Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
I2/TBPB mediated oxidative reaction of aryl acetaldehydes with amidines: synthesis of 1,2,5-triaryl-1H-imidazoles†
Jing Wang a,
Fang-Dong Zhanga,
Dong Tangb,
Ping Wua,
Xue-Guo Zhanga and
Bao-Hua Chen
a,
Fang-Dong Zhanga,
Dong Tangb,
Ping Wua,
Xue-Guo Zhanga and
Bao-Hua Chen *a
*a
aState Key Laboratory of Applied Organic Chemistry, Lanzhou University Gansu, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, Lanzhou 730000, People's Republic of China. E-mail: chbh@lzu.edu.cn; Fax: +86-931-891-2582
bDepartment of Chemistry, Lishui University, Lishui 323000, People's Republic of China
First published on 9th May 2017
Abstract
A direct method for the synthesis of 1,2,5-triaryl-1H-imidazoles was achieved easily from cyclization of aryl acetaldehydes with amidines catalyzed by I2. Various substitued groups can be employed, and this reaction proceeds smoothly in moderate to good yields.
Introduction
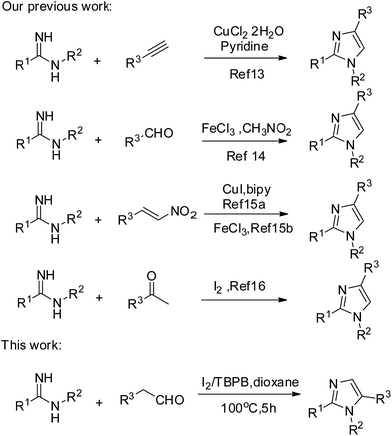
As one of the most valuable types of N-heterocyclic compound, imidazoles have been found in numerous natural products1 and functional materials.2 They also have good biological activities, such as antitumor,3 antimicrobial,4 antihypertensive5 and protein kinase inhibitory activities.6 In addition, they can also act as organocatalysts,7 ionic liquids,8 and precursors of N-heterocyclic carbenes.9Due to their indisputable importance and great application prospects, more and more researchers are dedicating effort to constructing imidazoles. As for tri-substituted imidazoles, a number of methods have been developed. The existing synthetic methodologies have mostly focused on 1,2,4-triarylated imidazoles10 and 2,4,5-triarylated imidazoles.11 The most common route for producing 1,2,4-triarylated imidazoles is combining amidines with terminal alkynes and nitroolefins with ketones. Besides, most of the reported methods for obtaining 2,4,5-triarylated imidazoles involve the condensation of 1,2-diketones and aldehydes with amines or ammonia. However, until now only a few methods have been developed for making 1,2,5-triarylated imidazoles.12 Therefore, it is a big challenge for organic chemists to find an efficient and simple way to construct 1,2,5-triarylated imidazoles.
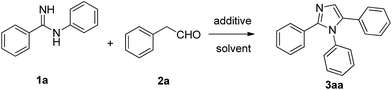
Our group is dedicated to the efficient synthesis of 1,2,4-triarylated imidazoles by employing amidines and alkynes via metal-catalyzed oxidative processes.13 In recent years, our group has been interested in the synthesis of 1,2,4-triarylated imidazoles by employing amidines and aldehydes14/nitroolefins15/ketones.16 We herein report a more environment-friendly and efficient methodology to construct 1,2,5-triaryl-1H-imidazoles17 which uses I2/TBPB mediated oxidative formal [3 + 2] cycloaddition of aryl acetaldehydes with amidines Scheme 1.18
Results and discussion
To elucidate the optimal reaction conditions, we initially studied the reaction of N-phenylbenzimidamide 1a (0.20 mmol) with phenylacetaldehyde 2a (0.24 mmol) in the presence of I2 (20 mol%) in dioxane at 100 °C in air for 5 h. As expected, the desired product 1,2,5-triphenyl-1H-imidazole 3aa was obtained in 40% yield (Table 1, entry 1). In order to improve the yield of 3aa, we further screened different oxidants, such as TBHP, TBPB, DTBP, H2O2, K2S2O8 and O2 for this reaction in the presence of iodine (Table 1, entries 2–7). Among them, TBPB was found to be the most optimal oxidant for the transformation. Next, the reaction proceeded less efficiently in other iodine-containing catalysts such as KI, TBAI, NIS, and PIDA (Table 1, entries 8–11). Then, the reaction did not give a superior result with variations in temperature (80, 110 °C, Table 1, entries 12–13). Furthermore, different solvents such as DMF, DMSO, toluene and DCB were failed to improve the yield (Table 1, entries 14–17). After several experimental iterations, the optimal reaction conditions emerged with N-phenylbenzimidamide 1a (0.20 mmol) with phenylacetaldehyde 2a (0.24 mmol) in the presence of I2 (20 mol%) and TBPB (1 eq.) in dioxane (2 mL) at 100 °C in air for 5 h (Table 1, entry 3)| Entry | Catalyst (mol%) | Oxidant (equiv.) | Solvent (mL) | T (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.20 mmol), 2a (0.24 mmol), catalyst (20 mol%), oxidant (1 eq), solvent (2 mL), in air for 5 h; TBHP = tert-butyl hydroperoxide (70% in water); TBPB = tert-butyl peroxybenzoate; DTBP = di-tert-butyl peroxide; TBAI = tetrabutylammonium iodide; NIS = niodosuccinimide; PIDA = iodosobenzene diacetate.b Isolated yield. | |||||
| 1 | I2(20) | — | Dioxane | 100 | 40 |
| 2 | I2(20) | TBHP(1) | Dioxane | 100 | 78 |
| 3 | I2(20) | TBPB(1) | Dioxane | 100 | 93 |
| 4 | I2(20) | DTBP(1) | Dioxane | 100 | 38 |
| 5 | I2(20) | H2O2(1) | Dioxane | 100 | 42 |
| 6 | I2(20) | K2S2O8(1) | Dioxane | 100 | 12 |
| 7 | I2(20) | O2 | Dioxane | 100 | 41 |
| 8 | KI(20) | TBPB(1) | Dioxane | 100 | 69 |
| 9 | TBAI(20) | TBPB(1) | Dioxane | 100 | 54 |
| 10 | NIS(20) | TBPB(1) | Dioxane | 100 | 65 |
| 11 | PIDA(20) | TBPB(1) | Dioxane | 100 | 31 |
| 12 | I2(20) | TBPB(1) | Dioxane | 80 | 66 |
| 13 | I2(20) | TBPB(1) | Dioxane | 110 | 68 |
| 14 | I2(20) | TBPB(1) | DMF | 100 | 42 |
| 15 | I2(20) | TBPB(1) | DMSO | 100 | 50 |
| 16 | I2(20) | TBPB(1) | Toluene | 100 | 88 |
| 17 | I2(20) | TBPB(1) | DCB | 100 | 85 |
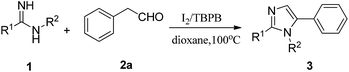
With the optimized conditions in hand, we proceeded to examine the substrate scope (Table 2). First, we studied the R1-substituted arylamidines. A variety of 1,2,5-trisubstituted imidazoles could be obtained by employing various arylamidines (1) and phenylacetaldehyde (2a) giving 44–85% yields (Table 2, entries 1–5). Generally, electron donating groups substituents (4-Me, 4-MeO) as well as some electron withdrawing groups (4-CF3, 2-Cl) provided moderate yields (Table 2, entries 1–4). In addition, the substrate N-p with the pyridine ring could also be applied to this strategy, even with a relatively low yield (Table 2, entry 5).
| Entry | R1 | R2 | Product | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1 (0.20 mmol), 2a (0.24 mmol), I2 (20 mol%), TBPB(1 eq.), dioxane (2 mL), 100 °C in air for 5 h, unless otherwise stated.b Isolated yields. | ||||
| 1 | 4-MeOC6H4 | Phenyl | 3ba | 81 |
| 2 | 4-Me C6H4 | Phenyl | 3ca | 85 |
| 3 | 4-CF3 C6H4 | Phenyl | 3da | 78 |
| 4 | 2-Cl C6H4 | Phenyl | 3ea | 79 |
| 5 | 2-Pyridyl | Phenyl | 3fa | 44 |
| 6 | Phenyl | 4-MeO C6H4 | 3ga | 95 |
| 7 | Phenyl | 4-Me C6H4 | 3ha | 92 |
| 8 | Phenyl | 2-Me C6H4 | 3ia | 90 |
| 9 | Phenyl | 3,4-diMe C6H3 | 3ja | 91 |
| 10 | Phenyl | 2-Et C6H4 | 3ka | 86 |
| 11 | Phenyl | 4-F C6H4 | 3la | 86 |
| 12 | Phenyl | 4-Cl C6H4 | 3ma | 89 |
| 13 | 4-MeO C6H4 | 4-Me C6H4 | 3na | 92 |
| 14 | 4-Me C6H4 | 4-Me C6H4 | 3oa | 91 |
| 15 | (3-Br,4-Me)C6H3 | 4-Me C6H4 | 3pa | 78 |
| 16 | 4-Cl C6H4 | 4-Me C6H4 | 3qa | 76 |
| 17 | 4-Br C6H4 | 4-Me C6H4 | 3ra | 72 |
We next examined the substrate scope of this reaction using R2-substituted arylamidines. Electron-rich-substituted arylamidines such as 4-OMe, 4-Me, 2-Me, 3,4-diMe were easily converted into the corresponding products in excellent yields (Table 2, entries 6–9). Moreover, the substrate with ethyl at the ortho-position afforded the corresponding product 3ka in 86% yield (Table 3, entry 10). Electron-deficient arylamidines bearing halide (4-F, 4-Cl) groups reacted under the standard conditions to afford the desired products in moderate yields (Table 2, entries 11–12).
| Entry | R3 | Product | Yield(%)b |
|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2 (0.24 mmol), I2 (20 mol%), TBPB(0.2 mmol), dioxane (2 mL), 100 °C in air for 5 h, unless otherwise stated.b Isolated yields. | |||
| 1 | 4-MeO C6H4 | 3ab | 85 |
| 2 | 4-Me C6H4 | 3ac | 83 |
| 3 | 3-Me C6H4 | 3ad | 81 |
| 4 | 2-Me C6H4 | 3ae | 78 |
| 5 | 3,4-diMe C6H3 | 3af | 80 |
| 6 | 4-Et C6H4 | 3ag | 81 |
| 7 | 4-F C6H4 | 3ah | 78 |
| 8 | 2-F C6H4 | 3ai | 72 |
In addition, bifunctional amidines were also studied, and were well compatible in this transformation in 72–92% yields (Table 2, entries 13–17).
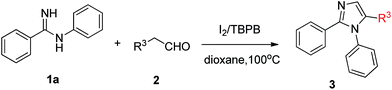
To further study the scope and generality of the present protocol, various aryl acetaldehyde 2 were studied for the cycloaddition reactions with N-phenylbenzimidamide 1a under the optimized reaction conditions (Table 3). Substrates bearing electron-donating groups (4-OMe, 4-Me, 3-Me, 2-Me, 3,4-diMe and 2-Et) at the aromatic ring produced the corresponding products in moderate yields (Table 3, entries 1–6). The presence of electron-withdrawing substituents (2-F, 4-F) reduced the efficiency of the reaction, as the corresponding products could be isolated in slightly lower yields (Table 3, entries 7–8).
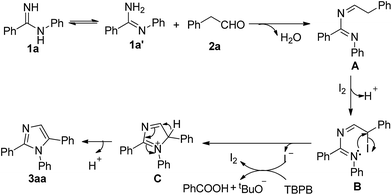
Based on the results and the literature reports,19 a plausible mechanism was proposed as shown in Scheme 2. Initially, the intermediate A is produced by the condensation reaction of N-phenylbenzamidine 1a with phenylacetaldehyde 2a. Subsequently, the iodo compound B is formed from the A after attack by a molecule of iodine, at the same time with release of H+. There follows an intramolecular cyclization of the iodo compound B to form the immediate C by leaving of I− along with a catalytic cycle of I− being oxidized to I2 by TBPB. Then, a subsequent oxidation of C to give the desired product 3aa.
Conclusion
In conclusion, we have successfully described a practical and efficient strategy for one-pot synthesis of 1,2,5-triaryl-1H-imidazoles which uses I2/TBPB mediated oxidative formal [3 + 2] cycloaddition of aryl acetaldehydes with amidines. The reaction is carried out smoothly and the corresponding products are formed in good to excellent yields with excellent regioselectivity. Importantly, operational simplicity, inexpensive catalysts, an excellent functional groups tolerance to the desired compounds from easily available starting materials. And further studies with respect to the details on the subject remain in progress.Acknowledgements
We are grateful to the project sponsored by the National Science Foundation of P. R. China (No. 21372102 and 21403256).Notes and references
- (a) Z. Jin, Nat. Prod. Rep., 2005, 22, 196–229 RSC; (b) Z. Jin, Nat. Prod. Rep., 2006, 23, 464–496 RSC; (c) Z. Jin, Nat. Prod. Rep., 2009, 26, 382–445 RSC; (d) M. Roue, I. Domart-Coulon, A. Ereskovsky, C. Djediat, T. Perez and M. L. Bourguet-Kondracki, J. Nat. Prod., 2010, 73, 1277–1282 CrossRef CAS PubMed.
- (a) Y. Yuan, J.-X. Chen, F. Lu, Q.-X. Tong, Q.-D. Yang, H.-W. Mo, T.-W. Ng, F.-L. Wong, Z.-Q. Guo, J. Ye, Z. Chen, X.-H. Zhang and C.-S. Lee, Chem. Mater., 2013, 25, 4957–4965 CrossRef CAS; (b) N. Nagarajan, G. Velmurugan, A. Prakash, N. Shakti, M. Katiyar, P. Venuvanalingam and R. Renganathan, Chem.–Asian J., 2014, 9, 294–304 CrossRef CAS PubMed; (c) T. H. Chiang, Y.-C. Lin, Y.-F. Chen and E.-Y. Chen, J. Appl. Polym. Sci., 2016, 133 Search PubMed; (d) J. J. Huang, Y. H. Hung, P. L. Ting, Y. N. Tsai, H. J. Gao, T. L. Chiu, J. H. Lee, C. L. Chen, P. T. Chou and M. K. Leung, Org. Lett., 2016, 18, 672–675 CrossRef CAS PubMed.
- (a) C. D. Mohan, V. Srinivasa, S. Rangappa, L. Mervin, S. Mohan, S. Paricharak, S. Baday, F. Li, M. K. Shanmugam, A. Chinnathambi, M. E. Zayed, S. A. Alharbi, A. Bender, G. Sethi, Basappa and K. S. Rangappa, PLoS One, 2016, 11, e0153155 Search PubMed; (b) S. Subramanian, H.-S. Yang, M. Manickam, J. Yun and S.-H. Jung, Bull. Korean Chem. Soc., 2016, 37, 632–637 CrossRef CAS; (c) J. Yong, C. Lu and X. Wu, Lett. Org. Chem., 2016, 13, 283–288 CrossRef CAS.
- (a) S. Q. Wen, P. Jeyakkumar, S. R. Avula, L. Zhang and C. H. Zhou, Bioorg. Med. Chem. Lett., 2016, 26, 2768–2773 CrossRef CAS PubMed; (b) Y. A. Ammar, M. A. El-Sharief, M. M. Ghorab, Y. A. Mohamed, A. Ragab and S. Y. Abbas, Curr. Org. Synth., 2016, 13, 466–475 CrossRef CAS PubMed.
- B. Hanumantha Rao, I. V. Subramanyeswara Rao, V. Ravi Kanth, K. V. Prasada Rao, K. Balamurali Krishna and B. Syama Sundar, Sci. Pharm., 2015, 83, 465–478 CrossRef CAS PubMed.
- L. Z. Bendjeddou, N. Loaec, B. Villiers, E. Prina, G. F. Spath, H. Galons, L. Meijer and N. Oumata, Eur. J. Med. Chem., 2017, 125, 696–709 CrossRef CAS PubMed.
- L. Hojabri, A. Hartikka, F. M. Moghaddam and P. I. Arvidsson, Adv. Synth. Catal., 2007, 349, 740–748 CrossRef CAS.
- J. Dupont, R. F. de Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667–3692 CrossRef CAS PubMed.
- (a) F. E. Hahn and M. C. Jahnke, Angew. Chem., Int. Ed., 2008, 47, 3122–3172 CrossRef CAS PubMed; (b) K. M. Hindi, M. J. Panzner, C. A. Tessier, C. L. Cannon and W. J. Youngs, Chem. Rev., 2009, 109, 3859–3884 CrossRef CAS PubMed; (c) B. Alič and G. Tavčar, J. Fluorine Chem., 2016, 192, 141–146 CrossRef.
- (a) M. Adib, S. Ansari, S. Feizi, J. Damavandi and P. Mirzaei, Synlett, 2009, 2009, 3263–3266 CrossRef; (b) L. Hong, Y. Shao, L. Zhang and X. Zhou, Chem.–Eur. J., 2014, 20, 8551–8555 CrossRef CAS PubMed; (c) J. Cao, X. Zhou, H. Ma, C. Shi and G. Huang, RSC Adv., 2016, 6, 57232–57235 RSC; (d) Y. Li, Y. Fu, C. Ren, D. Tang, P. Wu, X. Meng and B. Chen, Org. Chem. Front., 2015, 2, 1632–1636 RSC.
- (a) X. Guo, J. Shao, H. Liu, B. Chen, W. Chen and Y. Yu, RSC Adv., 2015, 5, 51559–51562 RSC; (b) C. Y. Chen, W. P. Hu, P. C. Yan, G. C. Senadi and J. J. Wang, Org. Lett., 2013, 15, 6116–6119 CrossRef CAS PubMed; (c) J. Banothu, R. Gali, R. Velpula and R. Bavantula, Arabian J. Chem., 2013 DOI:10.1016/j.arabjc.2013.10.022; (d) X. Xu and Y. Li, Res. Chem. Intermed., 2014, 41, 4169–4176 CrossRef; (e) H. Ramezanalizadeh and F. Manteghi, Monatsh. Chem., 2016 DOI:10.1007/s00706-016-1776-9.
- (a) X. Zhou, Z. Jiang, L. Xue, P. Lu and Y. Wang, Eur. J. Org. Chem., 2015, 2015, 5789–5797 CrossRef CAS; (b) J. Zhang, Q. Gao, X. Wu, X. Geng, Y. D. Wu and A. Wu, Org. Lett., 2016, 18, 1686–1689 CrossRef CAS PubMed; (c) B. Sezen and D. Sames, J. Am. Chem. Soc., 2003, 125, 10580–10585 CrossRef CAS PubMed; (d) A. R. Katritzky, L. Zhu, H. Lang, O. Denisko and Z. Wang, Tetrahedron, 1995, 51, 13271–13276 CrossRef CAS; (e) J. Grimshaw and S. A. Hewitt, J. Chem. Soc., Perkin Trans. 1, 1990, 2995–2998, 10.1039/p19900002995.
- J. Li and L. Neuville, Org. Lett., 2013, 15, 1752–1755 CrossRef CAS PubMed.
- X. Liu, D. Wang, Y. Chen, D. Tang and B. Chen, Adv. Synth. Catal., 2013, 355, 2798–2802 CrossRef CAS.
- (a) D. Tang, P. Wu, X. Liu, Y. X. Chen, S. B. Guo, W. L. Chen, J. G. Li and B. H. Chen, J. Org. Chem., 2013, 78, 2746–2750 CrossRef CAS PubMed; (b) X. Liu, D. Wang and B. Chen, Tetrahedron, 2013, 69, 9417–9421 CrossRef CAS.
- J. Qu, P. Wu, D. Tang, X. Meng, Y. Chen, S. Guo and B. Chen, New J. Chem., 2015, 39, 4235–4239 RSC.
- D. El Abed, C. Adiche and M. Hamadouche, Heterocycles, 2016, 92, 1614 CrossRef.
- (a) J. Li and L. Neuville, Org. Lett., 2013, 15, 6124–6127 CrossRef CAS PubMed; (b) J. Li, S. Benard, L. Neuville and J. Zhu, Org. Lett., 2012, 14, 5980–5983 CrossRef CAS PubMed; (c) R. Navratil, J. Tarabek, I. Linhart and T. Martinu, Org. Lett., 2016, 18, 3734–3737 CrossRef CAS PubMed.
- (a) X. Zhang, M. A. Campo, T. Yao and R. C. Larock, Org. Lett., 2005, 7, 763–766 CrossRef CAS PubMed; (b) G. Bharathiraja, S. Sakthivel, M. Sengoden and T. Punniyamurthy, Org. Lett., 2013, 15, 4996–4999 CrossRef CAS PubMed; (c) S. K. Lee and J. K. Park, J. Org. Chem., 2015, 80, 3723–3729 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra01966a |
| This journal is © The Royal Society of Chemistry 2017 |