Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A series of five-coordinated copper coordination polymers for efficient degradation of organic dyes under visible light irradiation†
Lu-Lu Shi,
Tian-Rui Zheng,
Min Li,
Lin-Lu Qian,
Bao-Long Li * and
Hai-Yan Li
* and
Hai-Yan Li
State and Local Joint Engineering Laboratory for Functional Polymeric Materials, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, PR China. E-mail: libaolong@suda.edu.cn
First published on 27th April 2017
Abstract
The copper(II) coordination polymers {[Cu4(OH)2(bix)2(1,2,4-btc)2(H2O)2]·3H2O}n (1), [Cu2(bix)(ip)2]n (2), [Cu2(bix)(Meip)2]n (3), {[Cu2(bix)(pbda)2]·H2O}n (4) and {[Cu2(bix)1.5(2,5-pydc)2(H2O)]·2H2O}n (5) were synthesized using the hydrothermal method (bix = 1,4-bis(2-methyl-imidazol-1-ylmethyl)benzene, 1,2,4-btc = 1,2,4-benzenetricarboxylate, ip = isophthalate, Meip = 5-methyl-isophthalate, pbda = 1,4-benzenediacatate, 2,5-pydc = 2,5-pyridine-dicarboxylate). 1 exhibits a (3,5)-connected 2D network with the point symbol of (42·6)(42·67·8) based on the [Cu2(μ-OH)] dimer. 2 exhibits the 2-fold interpenetrating three-dimensional pcu network based on the [Cu2(COO)4] dimers. 3 exhibits the 2-fold interpenetrating three-dimensional pcu network based on the [Cu2(COO)2] and [Cu2(COO)4] dimers. 4 shows an unusual 6-connected self-catenated 3D network with the point symbol of (44·610·8) based on [Cu2(COO)4] dimers. 5 exhibits a (3,4)-connected 2D network with the point symbol of (42·6)(42·63·8). 1–5 are semiconducting in nature, with Eg of 2.21 eV (1), 2.35 eV (2), 2.41 eV (3), 2.13 eV (4), and 2.62 eV (5). 1–5 are highly efficient and universal photocatalysts for the degradation of the organic dyes methylene blue (MB), rhodamine B (RhB) and methyl orange (MO) under visible light irradiation, and are very stable and easily separated from the reaction system for reuse. This work shows that the coordinated unsaturated copper coordination polymers should be very promising photocatalysts for degradation of organic dyes.
Introduction
Today, water pollution has become a serious global environmental problem facing humans worldwide with the development of modern industry.1 In particular, the organic dyes released into water from dyeing, dyestuffs and the textile industry are the main contamination of wastewater. Photocatalytic degradation of organic pollutants is an effective technique to eliminate organic pollutants. Traditional semiconductors such as metal oxides TiO2, ZnO, metal sulfides and composites are usually employed as photocatalysts for photocatalytic degradation of organic dyes.2 However, the high band gaps (Eg) of these semiconductors (usually above 3 eV, 3.2 eV for TiO2, 3.4 eV for ZnO), make them only absorb a small amount of ultraviolet light from sunlight.3 The composite semiconductors such as doping the metal ions, or coupling metal oxides (for example TiO2) with other semiconductors with suitable electronic band (such as CuxS, CuInS2, ZnO, SnO2) are useful strategy for developing semiconductor composite photocatalysts under visible light or sunlight irradiation.4 Therefore, apart from the developing the composite semiconductor photocatalysts, searching and development of the efficient and environment friendly new photocatalysts under visible light or sunlight irradiation is very important.In the last two decades, coordination polymers have attracted great interest in inorganic chemistry and materials science because of their fascinating topologies and interesting properties for potential applications as functional materials.5,6 Coordination interactions between metal and ligand result in different metal–ligand charge transfers and affect bandgap width of coordination polymers. Coordination polymers with high or narrow band gaps can absorb UV or visible light as photocatalysts. Recent researches show that these coordination polymers are quite effective photocatalysts for the degradation of organic dyes under UV or even visible light irradiation.7–14 But relative few coordination polymers exhibit effective photocatalytic activity under visible light irradiation due to most of coordination polymers with poor photoresponse in the visible region.10–14 Therefore, the designation and synthesis of coordination polymers with narrow band gaps is very promising prospect in developing efficient visible light photocatalysts.
Copper, as an abundant and cheap element, has been widely studied since the beginning of chemistry. Copper salt and its coordination polymers are good catalysts for catalytic organic reactions and degradation of organic pollutants.14–17 For example, a semi-conductive copper framework {[CuICuII2(DCTP)2]NO3·1.5DMF}n (DCTP = 4′-(3,5-dicarboxyphenyl)-4,2′:6′,4′′-terpyridine) demonstrated hydrogen production in the presence of Pt co-catalyst and the degradation of methylene blue.15 Up to now, most coordination polymers show selective photocatalytic degradation of one organic dye.7–12 Few coordination polymers can photocatalytic degradation of two organic dyes.13 Universal and highly efficient photocatalysts are rare.14
In previous work, we synthesized many coordination polymers with unusual topologies and interesting properties.17,18 Some copper coordination polymers show good photocatalytic activity in the degradation of organic dyes under UV irradiation.17 For examples, {[Cu(tmtz)(H2O)4][Cu2(tmtz)2(sip)2]·4H2O}n (tmtz = 1,4-bis(1,2,4-triazol-1-ylmethyl)-2,3,4,5-tetramethylbenzene, sip = 5-sulfoisophthalate) exhibits good photocatalytic activity for the degradation of methyl orange.17a [Cu4(OH)2(btre)(1,2,3-btc)2(H2O)2]·2H2O}n (btre = 1,2-bis(1,2,4-triazol-4-yl)ethane, 1,2,3-btc = 1,2,3-benzenetricarboxylate) presents the good photocatalytic activity for the degradation of methyl orange.17b {[Cu4(OH)2(itp)2(btc)2(H2O)4][Cu(H2O)4(Hbtc)]2·4H2O}n, {[Cu4(OH)2(itp)2(btc)2]·2EtOH·2H2O}n and {[Cu4(OH)2(itp)2(sip)2(H2O)4]·4H2O}n (btc = 1,2,3-benzenetricarboxylate, itp = 1-imidazol-1-yl-3-(1,2,4-triazol-4-yl)propane) can act as universal and highly efficient photocatalysts for the degradation of organic dyes such as methyl orange (MO), methylene blue (MB) and rhodamine B (RhB).17c
In the present work, in our effort to obtain effective visible light photocatalysts properties, we synthesized five intriguing copper(II) coordination polymers {[Cu4(OH)2(bix)2(1,2,4-btc)2(H2O)2]·3H2O}n (1), [Cu2(bix)(ip)2]n (2), [Cu2(bix)(Meip)2]n (3), {[Cu2(bix)(pbda)2]·H2O}n (4) and {[Cu2(bix)1.5(2,5-pydc)2(H2O)]·2H2O}n (5) using the hydrothermal method (bix = 1,4-bis(2-methyl-imidazol-1-ylmethyl)benzene, 1,2,4-btc = 1,2,4-benzenetricarboxylate, ip = isophthalate, Meip = 5-methyl-isophthalate, pbda = 1,4-benzenediacatate, 2,5-pydc = 2,5-pyridine-dicarboxylate). 1–5 exhibit diverse structures. 1–5 are highly efficient and universal photocatalysts for the degradation of the organic dyes methyl orange (MO), methylene blue (MB) and rhodamine B (RhB) under visible light irradiation.
Experimental section
Materials and physical measurements
1,4-Bis(2-methyl-imidazol-1-ylmethyl)benzene (bix) was synthesized according the literature method.19 All reagents were of analytical grade and used without further purification. Elemental analyses for C, H and N were performed on a Perkin-Elmer 240C analyser. IR spectra were obtained for KBr pellets on a Nicolet 170SX FT-IR spectrophotometer in the 4000–400 cm−1 region. XPRD were performed on a D/MAX-3C diffractometer with the Cu Kα radiation (λ = 1.5406 Å) at room temperature.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was placed in a Teflon-lined stainless steel vessel, heated to 70 °C for five days, then cooled to room temperature over 12 h. The blue crystals of 2 were obtained. Anal. calcd for C32H26Cu2N4O8 (2): C, 53.26; H, 3.63; N, 7.77; found: C, 53.14; H, 3.60; N, 7.73%. IR (cm−1, KBr): 3443 m, 1629 s, 1576 w, 1540 w, 1506 w, 1396 s, 1384 s, 1277 w, 1161 w, 1133 w, 1083 w, 1000 w, 848 w, 803 w, 760 w, 742 m, 718 s, 666 w, 475 w.
1, v/v) was placed in a Teflon-lined stainless steel vessel, heated to 70 °C for five days, then cooled to room temperature over 12 h. The blue crystals of 2 were obtained. Anal. calcd for C32H26Cu2N4O8 (2): C, 53.26; H, 3.63; N, 7.77; found: C, 53.14; H, 3.60; N, 7.73%. IR (cm−1, KBr): 3443 m, 1629 s, 1576 w, 1540 w, 1506 w, 1396 s, 1384 s, 1277 w, 1161 w, 1133 w, 1083 w, 1000 w, 848 w, 803 w, 760 w, 742 m, 718 s, 666 w, 475 w.Crystal data collection and refinement
Suitable single crystals of 1–5 were carefully selected under an optical microscope and glued to thin glass fibers. The diffraction data were collected on Agilent Gemini Atlas CCD diffractometer with graphite monochromated Cu Kα radiation. Intensities were collected by the ω scan technique. The structures were solved by direct methods and refined with full-matrix least-squares technique (SHELXTL-97).20 The parameters of the crystal data collection and refinement of 1–5 are given in Table S1 (ESI†). Selected bond lengths and bond angles are listed in Table S2 (ESI†).Photocatalytic reaction measurement
The experiment was carried out a PCR-I multipurpose photoreactor (Beijing China Education Au-Light Company Limited, China) equipped a CEL-HXF300 Xe lamp with UV cut-off filter (providing visible light with λ > 400 nm), which contains a cylindrical reactor (inner diameter 4.5 cm) surrounded by a circulating water jacket to cool the reactor (20 °C), in a 40 cm × 40 cm × 50 cm closed metal-box. 1 (40 mg), 2 (40 mg), 3 (40 mg), 4 (40 mg) or 5 (40 mg) and 0.50 mL 30% H2O2 were added into 100 mL of a methyl orange (MO) solution (10 mg L−1), or methylene blue (MB) solution (10 mg L−1), or rhodamine B (RhB) solution (10 mg L−1). The suspension solution was stirred in the dark for about 30 min. Then, the mixture was stirred continuously under visible light irradiation from the lamp at a distance of 28 cm between the liquid surface and the cut-off filter. The light intensity of the liquid surface was 3850 W m−2 which was determined by PL-MW2000 photoradiometer (Beijing Perfect Light Company Limited). At a given interval, aliquots of the reaction mixture were periodically taken and analyzed with a UV-vis spectrophotometer at an absorption wavelength of 465 nm for MO, 664 nm for MB and 552 nm for RhB. The blank experiment and the control experiment with photocatalysts 1–5 in the absence of H2O2 were performed.Results and discussion
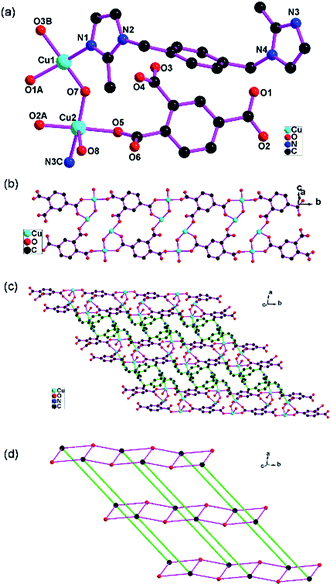
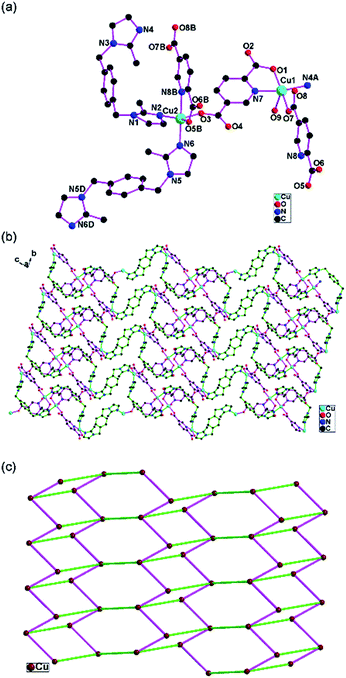
Crystal structure of {[Cu4(OH)2(bix)2(1,2,4-btc)2(H2O)2]·3H2O}n (1)
1 exhibits a (3,5)-connected 2D network based on the [Cu2(μ-OH)] dimer. The asymmetric unit of 1 consists of two Cu(II) atoms, one bix, one 1,2,4-btc, one μ-OH, one coordinated water and disordered lattice water molecules. The Cu1 atom is four-coordinated with two carboxylate oxygen atoms from two 1,2,4-btc (O1A, O3B), one μ-OH oxygen atom (O7) and one imidazole nitrogen atoms from one bix (N1) in the distorted square geometry. The Cu2 atom is five-coordinated with two carboxylate oxygen atoms from two 1,2,4-btc (O2A, O5), one μ-OH oxygen atom (O7) and one imidazole nitrogen atoms from one bix (N3C) in the equation plane and one water oxygen atom (O8) in the apical position in the distorted square-pyramidal configuration (Fig. 1a). The structural distortion index τ for Cu2 atom is 0.202, indicating that the coordination environment of the metal atom is closer to square-pyramidal geometry than trigonal-bipyramidal configuration.21One carboxylate group (O1O2) of the 1,2,4-btc ligand exhibits bidentate bridging mode and connects two Cu(II) atoms (Cu1D, Cu2D). Two other carboxylate groups (O3O4, O5O6) show the monodentate mode and coordinate one Cu(II) atom (Cu1B or Cu2). Each 1,2,4-btc ligand links four Cu(II) atoms (Fig. S1 in ESI†). The μ-OH oxygen atom (O7) connects two Cu(II) atoms and form [Cu2(μ-OH)] dimer. The [Cu2(μ-OH)] dimers are connected by 1,2,4-btc ligands and extend to form the one-dimensional “ladder-like” chain (Fig. 1b).
The bix ligand shows the anti-conformation and bridges two Cu(II) atoms. The one-dimensional “ladder-like” chains are further joined by bix ligands to construct an unusual two-dimensional network (Fig. 1c). The topological analysis of 1 has been performed. If the [Cu2(μ-OH)] dimer is simplified as one node, the [Cu2(μ-OH)] dimers are 5-connected because each [Cu2(μ-OH)] dimer connects three 1,2,4-btc and two bix ligands. The 1,2,4-btc ligands are 3-connected. The bix ligands are 2-connected. The two-dimensional network can be simplified as a (3,5)-connected network (Fig. 1d) with the point symbol of (42·6)(42·67·8).22 The three-dimensional supermolecular architecture (Fig. S2 in ESI†) is formed through the hydrogen bond interactions between the carboxylate oxygen atoms and water molecules (O6⋯O8 2.690(5) Å, O6⋯O9 2.784(7) Å) (Table S3 in ESI†).
Crystal structure of [Cu2(bix)(ip)2]n (2)
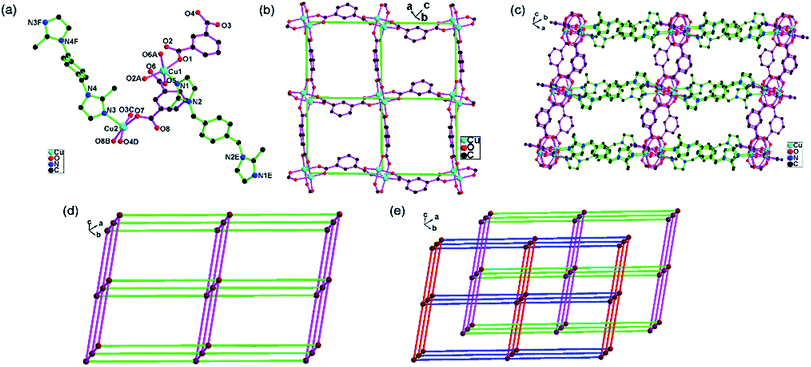
2 exhibits the 2-fold interpenetrating three-dimensional pcu network based on the [Cu2(COO)4] dimers. The asymmetric unit of 2 consists of two Cu(II) atoms (Cu1, Cu2), two halves bix (N1N2, N3N4) and two ip ligands (O1–O4, O5–O8). The Cu1 atom is coordinated by four carboxylate oxygen atoms from four ip ligands (O1, O5, O2A, O6A) in the equation positions and one imidazole nitrogen atom from one bix ligand (N1) in the apical position in the distorted square-pyramidal configuration (Fig. 2a). The Cu2 atom is coordinated by four carboxylate oxygen atoms from four ip ligands (O3B, O4D, O7, O8C) and one imidazole nitrogen atom from one bix ligand (N3) in the distorted square-pyramidal configuration. The structural distortion index τ for Cu1 and Cu2 atoms are 0.007 and 0.014, respectively.21There are two independent ip ligands. All carboxylate groups (O1O2, O3O4, O5O6, O7O8) of the ip ligands exhibit bridging mode structure (Fig. S3 in ESI†). The ip ligands link the Cu(II) atoms and construct the [Cu2(ip)2]n two-dimensional network (Fig. 2b). The adjacent [Cu2(ip)2]n two-dimensional networks are connected by bix ligands and construct the three-dimensional network (Fig. 2c).
Topologically, the [Cu2(COO)4] dimers are simplified as 6-connected nodes (Fig. S4 in ESI†). The ip and bix ligands are 2-connected. The 3D network can be described as a 6-connected 3D pcu network based on the [Cu2(COO)4] dimers (Fig. 2d). Moreover, two identical 3D networks are still mutually interpenetrated to generate the 2-fold interpenetrating 3D pcu network because of the porous nature of the single network (Fig. 2e).
Crystal structure of [Cu2(bix)(Meip)2]n (3)
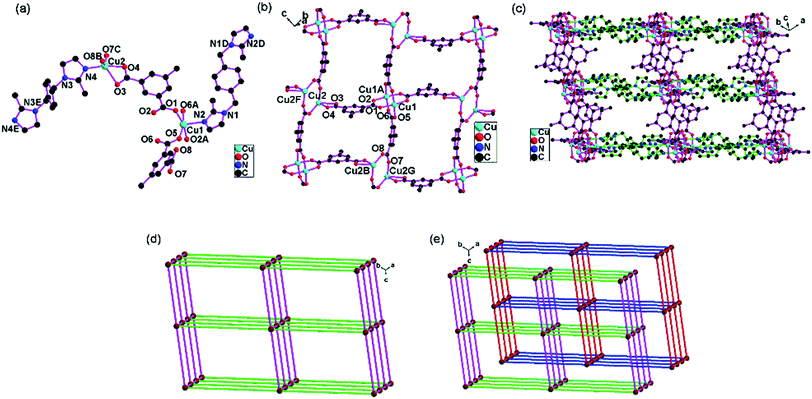
3 exhibits the 2-fold interpenetrating three-dimensional pcu network based on the [Cu2(COO)2] and [Cu2(COO)4] dimers. The asymmetric unit of 3 consists of two Cu(II) atoms (Cu1, Cu2), two halves bix (N1N2, N3N4) and two Meip ligands (O1–O4, O5–O8). The Cu1 atom is coordinated by four carboxylate oxygen atoms from four Meip ligands (O1, O5, O2A, O6A) in the equation positions and one imidazole nitrogen atom from one bix ligand (N2) in the apical position in the distorted square-pyramidal configuration (Fig. 3a) with the structural distortion index τ 0.003. The Cu2 atom is coordinated by four carboxylate oxygen atoms from four Meip ligands (O3, O4, O7C, O8B) and one imidazole nitrogen atom from one bix ligand (N4) in the middle of distorted square-pyramidal configuration and trigonal-bipyramidal configuration with the structural distortion index τ 0.499.21There are two kinds of Meip ligands. The carboxylate groups (O1O2, O5O6, O7O8) of the Meip ligands exhibit bridging mode and connect two Cu(II) atoms (Fig. S5 in ESI†). One carboxylate group (O3O4) of one Meip ligand shows the chelating mode and links one Cu2 atom. The Cu1 and Cu1A atoms are connected by four Meip ligands to form [Cu2(COO)4] paddle wheel structure. However the Cu2 and Cu2F atoms are joined by two Meip ligands to form the [Cu2(COO)2] dimer. Two kinds of Meip ligands link the Cu(II) atoms and construct the [Cu2(Meip)2]n two-dimensional network (Fig. 3b). The adjacent [Cu2(Meip)2]n two-dimensional networks are connected by bix ligands and construct the three-dimensional network (Fig. 3c).
Topologically, the [Cu2(COO)2] and [Cu2(COO)4] dimers are deemed as 6-connected nodes (Fig. S6 and S7 in ESI†). The Meip and bix ligands are 2-connected. The 3D network can be described as a 6-connected 3D pcu network based on the [Cu2(COO)2] and [Cu2(COO)4] dimers (Fig. 3d). Moreover, two identical 3D networks are still mutually interpenetrated to generate the 2-fold interpenetrating 3D pcu network because of the porous nature of the single network (Fig. 3e).
Crystal structure of {[Cu2(bix)(pbda)2]·H2O}n (4)
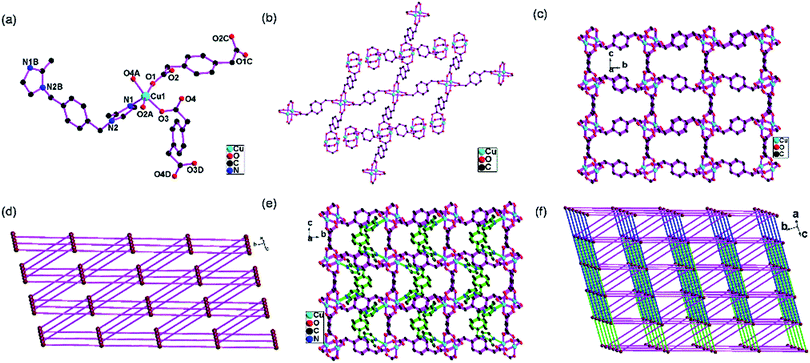
4 shows an unusual 6-connected self-catenated 3D network based on [Cu2(COO)4] dimers. The asymmetry unit consists of one Cu(II), half bix and two halves pbda. Each Cu(II) atom is coordinated by one imidazole nitrogen atom from one bix ligand (N1) and four carboxylate oxygen atoms from four pbda ligands (O1, O3, O2A, O4A) in a distorted square-pyramidal coordination geometry (Fig. 4a). The structural distortion index τ is 0.0025, indicating that the coordination environment of Cu(II) atom is very closer to a square-pyramidal than a trigonal-bipyramidal configuration.21 Each carboxylate group of one pbda ligand shows bidentate bridging mode. Each pbda ligand acts as a tetradentate bridge and joins four Cu(II) atoms (Fig. S8 in ESI†).Two Cu(II) atoms are connected by four pbda ligands and form the [Cu2(COO)4] paddle wheel structure. Each [Cu2(COO)4] dimer connects four [Cu2(COO)4] through pbda ligands (Fig. 4b) and extend to construct the [Cu2(pbda)2]n 3D network (Fig. 4c). Topologically, the [Cu2(COO)4] node is 4-connected and pbda ligand is 2-connected, if the [Cu2(COO)4] is simplified as one node. This [Cu2(pbda)2]n 3D network is simplified as a 4-connected 3D network with the point symbol of (65·8) (Fig. 4d).22
The bix ligands show the gauche-conformation. The self-catenated [Cu2(pbda)2]n 3D network is linked by bix ligands to construct new self-catenated [Cu2(bix)(pbda)2]n 3D network (Fig. 4e). Topologically, the [Cu2(COO)4] nodes are 6-connected, pbda and bix ligands are 2-connected, if the [Cu2(COO)4] is simplified as one node. This [Cu2(bix)(pbda)2]n 3D network is simplified as a 6-connected self-catenated 3D network with the point symbol of (44·610·8) (Fig. 4f).22 The self-catenated motifs are few in the literature.23 This self-catenated 3D network is not reported, as to best of our knowledge.
Crystal structure of {[Cu2(bix)1.5(2,5-pydc)2(H2O)]·2H2O}n (5)
5 exhibits a (3,4)-connected 2D network with the point symbol of (42·6)(42·63·8). The asymmetric unit of 5 consists of two Cu(II) atoms, one and a half bix, two 2,5-pydc, one coordinated water and disordered lattice water molecules. The Cu1 atom is five-coordinated with two carboxylate oxygen atoms (O1, O7) from two 2,5-pydc, two nitrogen atoms from one bix (N4A) and one 2,5-pydc (N7) in the equation plane and one water oxygen atom (O9) in the apical position in a distorted square-pyramidal configuration. The Cu2 atom is five-coordinated with one carboxylate oxygen atom from one 2,5-pydc (O5B), three nitrogen atoms from one 2,5-pydc (N8B) and two bix ligands (N2, N6) in the equation plane and one carboxylate oxygen atom (O3) from one 2,5-pydc in the apical position in the distorted square-pyramidal configuration (Fig. 5a). The structural distortion index τ for Cu1 and Cu2 atoms are 0.087 and 0.377, respectively, indicating that the coordination environment of the Cu1 and Cu2 atom is closer to square-pyramidal geometry than trigonal-bipyramidal configuration and Cu1 atom is almost perfect square-pyramidal configuration.21There are two independent 2,5-pydc ligands. All carboxylate groups (O1O2, O3O4, O5O6, O7O8) of the 2,5-pydc ligand exhibit monodentate mode. Each 2,5-pydc ligand connects two Cu(II) atoms through two carboxylate oxygen atoms and one pyridine nitrogen atom (O1O3N7, O5O7N8). All bix ligands act as bidentate bridging ligands and link two Cu(II) atoms (Fig. S9 in ESI†). The Cu(II) atoms are connected by 2,5-pydc and bix ligands to form a 2D network (Fig. 5b).
The topological analysis of 5 has been performed. Each Cu1 atom coordinates one bix and two 2,5-pydc ligands and is 3-connected. Each Cu2 atom coordinates two bix and two 2,5-pydc ligands and is 4-connected. All 2,5-pydc and bix ligands are 2-connected nodes. The two-dimensional network of 5 can be simplified as a (3,4)-connected network (Fig. 5c) with the point symbol of (42·6)(42·63·8).22 There are hydrogen bonding interactions between the coordination water and carboxylate oxygen atoms, between the coordination water and lattice water, between the lattice water and carboxylate oxygen atoms (Table S3 in ESI†).
PXRD and solid-state diffuse-reflection study
The measured and simulated PXRDs confirm the purity of 1–5 (Fig. S10–S14 in ESI†). The optical diffuse reflection spectra of crystalline solids 1–5 were measured at room temperature. The absorption data were calculated from the reflectance using the Kubelka–Munk function. The energy band gaps (Eg) obtained by extrapolation of the linear portion of the absorption edges were estimated to be 2.21 eV (1), 2.35 eV (2), 2.41 eV (3), 2.13 eV (4), and 2.62 eV (5), indicating their semiconductor nature (Fig. 6).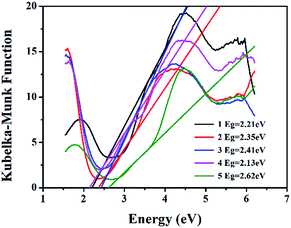 | ||
| Fig. 6 Solid-state optical diffuse-reflection spectra of 1–5 derived from diffuse reflectance data at room temperature. | ||
Catalytic activity for the degradation of methylene blue (MB), rhodamine B (RhB) and methyl orange (MO)
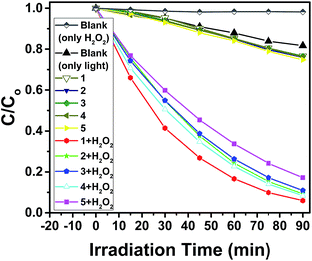
To study the photocatalytic activity of 1–5, we selected the organic dyes methylene blue (MB), rhodamine B (RhB) and methyl orange (MO) as the model dye contaminants to evaluate the photocatalytic effectiveness in the purification of wastewater. The photocatalytic behaviors of 1–5 for the degradation of MB under visible light irradiation were shown in Fig. 7, 8 and Fig. S15–S18 (in ESI†). There is almost no degradation for the blank experiment (only H2O2, without light) for degradation of MB, RhB and MO. There is no significant degradation for the blank experiment (only light, without H2O2) and the control experiment with photocatalysts 1–5 in the absence of H2O2 under visible light irradiation. Meanwhile, the absorption peaks of MB were declined obviously with increasing time when 1–5 as heterogeneous catalysts in the presence of H2O2 under visible light irradiation for degradation of MB. After 90 min, the degradation efficiencies of MB reached 94.0% for 1, 90.7% for 2, 89.1 for 3, 91.6 for 4 and 82.8% for 5. Clearly, 1–5 were good photocatalysts for the degradation of MB. The catalytic efficiencies for degradation of MB are 1 > 4 > 2 > 3 > 5.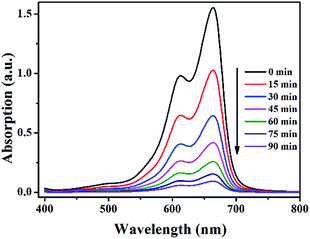 | ||
| Fig. 7 The UV-vis absorption spectra of MB solution during photocatalytic degradation of the MB solution using catalyst 1 in the presence of H2O2 under visible light irradiation. | ||
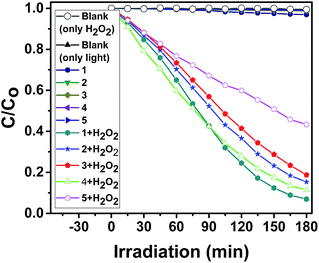
The photocatalytic behaviors of 1–5 for the degradation of RhB under visible light irradiation were shown in Fig. 9, 10 and Fig. S19–S22 (in ESI†). The absorption peaks of RhB were declined obviously with increasing time when 1–5 as heterogeneous catalysts in the presence of H2O2 under visible light irradiation. After 180 min, the degradation efficiencies of RhB reached 93.0% for 1, 84.7% for 2, 81.3 for 3, 88.6 for 4 and 56.7% for 5. There is no significant degradation for the blank experiment (only light, without H2O2) and the control experiment with photocatalysts 1–5 in the absence of H2O2 under visible light irradiation as comparison. 1–5 were effective photocatalysts for the degradation of RhB. The catalytic efficiencies for degradation of RhB are 1 > 4 > 2 > 3 > 5.
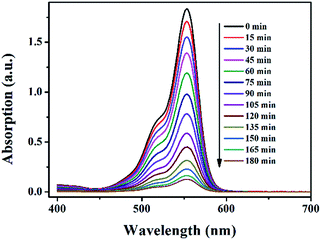 | ||
| Fig. 9 The UV-vis absorption spectra of RhB solution during photocatalytic degradation of the RhB solution using catalyst 1 in the presence of H2O2 under visible light irradiation. | ||
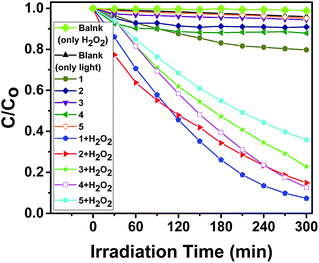
The photocatalytic behaviors of 1–5 for the degradation of MO under visible light irradiation were shown in Fig. 11, 12 and Fig. S23–S26 (in ESI†). The degradation efficiencies of MO reached 92.7% for 1, 85.1% for 2, 77.2 for 3, 87.3 for 4 and 64.1% for 5 after 300 min when 1–5 as heterogeneous catalysts in the presence of H2O2 under visible light irradiation. As comparison, there is no significant degradation for the blank experiment (only light, without H2O2) and the control experiment with photocatalysts 1–5 in the absence of H2O2 under visible light irradiation. Therefore, 1–5 were effective photocatalysts for the degradation of MO. The catalytic efficiencies for the degradation of MO are 1 > 4 > 2 > 3 > 5.
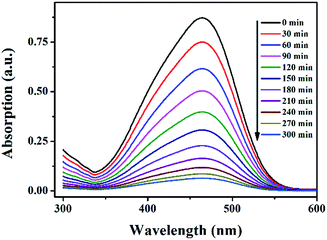 | ||
| Fig. 11 The UV-vis absorption spectra of MO solution during photocatalytic degradation of the MO solution using catalyst 1 in the presence of H2O2 under visible light irradiation. | ||
After the photocatalytic degradation of the organic dyes MB, RhB and MO solutions, the photocatalysts 1–5 can be easily separated by simple centrifugation because they are undissolved in water. After the photocatalysis, the PXRD patterns of 1–5 are in good agreement with that of the original compounds, implying that 1–5 maintain their structural integrity after the photocatalysis reaction (Fig. S10–S14 in ESI†). The photocatalysts 1–5 can be recovered from the catalytic system and reused for five catalytic cycles (Fig. S27–S31 in ESI†). The photocatalytic experiment proves that 1, 2 and 4 are highly efficient and universal photocatalysts for degradation of the organic dyes MB, RhB and MO (Fig. 13).
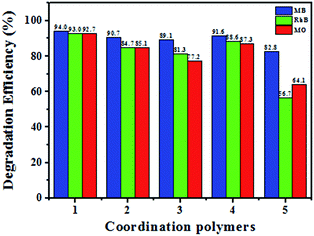 | ||
| Fig. 13 Photocatalytic degradation efficiency of the MB, RhB and MO using catalysts 1–5 in the presence of H2O2 under visible light irradiation. | ||
H2O2 is a good oxide and the precursor of hydroxyl radical (˙OH), which is an effective and highly active oxidizing species for decomposing organic dyes into nonpolluting simple molecules, or CO2 and H2O.24 In order to find out whether hydroxyl radical (˙OH) is participated in the photocatalytic degradation process or not, the photocatalytic reaction using catalyst 1 in the presence of H2O2 and good hydroxyl radical (˙OH) scavenger mannitol (1.0 g) for degradation of MB, RhB and MO under visible light irradiation was performed for comparison. The degradation efficiencies of MB using catalyst 1 in the absence of H2O2, in the presence of H2O2, in the presence of H2O2 and mannitol for degradation of MB were 23.4%, 94.0% and 39.4%, after 90 min (Fig. S32 in ESI†). The degradation efficiencies of RhB using catalyst 1 in the absence of H2O2, in the presence of H2O2, in the presence of H2O2 and mannitol for degradation of RhB were 3.1%, 93.1% and 12.6%, after 180 min (Fig. S33 in ESI†). The degradation efficiencies of MO using catalyst 1 in the absence of H2O2, in the presence of H2O2, in the presence of H2O2 and mannitol for degradation of MO were 20.3%, 92.7% and 28.0%, after 300 min (Fig. S34 in ESI†). The experimental results show that H2O2 can greatly increase the photocatalytic efficiency and the good hydroxyl radical (˙OH) scavenger mannitol can inhibit the photocatalytic reaction. Therefore, hydroxyl radical (˙OH) should participate in the photocatalytic degradation process.
During the photocatalytic process, upon irradiation by visible light, the absorption of energy is equal to or greater than the energy band gap of photocatalysts (hν ≧ 2.21 eV for 1, 2.35 eV for 2, 2.41 eV for 3, 2.13 eV for 4, 2.62 eV for 5), the electrons in the highest occupied molecular orbital (HOMO) are excited to the lowest unoccupied orbital (LUMO). The charge transfer excited state of photocatalyst can be deactivated by oxidizing the contaminant directly and/or oxygenating H2O or H2O2 into a hydroxyl radical (˙OH). The hydroxyl radical (˙OH) can oxidize the organic dyes into nonpolluting simple molecules, or CO2 and H2O to complete the photocatalytic process.7–17
The above photocatalytic reaction shows that the catalytic efficiencies for the degradation of MB, RhB and MO are 1 > 4 > 2 > 3 > 5. The lower energy band gap (Eg) of the photocatalysts (Coordination Polymers) usually can present the high catalytic efficiency. The other factors such as coordination configuration of the metal centers and the structures of the photocatalysts (Coordination Polymers) also play the important role in the degradation rates of the photocatalysts. The Cu(II) atoms in 2–4 all have an unsaturated five-coordinated distorted square-pyramidal configuration. We speculate that the thermodynamic stable [Cu2(COO)4] paddle wheel structures are favourably formed in 2–4 and the Cu(II) atoms in [Cu2(COO)4] paddle wheel structures all exhibit the distorted square-pyramidal configuration because each copper(II) atom in [Cu2(COO)4] hinders the coordination of the other copper(II) atom. The Cu(II) atoms in 5 maybe accidently show an unsaturated five-coordinated distorted square-pyramidal configuration. But the Cu1 atoms and Cu2 atoms in 1 have an unsaturated four-coordinated distorted square geometry and an unsaturated five-coordinated distorted square-pyramidal configuration, respectively. We speculate that each copper(II) atom in the [Cu2(μ-OH)] dimer hiders the coordination of the other copper(II) atom and the copper(II) show the unsaturated four-coordinated and five-coordinated configurations in 1. The highly photocatalytic activity of 1 can be attributed to the lower energy band gap and the Cu(II) atom with unsaturated four-coordinated distorted square geometry.
Chemistry researchers usually select one organic dye as model contaminant to study the photocatalytic properties of photocatalysts.7–12 Only few researches choose two or more model dyes.13,14 The photocatalysts usually exhibit the selectivity of photocatalytic activities for degradation of unique organic dye. Highly efficient and universal photocatalysts are seldom observed.13,14 For example, Wang and coworkers reported five polyoxometalate-based Cu(II) MOFs which show the selectivity photocatalytic degradation of three organic dyes methylene blue (MB), rhodamine B (RhB) and methyl orange (MO) under UV irradiation. Three compounds are good candidates for the photocatalytic degradation of MB, and one compound is a good photocatalyst for the degradation of RhB.13c Zhang and coworkers synthesized two isomeric two-dimensional copper(I) coordination polymers Cu(ptz) which showed high photocatalytic efficiency for the degradation of MB, RhB, and MO in aqueous solution under xenon arc lamp irradiation.14b Wang and coworkers reported a 3D polyoxomolybdate–organic framework, {[Cu3(H3tpb)2(tpb)(Mo4O12)]·4H2O}n which exhibits remarkable photocatalytic activities for decomposition of MO, methylene red (MR), MB, methylene violet (MV) and RhB under UV light.14c Yu, Zhou and coworkers synthesized five molybdophosphate coordination polymers. One coordination polymer as a representative catalyst exhibits a universal highly efficient degradation ability for typical dyes MB, MO, and RhB under UV light.14d Wu and coworkers reported four anionic zwitterion–POM hybrid materials which demonstrate remarkable efficiency for selective scavenging and photolysis of cationic dyes from polluted water.14e Zhu and coworkers synthesized three copper(II) porous coordination polymers which show high catalytic activities for the degradation of rhodamine B (RhB), methylene blue (MB), safranine T (ST), and orange II (OII) dyes in aqueous solution.14f
In the present work, five copper(II) coordination polymers based on 1,4-bis(2-methyl-imidazol-1-ylmethyl)benzene (bix) and different multicarboxylate 1,2,4-benzenetricarboxylate (1,2,4-btc), isophthalate (ip), 5-methyl-isophthalate (Meip), 1,4-benzenediacatate (pbda) and 2,5-pyridine-dicarboxylate (2,5-pydc) were synthesized. 1–5 exhibit diverse structures and are highly efficient and universal photocatalysts for the degradation of the organic dyes methylene blue (MB), rhodamine B (RhB) and methyl orange (MO) under visible light irradiation. The structures and properties of coordination polymers based on bix ligand in literatures are summarized here for discussion and comparison.25 [Cu(Hbtc)(bix)0.5]n (H3btc = 1,3,5-benzenetricarboxylic acid) exhibits a 3D (4,5)-connected net and catalytic degradation of methyl orange in photo Fenton like process in the presence of sodium persulfate under UV irradiation.25a A pair of Cu(I) compound isomers [Cu2(CN)2(bix)] generate a three-fold interpenetrated 3D uninodal (10, 3) network with ThSi2 and utq topology and show the strong green photoluminescence peak at 565 nm.25b Barbour and coworkers reported the single-crystal to single-crystal interconversion of four distinct molecular crystal forms consisting of neutral, dinuclear metal complexes using bix, CuCl2 and different solvents.25c A discrete rectangular complex [Ag2(bix)2](BF4)2·2CH3CN retains its solvent-templated channel structure on guest removal to yield a porous, gas sorbing material for CO2, H2, CH4 and N2 at room temperature (30 °C).25d {[Ag2(bix)3](BF4)2} shows an infinite two-dimensional Borromean coordination framework.25e A 2D corrugated (6,3) network [Zn(dpc)(bix)0.5]n and a 3D microporous framework [Pb(dpc)(bix)0.5]n (dpc = dipicolinate) show luminescence emission peaks at 416 and 441 nm, respectively.25f A 3D → 3D interpenetrated [Ag2(bix)1.5(pzdc)·3H2O]n and a 2D → 3D polycatenated [Ag(bix)(mac)0.5·0.5H2O]n (H2pydc = pyrazine-2,3-dicarboxylic acid, H2mac = L-malic acid) exhibit luminescence emission peaks at 476 and 426 nm, respectively.25g A one-dimensional {[Ag3(bix)2(ndca)1.5]·H2O}n (H2ndca = naphthalene-1,4-dicarboxylicacid) which exhibits a luminescence emission peak at 446 nm.25h A 2-fold interpenetrated 3D pcu network {[Zn(fum)(bix)0.5]·0.5H2O}n (H2fum = fumaric acid) displays a luminescence emission peak at 453 nm.25i {[M(bcpb)(bix)0.5]·xH2O}n (M = Co(II), Cu(II), Ni(II)), (H2bcpb = 3,5-bis(4-carboxyphenyl)pyridine) are isomorphic and show complicated 3D (3,5)-coordinated amd networks, which could be viewed as two interpenetrated ths nets.25j {[Ni(tptc)0.5(bix)]·0.25H2O}n (H4tptc = terphenyl-2,5,2′,5′-tetracarboxylic acid) shows an unprecedented 3D (4,4)-connected framework with the point Schläfli symbol of (4·64·82)2(42·84).25k [Cd(bix)0.5(ndc)]·1.5H2O (H2ndc = naphthalene-2,6-dicarboxylic acid) forms a 3-fold interpenetrated pcu network constructed from paddle-wheel second building blocks.25l [Cd(L)(bix)]n (H2L = 4′-((2-carboxyphenoxy)methyl)biphenyl-2-carboxylic acid) displays a 2D → 3D interdigitated framework and luminescent emissions at 426 nm.25m [Zn2(μ2-OH)(boaba)(bix)]n (H3boaba = 3,5-bis-oxyacetate-benzoicacid) exhibits a (3,5)-connected bimodal gra net with binuclear [Zn2(μ2-OH)(COO)] clusters which shows blue photo-luminescence emissions at 416 nm.25n
Conclusions
In summary, five structural diversity of five-coordinated copper(II) coordination polymers were synthesized. 1–5 are semiconducting in nature, with Eg of 2.21 eV (1), 2.35 eV (2), 2.41 eV (3), 2.13 eV (4), and 2.62 eV (5). 1–5 are highly efficient and universal photocatalysts for the degradation of the organic dyes methylene blue (MB), rhodamine B (RhB) and methyl orange (MO) under visible light irradiation, and are very stable and easily separated from the reaction system for reuse. This work shows that the coordinated unsaturated copper coordination polymers should be very promising photocatalysts for degradation of organic dyes under visible light irradiation.Acknowledgements
This work is supported by the Natural Science Foundation of China (No. 21171126), the Natural Science Foundation of Jiangsu Province (No. BK20161212), the Priority Academic Program Development of Jiangsu Higher Education Institutions, State and Local Joint Engineering Laboratory for Functional Polymeric Materials.References
- W. W. Li, H. Q. Yu and Z. He, Energy Environ. Sci., 2013, 7, 911–924 Search PubMed.
- (a) T. Aarthi and G. Madras, Catal. Commun., 2008, 9, 630–634 CrossRef CAS; (b) T. Jing, Y. Dai, W. Wei, X. C. Ma and B. B. Huang, Phys. Chem. Chem. Phys., 2014, 16, 18596–18604 RSC; (c) J. Di, J. Xia, Y. Ge, H. Li, H. Ji, H. Xu, Q. Zhang, H. Li and M. Li, Appl. Catal., B, 2015, 168, 51–61 CrossRef; (d) H. Wang, M. Miyauchi, Y. Ishikawa, A. Pyatenko, N. Koshizaki, Y. Li, L. Li, X. Li, Y. Bando and D. Golberg, J. Am. Chem. Soc., 2011, 133, 19102–19109 CrossRef CAS PubMed; (e) Z. Sha, J. Sun, H. S. O. Chan, S. Jaenicke and J. Wu, RSC Adv., 2014, 4, 64977–64984 RSC.
- (a) M. N. Chong, B. Jin and C. W. K. Chow, Water Res., 2010, 44, 2997–3027 CrossRef CAS PubMed; (b) E. Piera, M. I. Tejedor-Tejedor, M. E. Zorn and M. A. Anderson, Appl. Catal., B, 2003, 46, 671–685 CrossRef CAS.
- (a) X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed; (b) A. Duta, A. Enesca, C. Bogatu and E. Gyorgy, Mater. Sci. Semicond. Process., 2016, 42, 94–97 CrossRef CAS; (c) A. Enesca, M. Baneto, D. Perniu, L. Isac, C. Bogatu and A. Duta, Appl. Catal., B, 2016, 186, 69–76 CrossRef CAS; (d) A. Enesca, L. Isac and A. Duta, Appl. Catal., B, 2015, 162, 352–363 CrossRef CAS; (e) E. Thimsen, Q. Peng, A. B. F. Martinson, M. J. Pellin and J. W. Elam, Chem. Mater., 2011, 23, 4411–4413 CrossRef CAS; (f) S. H. Hwang, C. Kim and J. Jang, Catal. Commun., 2011, 12, 1037–1041 CrossRef CAS; (g) S. Chen, W. Zhao, W. Liu and S. Zhang, Appl. Surf. Sci., 2008, 255, 2478–2484 CrossRef CAS.
- (a) Z. C. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815–5840 RSC; (b) P. Q. Liao, D. D. Zhou, A. X. Zhu, L. Jiang, R. B. Lin, J. P. Zhang and X. M. Chen, J. Am. Chem. Soc., 2012, 134, 17380–17381 CrossRef CAS PubMed; (c) M. Du, C. P. Li, C. S. Liu and S. M. Fang, Coord. Chem. Rev., 2013, 257, 1282–1305 CrossRef CAS; (d) L. Fu, Y. Liu, M. Pan, X. J. Kuang, C. Yan, K. Li, S. C. Wei and C. Y. Su, J. Mater. Chem. A, 2013, 1, 8575–8580 RSC; (e) M. X. Li, Y. F. Zhang, X. He, X. M. Shi, Y. P. Wang, M. Shao and Z. X. Wang, Cryst. Growth Des., 2016, 16, 2912–2922 CrossRef CAS.
- (a) X. Zhao, X. H. Bu, Q. G. Zhai, H. Tran and P. Y. Feng, J. Am. Chem. Soc., 2015, 137, 1396–1399 CrossRef CAS PubMed; (b) D. S. Li, J. Zhao, Y. P. Wu, B. Liu, L. Bai, K. Zou and M. Du, Inorg. Chem., 2013, 52, 8091–8098 CrossRef CAS PubMed; (c) S. L. Huang, A. Q. Jia and G. X. Jin, Chem. Commun., 2013, 49, 2403–2405 RSC; (d) Z. Z. Lu, R. Zhang, Y. Z. Li, Z. J. Guo and H. G. Zheng, J. Am. Chem. Soc., 2011, 133, 4172–4174 CrossRef CAS PubMed; (e) P. Yang, X. He, M. X. Li, Q. Ye, J. Z. Ge, Z. X. Wang, S. R. Zhu, M. Shao and H. L. Cai, J. Mater. Chem., 2012, 22, 2398–2400 RSC; (f) H. B. Zhou, J. H. Yao, X. P. Shen, H. Zhou and A. H. Yuan, RSC Adv., 2014, 4, 61–70 RSC; (g) X. X. Liu, C. D. Shi, C. W. Zhai, M. L. Cheng, Q. Liu and G. X. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 4585–4591 CrossRef CAS PubMed.
- (a) Y. Hu, F. Luo and F. F. Dong, Chem. Commun., 2011, 47, 761–763 RSC; (b) X. L. Wang, J. Luan, H. Y. Lin, C. Xu, G. C. Liu, J. W. Zhang and A. X. Tian, CrystEngComm, 2013, 15, 9995–10006 RSC; (c) Y. Q. Jiao, C. Qin, H. Y. Zang, W. C. Chen, C. G. Wang, T. T. Zheng, K. Z. Shao and Z. M. Su, CrystEngComm, 2015, 17, 2176–2189 RSC; (d) L. Liu, J. Ding, M. Li, X. Lv, J. Wu, H. Hou and Y. Fan, Dalton Trans., 2014, 43, 12790–12799 RSC; (e) L. L. Liu, C. X. Yu, F. J. Ma, Y. R. Li, J. J. Han, L. Lin and L. F. Ma, Dalton Trans., 2015, 44, 1636–1645 RSC; (f) S. Y. Hao, S. X. Hou, K. V. Heckeb and G. H. Cui, Dalton Trans., 2017, 46, 1951–1964 RSC; (g) J. W. Cui, S. X. Hou, K. V. Heckeb and G. H. Cui, Dalton Trans., 2017, 46, 2892–2903 RSC.
- (a) J. X. Meng, Y. G. Li, H. Fu, X. L. Wang and E. B. Wang, CrystEngComm, 2011, 13, 649–655 RSC; (b) Q. Wu, W. L. Chen, D. Liu, C. Liang, Y. G. Li, S. W. Lin and E. B. Wang, Dalton Trans., 2011, 40, 56–61 RSC; (c) X. He, K. Fang, X. H. Guo, J. Han, X. P. Lu and M. X. Li, Dalton Trans., 2015, 44, 13545–13549 RSC; (d) F. Q. Wang, C. M. Wang, Z. C. Yu, Q. G. He, X. Y. Li, C. L. Shang and Y. N. Zhao, RSC Adv., 2015, 5, 70086–70093 RSC.
- (a) G. H. Cui, C. H. He, C. H. Jiao, J. C. Geng and V. A. Blatov, CrystEngComm, 2012, 14, 4210–4216 RSC; (b) M. Li, S. Zhao, Y. F. Peng, B. L. Li and H. Y. Li, Dalton Trans., 2013, 42, 9771–9776 RSC; (c) J. M. Hao, B. Y. Yu, K. V. Hecke and G. H. Cui, CrystEngComm, 2015, 17, 2279–2293 RSC; (d) W. Y. Yin, Z. L. Huang, X. Y. Tang, J. Wang, H. J. Cheng, Y. S. Ma, R. X. Yuan and D. Liu, New J. Chem., 2015, 39, 7130–7139 RSC.
- (a) X. L. Wang, Z. H. Chang, H. Y. Lin, A. X. Tian, G. C. Liu, J. W. Zhang and D. N. Liu, CrystEngComm, 2015, 17, 895–903 RSC; (b) B. Mu and R. D. Huang, CrystEngComm, 2016, 18, 986–999 RSC; (c) X. L. Wang, Z. H. Chang, H. Y. Lin, A. X. Tian, G. C. Liu and J. W. Zhang, Dalton Trans., 2014, 43, 12272–12278 RSC; (d) L. L. Liu, C. X. Yu, J. M. Du, S. M. Liu, J. S. Cao and L. F. Ma, Dalton Trans., 2016, 45, 12352–12361 RSC; (e) X. L. Wang, X. M. Wu, G. C. Liu, H. Y. Lin and X. Wang, Dalton Trans., 2016, 45, 19341–19350 RSC.
- (a) B. Q. Song, X. L. Wang, J. Liang, Y. T. Zhang, K. Z. Shao and Z. M. Su, CrystEngComm, 2014, 16, 9163–9167 RSC; (b) B. Q. Song, X. L. Wang, C. Y. Sun, Y. T. Zhang, X. S. Wu, L. Yang, K. Z. Shao, L. Zhao and Z. M. Su, Dalton Trans., 2015, 44, 13818–13822 RSC; (c) G. N. Liu, M. J. Zhang, W. Q. Liu, H. Sun, X. Y. Li, K. Li, C. Z. Ren, Z. W. Zhang and C. C. Li, Dalton Trans., 2015, 44, 18882–18892 RSC; (d) J. Zhao, W. W. Dong, Y. P. Wu, Y. N. Wang, C. Wang, D. S. Li and Q. C. Zhang, J. Mater. Chem. A, 2015, 3, 6962–6969 RSC.
- (a) H. X. Yang, T. F. Liu, M. N. Cao, H. F. Li, S. Y. Gao and R. Cao, Chem. Commun., 2010, 46, 2429–2431 RSC; (b) M. C. Das, H. Xu, Z. Y. Wang, G. Srinivas, W. Zhou, Y. F. Yue, V. N. Nesterov, G. D. Qian and B. L. Chen, Chem. Commun., 2011, 47, 11715–11717 RSC; (c) L. Liu, D. Q. Wu, B. Zhao, X. Han, J. Wu, H. W. Hou and Y. T. Fan, Dalton Trans., 2015, 44, 1406–1411 RSC; (d) N. Hussain and V. K. Bhardwaj, Dalton Trans., 2016, 45, 7697–7707 RSC.
- (a) J. Lu, J. X. Lin, X. L. Zhao and R. Cao, Chem. Commun., 2012, 48, 669–671 RSC; (b) Y. Q. Chen, G. R. Li, Y. K. Qu, Y. H. Zhang, K. H. He, Q. Gao and X. H. Bu, Cryst. Growth Des., 2013, 13, 901–907 CrossRef CAS; (c) X. L. Wang, C. H. Gong, J. W. Zhang, G. C. Liu, X. M. Kan and N. Xu, CrystEngComm, 2015, 17, 4179–4189 RSC; (d) Y. P. Wu, X. Q. Wu, J. F. Wang, J. Zhao, W. W. Dong, D. S. Li and Q. C. Zhang, Cryst. Growth Des., 2016, 16, 2309–2316 CrossRef CAS.
- (a) T. Wen, D. X. Zhang, J. Liu, R. Lin and J. Zhang, Chem. Commun., 2013, 49, 5660–5662 RSC; (b) T. Wen, D. X. Zhang and J. Zhang, Inorg. Chem., 2013, 52, 12–14 CrossRef CAS PubMed; (c) X. T. Zhang, L. M. Fan, W. Zhang, Y. S. Ding, W. L. Fan and X. Zhao, Dalton Trans., 2013, 42, 16562–16568 RSC; (d) T. Wen, D. X. Zhang, J. Liu, R. Lin and J. Zhang, CrystEngComm, 2015, 17, 6110–6119 RSC; (e) S. Ou, J. P. Zheng, G. Q. Kong and C. D. Wu, Dalton Trans., 2015, 44, 7862–7869 RSC; (f) C. K. Wang, F. F. Xing, Y. L. Bai, Y. M. Zhao, M. X. Li and S. R. Zhu, Cryst. Growth Des., 2016, 16, 2277–2288 CrossRef CAS.
- Z. L. Wu, C. H. Wang, B. Zhao, J. Dong, F. Lu, W. H. Wang, W. C. Wang, G. J. Wu, J. Z. Cui and P. Cheng, Angew. Chem., Int. Ed., 2016, 55, 4938–4942 CrossRef CAS PubMed.
- (a) X. X. Lu, Y. H. Luo, Y. S. Liu, W. W. Ma, Y. Xu and H. Zhang, CrystEngComm, 2016, 18, 3650–3654 RSC; (b) H. Chen, P. X. Liu, N. Xu, X. Meng, H. N. Wang and Z. Y. Zhou, Dalton Trans., 2016, 45, 13477–13482 RSC; (c) L. J. Han, Y. J. Kong, T. J. Yan, L. T. Fan, Q. Zhang, H. J. Zhao and H. G. Zheng, Dalton Trans., 2016, 45, 18566–18571 RSC.
- (a) M. Li, S. Zhao, Y. F. Peng, B. L. Li and H. Y. Li, Dalton Trans., 2013, 42, 9771–9776 RSC; (b) Y. F. Peng, S. Zhao, K. Li, L. Liu, B. L. Li and B. Wu, CrystEngComm, 2015, 17, 2544–2552 RSC; (c) L. Liu, Y. F. Peng, X. X. Lv, K. Li, B. L. Li and B. Wu, CrystEngComm, 2016, 18, 2490–2499 RSC.
- (a) K. Li, X. X. Lv, L. L. Shi, L. Liu, B. L. Li and B. Wu, Dalton Trans., 2016, 45, 15078–15088 RSC; (b) X. X. Lv, L. L. Shi, K. Li, B. L. Li and H. Y. Li, Chem. Commun., 2017, 53, 1860–1853 RSC.
- B. F. Hoskins, R. Robson and D. A. Slizys, J. Am. Chem. Soc., 1997, 119, 2952–2953 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- (a) A. W. Addison, T. N. Rao, J. Reedijk, J. Van Rijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 7, 1349–1356 RSC; (b) B. L. Li, Z. Xu, Z. B. Cao, L. M. Zhu and K. B. Yu, Transition Met. Chem., 1999, 24, 622–627 CrossRef CAS.
- (a) V. A. Blatov, M. O'Keeffe and D. M. Proserpio, CrystEngComm, 2010, 12, 44–48 RSC; (b) E. V. Alexandrov, V. A. Blatov, A. V. Kochetkova and D. M. Proserpio, CrystEngComm, 2011, 13, 3947–3958 RSC.
- (a) J. Zhang, Y. G. Yao and X. H. Bu, Chem. Mater., 2007, 19, 5083–5089 CrossRef CAS; (b) P. David Martin, M. Ronald Supkowski and L. Robert LaDuca, Cryst. Growth Des., 2008, 8, 3518–3520 CrossRef; (c) X. H. Zhou, X. D. Du, G. N. Li, J. L. Zuo and X. Z. You, Cryst. Growth Des., 2009, 9, 4487–4496 CrossRef CAS; (d) Y. Gong, Y. C. Zhou, T. F. Liu, J. Lu, D. M. Proserpio and R. Cao, Chem. Commun., 2011, 47, 5982–5984 RSC; (e) S. Z. Zhan, M. Li, X. P. Zhou, J. Ni, X. C. Huang and D. Li, Inorg. Chem., 2011, 50, 8879–8892 CrossRef CAS PubMed; (f) W. Q. Kan, J. Yang, Y. Y. Liu and J. F. Ma, Inorg. Chem., 2012, 51, 11266–11278 CrossRef CAS PubMed; (g) K. Zhou, F. L. Jiang, L. Chen, M. Y. Wu, S. Q. Zhang, J. Ma and M. C. Hong, Chem. Commun., 2012, 48, 12168–12170 RSC; (h) C. Liu, G. H. Cui, K. Y. Zou, J. L. Zhao, X. F. Gou and Z. X. Li, CrystEngComm, 2013, 15, 324–331 RSC; (i) X. Q. Yao, J. S. Hu, M. D. Zhang, L. Qin, Y. Z. Li, Z. J. Guo and H. G. Zheng, Cryst. Growth Des., 2013, 13, 3381–3388 CrossRef CAS; (j) M. L. Deng, S. J. Tai, W. Q. Zhang, Y. C. Wang, J. X. Zhu, J. S. Zhang, Y. Ling and Y. M. Zhou, CrystEngComm, 2015, 17, 6023–6029 RSC; (k) J. M. Hu, V. A. Blatov, B. Yu, K. V. Heckec and G. H. Cui, Dalton Trans., 2016, 45, 2426–2429 RSC.
- (a) A. Pintar and J. Levec, Chem. Eng. Sci., 1992, 47, 2395–2400 CrossRef CAS; (b) A. Corma, H. Garcia and F. X. L. Xamena, Chem. Rev., 2010, 110, 4606–4655 CrossRef CAS PubMed; (c) M. N. Chong, B. Jin, C. W. K. Chow and C. Saint, Water Res., 2010, 44, 2997–3027 CrossRef CAS PubMed; (d) J. Kim, C. W. Lee and W. Choi, Environ. Sci. Technol., 2010, 44, 6849–6854 CrossRef CAS PubMed; (e) B. Ahmed, E. Limem, A. Abdel-Wahab and B. Nasr, Ind. Eng. Chem. Res., 2011, 50, 6673–6680 CrossRef CAS; (f) N. Inchaurrondo, J. Font, C. P. Ramos and P. Haure, Appl. Catal., B, 2016, 181, 481–494 CrossRef CAS.
- (a) T. F. Liu, G. H. Cui, C. H. Jiao, C. S. Li and X. C. Deng, Chin. J. Inorg. Chem., 2011, 27, 1417–1422 CAS; (b) R. Y. Huang, C. Xue, C. H. Zhu, Z. Q. Wang, H. Xu and X. M. Ren, RSC Adv., 2014, 4, 61200–61209 RSC; (c) L. Dobrzanska, G. O. Lloyd, C. Esterhuysen and L. J. Barbour, Angew. Chem., Int. Ed., 2006, 45, 5856–5859 CrossRef CAS PubMed; (d) L. Dobrzanska, G. O. Lloyd, H. G. Raubenheimer and L. J. Barbour, J. Am. Chem. Soc., 2005, 127, 13134–13135 CrossRef CAS PubMed; (e) L. Dobrzanska, H. G. Raubenheimer and L. J. Barbour, Chem. Commun., 2005, 5050–5052 RSC; (f) T. F. Liu, W. F. Wu, W. G. Zhang and G. H. Cui, Z. Anorg. Allg. Chem., 2011, 637, 148–153 CrossRef CAS; (g) F. J. Liu, D. Sun, H. J. Hao, R. B. Huang and L. S. Zheng, CrystEngComm, 2012, 14, 379–382 RSC; (h) F. J. Liu, D. Sun, H. J. Hao, R. B. Huang and L. S. Zheng, J. Mol. Struct., 2012, 1014, 70–73 CrossRef CAS; (i) J. X. Yang, Y. Y. Qin, J. K. Cheng and Y. G. Yao, Cryst. Growth Des., 2014, 14, 1047–1056 CrossRef CAS; (j) L. M. Fan, X. T. Zhang, W. Zhang, Y. S. Ding, W. L. Fan, L. M. Sun and X. Zhao, CrystEngComm, 2014, 16, 2144–2157 RSC; (k) L. M. Fan, X. T. Zhang, W. Zhang, Y. S. Ding, W. L. Fan, L. M. Sun, Y. Pang and X. Zhao, Dalton Trans., 2014, 43, 6701–6710 RSC; (l) Z. H. Yan, W. Wang, L. L. Zhang, X. W. Zhang, L. Wang, R. M. Wang and D. F. Sun, RSC Adv., 2015, 5, 16190–16198 RSC; (m) Y. C. He, J. Guo, J. Yang, H. Y. Liu, Y. Y. Liu, Q. Y. Zhai, Q. T. Shen and J. F. Ma, Polyhedron, 2015, 99, 156–169 CrossRef CAS; (n) Y. Y. Sun, S. W. Zhao, H. R. Ma, Y. Han, K. Liu and L. Wang, J. Solid State Chem., 2016, 238, 284–290 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Selected bond lengths and angles, hydrogen bond and additional figures for the crystal structures, XRD patterns, the UV-visible spectra, catalysts recycling, X-ray crystallographic file in CIF format. CCDC 1538437–1538441. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra03268d |
| This journal is © The Royal Society of Chemistry 2017 |