Open Access Article
Open Access ArticleCu-catalyzed aerobic oxidative C–CN bond cleavage of benzyl cyanide for the synthesis of primary amides†
Xiuling Chen *,
Yanhong Peng,
Yan Li,
Minghu Wu,
Haibing Guo,
Jian Wang and
Shaofa Sun*
*,
Yanhong Peng,
Yan Li,
Minghu Wu,
Haibing Guo,
Jian Wang and
Shaofa Sun*
Non-power Nuclear Technology Collaborative Innovation Center, School of Nuclear Technology and Chemistry & Life Science, Hubei University of Science and Technology, Xianning 437100, China. E-mail: cxl828800@163.com; sunshaofa@mail.hbust.com.cn; Fax: +86-715-8338007
First published on 28th March 2017
Abstract
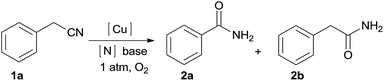
An efficient method via copper-catalyzed aerobic oxidative amidation of benzyl cyanide for primary amides is successfully developed. Using readily available NH4Cl as a nitrogen source and Cu/O2 as a catalytic oxidation system offers new opportunities for C–CN bond cleavage and primary amide bond formation.
Primary amides are prevalent organic motifs in peptide, protein synthesis, intensifiers of perfume, anti-block reagents, color pigments for inks, detergents, and lubricants.1 Generally, the synthetic procedures for the preparation of primary amides are rely on the reaction of carboxylic acid,2 or its derivatives with ammonia or ammonium salt.3 However, toxic and corrosive, materials, low functional groups tolerance and complex operation are generally required in conventional synthetic procedures. To overcome these problems, alternative methods for primary amide synthesis have been developed. The direct amidation of aldehydes,4 alcohols,5 or methylarenes6 to primary amides represents an interesting topic in this area, however, hazardous peroxides (TBHP), the toxic halogens, or an excess amount of molecular sieves were required. Other attractive approaches include the hydration of nitriles,7 oxidative amidation of terminal alkynes,8 transition-metal-catalyzed C–H activation and amidation9 and catalytic oxidation C–C bond cleavage of ketones into benzamides.10 Although great progress has been achieved in this field, there still remains significant challenges for synthetic organic chemists in reactivity, efficiencies, and high functional group tolerance.
Recently, C–C bond cleavage has become an attractive topic because of its potential usage in new chemical bond formation via inert starting materials.11 Also molecular oxygen has been considered as an ideal oxidant due to its atom-economical, environmentally benign, and abundance.12 However, few reactions are compatible with aerobic oxidation13 and C–C bond cleavage in one transformation.14 Therefore, the development of an efficient catalytic system towards aerobic oxidative unstrained C–C bonds cleavage is always highly desired. As our continued interest in Cu or Fe-catalyzed aerobic oxidative C–C bond cleavage reaction,15 we developed a Cu-catalyzed aerobic oxidative C–C bond cleavage of benzyl cyanide for the synthesis of primary amides. Using readily available NH4Cl as a nitrogen source and a Cu/O2 catalytic oxidation system offers new opportunities for primary amide bond formation. The developed methodology tolerated a wide range of functional groups and produced high yields for the synthesis of primary amides.
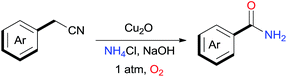 | (1) |
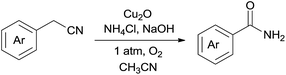
We carried out experiments to test the reactivity of benzyl cyanide 1a with ammonium salts in the presence of a metal catalyst under an oxygen atmosphere and the obtained results are compiled in Table 1. First, our studies started with the reaction between benzyl cyanide 1a and 5.0 equiv. of ammonia (25% aqueous) in CH3CN at 120 °C (Table 1, entries 1–5). When 5 mol% of CuCl was used as catalyst, the benzamide 2a was obtained in 38% yield along with the hydration of nitrile products 2b (Table 1, entry 1). We then screened the types of catalysts, and discovered that CuCl2 or Cu2O produces higher yields (Table 1, entries 4–5). However the products 2b was always by a product. Then, optimization studies were performed to improve the yield of 2a. To our surprise, when ammonia was replaced with NH4Cl, 2a was obtained in 50% yield, and only trace amounts 2b was observed (Table 1, entry 6). Four bases were tested using Cu2O as the catalyst in CH3CN, and NaOH showed the highest yield (Table 1, entries 6–9). Several copper salts and amine salts were screened (Table 1, entries 10–16), and the best result was obtained under Cu2O/NH4Cl/NaOH system (Table 1, entry 9). Noteworthily, NaOH is essential for this reaction. In the absence of NaOH, 2a could not be obtained at all (Table 1, entry 17). The reaction did not proceed in the absence of copper catalyst (Table 1, entry 18). The reaction was also dependent on dioxygen; when air was used as the oxidant, only trace of 2a was obtained (Table 1, entry 19). Other solvents such as, toluene and DMF gave lower yields (Table 1, entries 20–21).
| Entry | Cat | [N] source | Base | 2ab% | 2bb% |
|---|---|---|---|---|---|
| a Reaction conditions: 2-benzyl cyanide 1a (0.2 mmol), cat (5 mol%), N source (0.3 mmol), base (0.4 mmol), CH3CN (2 mL), in 25 mL Schlenk tube, 120 °C, O2 (1 atm), 30 h.b Yields were determined by GC using n-hexadecane as an internal standard.c Air was used as oxidant.d Toluene as solvent.e DMF as solvent. | |||||
| 1 | CuCl | NH3·H2O | — | 35 | 20 |
| 2 | Cu(OAc)2 | NH3·H2O | — | 25 | 30 |
| 3 | CuO | NH3·H2O | — | 40 | 41 |
| 4 | CuCl2 | NH3·H2O | — | 50 | 25 |
| 5 | Cu2O | NH3·H2O | 50 | 31 | |
| 6 | Cu2O | NH4Cl | Ba(OH)2 | 50 | <1 |
| 7 | Cu2O | NH4Cl | KOH | 58 | <1 |
| 8 | Cu2O | NH4Cl | Ca(OH)2 | 20 | <1 |
| 9 | Cu2O | NH4Cl | NaOH | 88 | <1 |
| 10 | CuCl | NH4Cl | NaOH | 71 | <1 |
| 11 | CuCl2 | NH4Cl | NaOH | 65 | <1 |
| 12 | Cu(OAc)2 | NH4Cl | NaOH | 58 | <1 |
| 13 | Cu2O | NH4OAc | NaOH | 63 | <1 |
| 14 | Cu2O | (NH4)2SO4 | NaOH | 50 | <1 |
| 15 | Cu2O | (NH4)2CO3 | NaOH | 71 | <1 |
| 16 | Cu2O | Urea | NaOH | <1 | <1 |
| 17 | Cu2O | NH4Cl | — | — | <1 |
| 18 | — | NH4Cl | NaOH | — | <1 |
| 19c | Cu2O | NH4Cl | NaOH | Trace | Trace |
| 20d | Cu2O | NH4Cl | NaOH | 20 | <1 |
| 21e | Cu2O | NH4Cl | NaOH | 13 | <1 |
With the optimal conditions in hand, the scope of copper-catalyzed aerobic oxidative amidation of substituted 2-benzyl cyanides with “NH2” producing primary amides was investigated, the results were compiled in Table 2. A variety of 2-benzyl cyanide with either an electron-donating or an electron-withdrawing group, were all converted readily to the corresponding primary amides efficiently. Thus, in addition to 2-benzyl cyanide, benzyl cyanide with electron-donating substituents methyl (–CH3), methoxy (–OCH3) reacted smoothly with NH4Cl/NaOH to provide the corresponding primary amides 2a–2c (Table 2, entries 1–3). However, when hydroxyl (–OH) substituted 2-benzyl cyanide was used as a substrate, none of the expected products were detected. This may have been due to the interaction between the hydroxyl and base or oxidizing agent, which would hinder the C–CN bond activation (Table 2, entry 4). Benzyl cyanide with fluoro (–F), chloro (–Cl), bromo (–Br), iodo (–I), trifluoromethyl (–CF3) or phenyl (–Ph) electron-withdrawing groups served as good substrates to produce the corresponding primary amides 2e–2j in excellent yields (Table 2, entries 5–10), and there is no sharp difference. Interestingly, when nitro (–NO2) substituted 2-benzyl cyanide were used, the corresponding 4-nitrobenzamide was obtained in high yield and with short time (Table 2, entry 11).
| Entry | Benzonitriles | Products | Yieldb |
|---|---|---|---|
| a Reaction conditions: substrate 1a–1q (0.2 mmol), Cu2O (5 mol%), NH4Cl (0.3 mmol), NaOH (0.4 mmol), CH3CN (2 mL), O2 in 25 mL Schlenk tube, 120 °C, 24 h.b Isolated yield.c 16 h.d 48 h. | |||
| 1 | 1a | 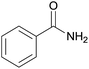 |
2a, 82% |
| 2 | 1b | 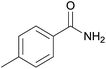 |
2b, 81% |
| 3 | 1c | 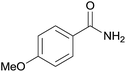 |
2c, 79% |
| 4 | 1d | 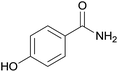 |
2d, trace |
| 5 | 1e | 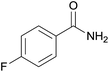 |
2e, 81% |
| 6 | 1f | 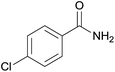 |
2f, 78% |
| 7 | 1g | 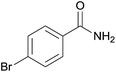 |
2g, 83% |
| 8 | 1h | 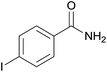 |
2h, 69% |
| 9 | 1i | 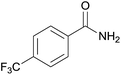 |
2i, 78% |
| 10 | 1j | 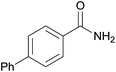 |
2j, 73% |
| 11c | 1k | 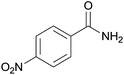 |
2k, 92% |
| 12 | 1l | 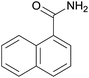 |
2l, 57% |
| 13 | 1m | 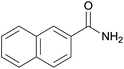 |
2m, 71% |
| 14 | 1n | 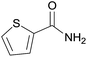 |
2n, 63% |
| 15 | 1o | 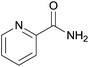 |
2o, 72% |
| 16 | 1p | 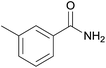 |
2p, 71% |
| 17d | 1q | 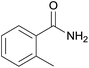 |
2q, 35% |
Even the 1-(naphthalen-2-yl)acetonitrile and 2-(naphthalen-2-yl)acetonitrile furnished the corresponding primary amide 2l and 2m in 57% and 71% yield respectively. The heteroaryl-substituted primary amide 2n and 2o were obtained from the corresponding 2-(thiophen-2-yl)acetonitrile or 2-(pyridin-2-yl)acetonitrile under the present Cu2O/NH4Cl/NaOH system. While, the reaction was influenced by the substituent steric effects. For example, the yield of 2q was decreased for ortho-substituted 2-benzyl cyanide contrast to para-substituted and meta-substituted 2-benzyl cyanide (Table 2, entry 2, entries 16–17).
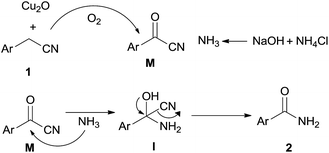
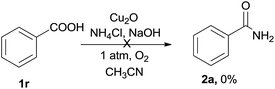
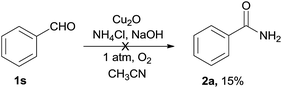
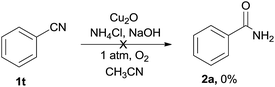
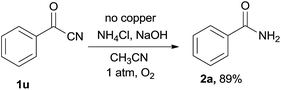
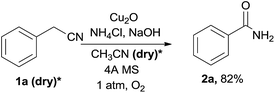
To get more information of the reaction mechanism, a few potential intermediates were subjected to the reaction under standard conditions. As shown below, phenylacetic acid 1r was used as a substrate under the standard reaction conditions, while 2a was not detected at all (eqn (2)). Benzaldehyde 1s was treated under the optimized conditions, 2a was obtained in 15% yield (eqn (3)), indicating that an aldehyde or acid was not the efficient intermediate. The reaction of benzonitrile 1t with NH4Cl/NaOH under standard conditions was also explored, and the desired benzamide 2a was not detected at all, showing that benzyl cyanide was not converted to benzonitrile via hydration of nitriles to primary amides (eqn (4)). The reaction of benzoyl cyanide 1u and NH4Cl/NaOH was performed in the absence of Cu2O, the corresponding primary amide 2a was obtained in 89% yield (eqn (5)). The results indicated that benzoyl cyanide 1u was the efficient intermediate for this transformation, at first benzyl cyanide was converted to benzoyl cyanide via sp3 C–H bond oxygenation. Under dry conditions and in the absence of air, the desired product 2a was also generated in 82% yield under the optimal conditions, thus the oxygen atoms of the product amides should originate from molecular dioxygen (eqn (6)).
 | (2) |
 | (3) |
 | (4) |
 | (5) |
 | (6) |
*The solvent CH3CN and 1a were dried, degassed by standard methods before use, and stored under nitrogen using standard Schlenk techniques.
Based on the above results and the reported literature,15a a possible mechanism for the copper-catalyzed aerobic oxidative amidation of benzyl cyanide to the synthesis of primary amides is suggested in Scheme 1. The catalytic cycle reaction involved sp3 C–H bond oxygenation of benzyl cyanide to the intermediate benzoyl cyanide M and with the Cu catalyst being regenerated, NH3 was generated from the heating of NH4Cl and NaOH. Then intermediate benzoyl cyanide M was attacked by NH3 to generate I, followed by C–CN bond cleavage affording the desired primary amide 2.
Conclusions
In summary, we have developed a simple and highly efficient method for the synthesis of primary amides via C–CN bond cleavage under copper-catalyzed aerobic oxidative conditions. Using readily available NH4Cl as a nitrogen source, conversion of benzyl cyanides to primary amides provides an opportunity to utilize inert starting materials to construct amides bond. The present method is practical and economical, and the starting materials are readily available.Acknowledgements
Financial supports by National Natural Science Foundation of China (21603068), Science and Technology Innovation Team Project of Hubei Provincial Department of Education (T201419), and research fund for the doctoral program of Hubei University of Science and Technology are gratefully appreciated.Notes and references
- (a) J. M. Humphrey and A. R. Chamberlin, Chem. Rev., 1997, 97, 2243 CrossRef CAS PubMed; (b) J. W. Bode, R. M. Fox and K. D. Baucom, Angew. Chem., Int. Ed., 1248, 2006, 45 Search PubMed; (c) B. L. Bray, Nat. Rev. Drug Discovery, 2003, 2, 587 CrossRef CAS PubMed.
- E. Valeur and M. Bradley, Chem. Soc. Rev., 2009, 38, 606 RSC.
- (a) M. B. Smith and J. March, March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, Wiley, Hoboken, NJ, 6th edn, 2007 Search PubMed; (b) M. B. Smith, Organic Synthesis, Mc-Graw-Hill Companies, New York, 2nd edn, 2002 Search PubMed; (c) H. Lundberg, F. Tinnis, J. Zhang, A. G. Algarra, F. Himo and H. Adolfsson, J. Am. Chem. Soc., 2017, 139, 2286 CrossRef CAS PubMed.
- (a) U. B. Patil, A. S. Singh and J. M. Nagarkar, RSC Adv., 2014, 4, 1102 RSC; (b) M. A. Ali and T. Punniyamurthy, Adv. Synth. Catal., 2010, 352, 288 CrossRef CAS; (c) S. C. Ghosh, J. S. Y. Ngiam, A. M. Seayad, D. T. Tuan, C. L. L. Chai and A. Chen, J. Org. Chem., 2012, 77, 8007 CrossRef CAS PubMed; (d) J. J. Shi and J.-M. Fang, J. Org. Chem., 2003, 68, 1158 CrossRef PubMed; (e) G. Wang, Q. Yu, S. Chen and X. Yu, Org. Biomol. Chem., 2014, 12, 414 RSC.
- (a) T. Zweifel, J.-V. Naubron and H. Grützmacher, Angew. Chem., Int. Ed., 2009, 48, 559 CrossRef CAS PubMed; (b) Y. Wang, D. Zhu, L. Tang, S. Wang and Z. Wang, Angew. Chem., Int. Ed., 2011, 50, 8917 CrossRef CAS PubMed; (c) J. F. Soulé, H. Miyamura and S. Kobayashi, J. Am. Chem. Soc., 2011, 133, 18550 CrossRef PubMed; (d) K. Yamaguchi, H. Hobayashi, T. Oishi and N. Mizuno, Angew. Chem., Int. Ed., 2012, 51, 544 CrossRef CAS PubMed; (e) R. Nie, J. Shi, S. Xia, L. Shen, P. Chen, Z. Hou and F. S. Xiao, J. Mater. Chem., 2012, 22, 18115 RSC; (f) K. Yamaguchi, H. Kobayashi, Y. Wang, T. Oishi, Y. Ogasawara and N. Mizuno, Catal. Sci. Technol., 2013, 3, 318 RSC; (g) R. Nie, J. Shi, S. Xia, L. Shen, P. Chen, Z. Hou and F. Xiao, J. Mater. Chem., 2012, 22, 18115 RSCX. Q. Li, W. K. Wang, Y. X. Han and C. Zhang, Adv. Synth. Catal., 2010, 352, 2588 CrossRef CAS; (h) R. Ohmura, M. Takahata and H. Togo, Tetrahedron Lett., 2010, 51, 4378 CrossRef CAS; (i) R. Das and D. Chakraborty, Catal. Commun., 2012, 26, 48 CrossRef CAS.
- (a) Y. Wang, K. Yamaguchi and N. Mizuno, Angew. Chem., Int. Ed., 2012, 51, 7250 CrossRef CAS PubMed; (b) Y. Huang, T. Chen, Q. Li, Y. Zhou and S. F. Yin, Org. Biomol. Chem., 2015, 13, 7289 RSC.
- (a) C. Singh, V. Kumar, U. Sharma, N. Kumar and B. Singh, Curr. Org. Synth., 2013, 10, 241 CrossRef CAS; (b) T. Tu, Z. Wang, Z. Liu, X. Feng and Q. Wang, Green Chem., 2012, 14, 921 RSC; (c) R. García-Álvarez, P. Crochet and V. Cadierno, Green Chem., 2013, 15, 46 RSC; (d) P. Marcé, J. Lynch, A. J. Blacker and J. M. J. Williams, Chem. Commun., 2016, 52, 1436 RSC; (e) H. Chen, W. Dai, Y. Chen, Q. Xu, J. Chen, L. Yu, Y. Zhao, M. Ye and Y. Pan, Green Chem., 2014, 16, 2136 RSC; (f) Y. Li, H. Chen, J. Liu, X. Wan and Q. Xu, Green Chem., 2016, 18, 4865 RSC.
- S. U. Dighe and S. Batra, Adv. Synth. Catal., 2016, 358, 500 CrossRef CAS.
- Y. Zhang, K. B. Teuscher and H. Ji, Chem. Sci., 2016, 7, 2111 RSC.
- (a) S. Shimokawa, Y. Kawagoe, K. Moriyama and H. Togo, Org. Lett., 2016, 18, 784 CrossRef CAS PubMed; (b) C. Tang and N. Jiao, Angew. Chem., Int. Ed., 2014, 53, 6528 CrossRef CAS PubMed.
- (a) M. Tobisu and N. Chatani, Chem. Soc. Rev., 2008, 37, 300 RSC; (b) S. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234 CrossRef CAS PubMed.
- S. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234 CrossRef CAS PubMed.
- (a) C. Zhang, P. Feng and N. Jiao, J. Am. Chem. Soc., 2013, 135, 15257 CrossRef CAS PubMed; (b) W. Kong, B. Li, X. Xu and Q. Song, J. Org. Chem., 2016, 81, 8436 CrossRef CAS PubMed; (c) W. Ding and Q. Song, Org. Chem. Front., 2015, 2, 765 RSC; (d) Q. Song, Q. Feng and K. Yang, Org. Lett., 2014, 16, 624 CrossRef CAS PubMed; (e) Q. Feng and Q. Song, J. Org. Chem., 2014, 79, 1867 CrossRef CAS PubMed; (f) Q. Feng and Q. Song, Adv. Synth. Catal., 2014, 356, 1697 CrossRef CAS.
- (a) H. Liu, C. Dong, Z. Zhang, P. Wu and X. Jiang, Angew. Chem., Int. Ed., 2012, 51, 12570 CrossRef CAS PubMed; (b) C. Hu, X. Yan, X. Zhou and Z. Li, Org. Biomol. Chem., 2013, 11, 8179 RSC; (c) K. Wu, Z. Huang, Y. Ma and A. Lei, RSC Adv., 2016, 6, 24349 RSC.
- (a) X. Chen, T. Chen, Q. Li, Y. Zhou, L. B. Han and S. F. Yin, Chem.–Eur. J., 2014, 20, 1 CrossRef CAS; (b) X. Chen, T. Chen, F. Ji, Y. Zhou and S. F. Yin, Catal. Sci. Technol., 2015, 5, 2197 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data for the products. See DOI: 10.1039/c7ra02207g |
| This journal is © The Royal Society of Chemistry 2017 |