.Acknowledgements
The authors are grateful for the support received from the National Natural Science Foundation of China (Grant No. 21204028, 21221063 and 51173063) and Project 2016159 supported by the Graduate Innovation Fund of Jilin University.Notes and references
- (a) L. Lu, L. Zhou, Z. Li, D. Li, S. Zhao, X. Li and J. Chen, IEEE Photonics Technol. Lett., 2015, 27, 2457 CrossRef; (b) R. G. Beausoleil, M. McLaren and N. P. Jouppi, IEEE J. Sel. Top. Quantum Electron., 2013, 19, 3700109 CrossRef; (c) A. Shacham, K. Bergman and L. P. Carloni, IEEE Trans. Comput., 2008, 57, 1246 CrossRef.
- F. Qiu, Q. Wang, D. Yang, G. Cao, Y. Guan and L. Zhuang, Eur. Polym. J., 2013, 49, 2247 CrossRef CAS.
- F. Qiu, J. Liu, G. Cao, Y. Guan, Q. Shen, D. Yang and Q. Guo, Opt. Commun., 2013, 296, 53 CrossRef CAS.
- (a) B. Lee, C. Lin, X. Wang, R. T. Chen, J. Luo and A. K. Y. Jen, Opt. Lett., 2009, 34, 3277 CrossRef CAS PubMed; (b) D. Perron, M. Wu, C. Horvath, D. Bachman and V. Van, Opt. Lett., 2011, 36, 2731 CrossRef PubMed; (c) A. M. Al-hetar, A. B. Mohammad, A. S. M. Supa'at, Z. A. Shamsan and I. Yulianti, Opt. Commun., 2011, 284, 1181 CrossRef CAS.
- C.-Y. Wu, P. Lin, R.-S. Huang, W.-C. Chao and M. M. H. Lee, Appl. Phys. Lett., 2006, 89, 121121 CrossRef.
- Z. Cao, L. Jin, Y. Liu, Z. Jiang and D. Zhang, J. Appl. Polym. Sci., 2013, 127, 607 CrossRef CAS.
- (a) Y.-C. Chen, H.-L. Chang, R.-H. Lee, S. A. Dai, W.-C. Su and R.-J. Jeng, Polym. Adv. Technol., 2009, 20, 493 CrossRef CAS; (b) J.-S. Koo, P. G. R. Smith and R. B. Williams, Chem. Mater., 2002, 14, 5030 CrossRef CAS; (c) M. Jang, C. K. Park and N. Y. Lee, Sens. Actuators, B, 2014, 193, 599 CrossRef CAS; (d) A. Podzorov and G. Gallot, Appl. Opt., 2008, 47, 3254 CrossRef CAS PubMed; (e) C.-C. Chang and W.-C. Chen, Chem. Mater., 2002, 14, 4242 CrossRef CAS; (f) H. Ma, A. K.-Y. Jen and L. R. Dalton, Adv. Mater., 2002, 14, 1339 CrossRef CAS; (g) E. Kim, S. Y. Cho, D. M. Yeu and S. Y. Shin, Chem. Mater., 2005, 17, 962 CrossRef CAS; (h) W.-S. Kim, K.-S. Kim, Y.-J. Eo, K. B. Yoon and B.-S. Bae, J. Mater. Chem., 2005, 15, 465 RSC; (i) W. H. Wong, K. S. Chan and E. Y. B. Pun, Appl. Phys. Lett., 2005, 87, 011103 CrossRef; (j) C. M. Chen, X. Z. Zhang, H. M. Zhang, D. Zhang, S. K. Mu, A. L. Hou, X. L. Zhang, L. Li and D. M. Zhang, Microw. Opt. Tech. Lett., 2007, 49, 3040 CrossRef.
- (a) J. T. Bendler, C. A. Edmondson, M. C. Wintersgill, D. A. Boyles, T. S. Filipova and J. J. Fontanella, Eur. Polym. J., 2012, 48, 830 CrossRef CAS; (b) G. Deng, H. Huang, P. Si, H. Xu, J. Liu, S. Bo, X. Liu, Z. Zhen and L. Qiu, Polymer, 2013, 54, 6349 CrossRef CAS.
- Z. Cai, B. Wang, Y. Zheng, M. Li, Y. Li, C. Chen, D. Zhang, Z. Cui and Z. Shi, J. Mater. Chem. C, 2016, 4, 533 RSC.
- (a) Y. Wan, Y. Zhang, Z. Shi, W. Xu, X. Zhang, L. Zhao and Z. Cui, Polymer, 2012, 53, 967 CrossRef CAS; (b) J. Ding, J. Jiang, C. Blanchetiere and C. L. Callender, Macromolecules, 2008, 41, 758 CrossRef CAS; (c) K. T. Powell, C. Cheng and K. L. Wooley, Macromolecules, 2007, 40, 4509 CrossRef CAS PubMed; (d) S. Wong, H. Ma, A. K. Y. Jen, R. Barto and C. W. Frank, Macromolecules, 2004, 37, 5578 CrossRef CAS.
- (a) M.-C. Oh, K.-J. Kim, W.-S. Chu, J.-W. Kim, J.-K. Seo, Y.-O. Noh and H.-J. Lee, Polymer, 2011, 3, 975 CrossRef CAS; (b) H. Ma, J. Luo, S. H. Kang, S. Wong, J. W. Kang, A. K. Y. Jen, R. Barto and C. W. Frank, Macromol. Rapid Commun., 2004, 25, 1667 CrossRef CAS; (c) S. H. Kang, J. Luo, H. Ma, R. R. Barto, C. W. Frank, L. R. Dalton and A. K.-Y. Jen, Macromolecules, 2003, 36, 4355 CrossRef CAS; (d) M. Faccini, M. Balakrishnan, R. Torosantucci, A. Driessen, D. N. Reinhoudt and W. Verboom, Macromolecules, 2008, 41, 8320 CrossRef CAS.
- (a) H. Mochizuki, T. Mizokuro, N. Tanigaki, T. Hiraga and I. Ueno, Polym. Adv. Technol., 2005, 16, 67 CrossRef CAS; (b) T. Takahashi, K. Yonetake, K. Koyama and T. Kikuchi, Macromol. Rapid Commun., 2003, 24, 763 CrossRef CAS; (c) X. Chang, T. Ding, H. Tian and T. Wang, J. Appl. Polym. Sci., 2016, 43636 Search PubMed.
- Z. Cai, H. Yu, Y. Zhang, M. Li, X. Niu, Z. Shi, Z. Cui, C. Chen and D. Zhang, Polymer, 2015, 61, 140 CrossRef CAS.
- Y. Zheng, C. M. Chen, Y. L. Gu, D. M. Zhang, Z. Z. Cai, Z. S. Shi, X. B. Wang, Y. J. Yi, X. Q. Sun, F. Wang and Z. C. Cui, Opt. Mater. Express, 2015, 5, 1934 CrossRef CAS.
- H. R. Allcock, J. D. Bender and Y. Chang, Chem. Mater., 2003, 15, 473 CrossRef CAS.
- A. Tercjak, E. Serrano, M. Larranaga and I. Mondragon, J. Appl. Polym. Sci., 2008, 108, 1116 CrossRef CAS.
- B. A. Sweileh, H. R. Al-Qalawi and H. A. Mohammad, J. Appl. Polym. Sci., 2014, 131, 39904 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra01850a |
| This journal is © The Royal Society of Chemistry 2017 |