Open Access Article
Open Access ArticleImidazolium-based ionic liquid-catalyzed hydrosilylation of imines and reductive amination of aldehydes using hydrosilane as the reductant†
Bin Li *a,
Shilin Zhanga,
Weizhen Wua,
Lecheng Liangab,
Shaohua Jianga,
Lu Chen
*a,
Shilin Zhanga,
Weizhen Wua,
Lecheng Liangab,
Shaohua Jianga,
Lu Chen *a and
Yibiao Lia
*a and
Yibiao Lia
aSchool of Chemical & Environmental Engineering, Wuyi University, Jiangmen 529020, Guangdong Province, P. R. China. E-mail: binli@wyu.edu.cn; wyuchemcl@126.com
bGuangdong Wamo New Material Technology Co., Ltd, Jiangmen 529020, Guangdong Province, P. R. China
First published on 21st June 2017
Abstract
The first imidazolium-based ionic liquid-catalyzed hydrosilylation of imine and reductive amination of aldehydes with primary amines using a catalytic amount of 1-butyl-3-methylimidazolium tetrachloride iron [BMIm][FeCl4] and Ph2SiH2 as a reductant were performed under mild conditions. Good yields of secondary amines with high chemoselectivity and a tolerance for a wide range of functional groups were obtained.
The formation of amines is one of the most important transformations in chemistry because amines are versatile building blocks for various organic molecules and essential precursors to a variety of biologically active compounds.1 Although several methods are known for amine synthesis, catalytic reduction of imines or direct reductive amination of carbonyl derivatives in the presence of primary amines via sequential condensation/catalytic reduction are some of the most efficient methods through hydrogenation,2,3 or hydrogen transfer reactions.4,5 Recently, transition metal-catalyzed hydrosilylations have attracted attention as a competitive alternative method to the classical stoichiometric reduction, hydrogenation or hydrogen transfer reaction due to their mild conditions and good chemoselectivities.6,7 In contrast, although some catalysts have been included in the amine synthesis via hydrosilylation of imine8–12 and the synthetic method of more green direct reductive amination from primary amines with carbonyl derivatives under hydrosilylation conditions has some examples in the literature,13–17 the IL-catalyzed hydrosilylation of imines or direct reductive amination has not yet been developed.
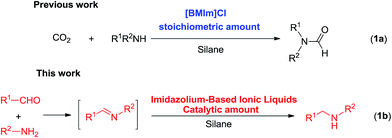
Ionic liquids (ILs), composed of organic cations and organic/inorganic anions, have attracted attention as a competitive alternative green solvent to the classical solvents mainly because of its unique features such as high thermal and chemical stability, negligible vapor pressure, easy separation, and tunable properties.18 ILs have been applied in many areas, especially in catalysis.19 On the other hand, ILs can provide specific functions as a result of cooperative or synergistic effects between ions due to the designable cations and anions from ILs.20 However, only few studies have been reported on IL-catalyzed hydrosilylations. Recently, Liu's group has shown that using a stoichiometric amount of imidazolium-based ionic liquids [BMIm]Cl, high efficiency for the synthesis of formamides of amines and carbon dioxide under hydrosilylation conditions was obtained (Scheme 1, 1a).21
Following our continuous efforts to perform selective reduction via hydrosilylation,22 herein, we report the first simple commercially available 1-alkyl-3-methylimidazolium-based ionic liquids and their use in catalytic amount for hydrosilylation of imines or reductive amination of aldehydes and primary amines (Scheme 1, 1b).
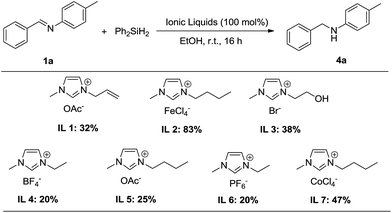
Initial studies focused on the reduction of imine 1a with several stoichiometric amounts of commercially available imidazolium-based ionic liquids and diphenylsilane (1.5 equiv.) as the hydride donor (Scheme 2). Preliminary tests on the ionic liquid 1 (IL 1: [AMIm]OAc) 1-allyl-3-methylimidazolium acetate indicated low conversion to the secondary amine 4a at room temperature. In contrast, 1-butyl-3-methylimidazolium tetrachloride iron (IL 2: [BMIm]FeCl4) was very efficient for this reaction, affording secondary amine 4a in 83% yield within 16 h at room temperature. The other five imidazolium-based ionic liquids IL 3–7 were also examined for catalyzing this reaction, but lower catalytic activity was observed. These findings indicated that different cations and anions have a significant influence on the activity of the ILs and on the catalytic hydrosilylation.
After the ionic liquids were tested in this catalytic system, IL 2 was determined to be the best catalyst and applied in this catalytic hydrosilylation of imine. The reaction could proceed in acetonitrile, toluene, even under neat conditions, but provided the desired product in lower yield (Table 1, entries 1–3). Among the variety of silanes tested in ethanol at room temperature for 16 h in the presence of 1 equiv. of [BMIm]FeCl4, PhSiH3, TMDS (1,1,3,3-tetramethylhydroxysiloxane), and PMHS (polymethylhydrosiloxane) were optimal hydrogen sources towards the formation of the secondary amine 4a (Table 1, entries 4–7). As PMHS is a less expensive and greener hydrosilylation reagent than other hydrosilanes, it was then evaluated for optimizing the conditions. When the reaction temperature increased to 80 °C, a 94% yield of amine 4a was obtained (Table 1 entry 8). Decreasing the amount of IL 2 to 20 mol% led to 66% yield (Table 1, entry 13). However, when this reaction was performed with Ph2SiH2 (1.5 equiv.) instead of PMHS (4 equiv.) in the presence of 20 mol% of IL 2 [BMIm]FeCl4, 91% yield of amine 4a was still obtained (Table 1, entry 15). Decreasing the amount of IL 2 to 10 mol% led to a lower yield using Ph2SiH2 as a hydrogen source (Table 1, entry 16).
| Entry | Amount of IL 2 (mmol) | Silane | Solvent (mL) | Temp. (°C) | GC-yieldb (%) |
|---|---|---|---|---|---|
| a Imine (0.5 mmol), [BMIm][FeCl4], silane (0.75 mmol), solvent (2 mL), 16 h.b Determined by GC.c Isolated yield of 4a. | |||||
| 1 | 0.5 | Ph2SiH2 | — | r.t. | 51 |
| 2 | 0.5 | Ph2SiH2 | CH3CN (2 mL) | r.t. | 50 |
| 3 | 0.5 | Ph2SiH2 | Toluene (2 mL) | r.t. | Trace |
| 4 | 0.5 | PhSiH3 | EtOH (2 mL) | r.t. | 97 |
| 5 | 0.5 | Ph3SiH | EtOH (2 mL) | r.t. | 25 |
| 6 | 0.5 | PMHS | EtOH (2 mL) | r.t. | 83 |
| 7 | 0.5 | TMDS | EtOH (2 mL) | r.t. | 91 |
| 8 | 0.5 | PMHS | EtOH (2 mL) | 80 | 94 |
| 9 | 0.5 | PMHS | EtOH (2 mL) | 90 | 91 |
| 10 | 0.4 | PMHS | EtOH (2 mL) | 80 | 93 |
| 11 | 0.3 | PMHS | EtOH (2 mL) | 80 | 89 |
| 12 | 0.2 | PMHS | EtOH (2 mL) | 80 | 75 |
| 13 | 0.1 | PMHS | EtOH (2 mL) | 80 | 66 |
| 14 | — | PMHS | EtOH (2 mL) | 80 | — |
| 15 | 0.1 | Ph2SiH2 | EtOH (2 mL) | 80 | 91 (85c) |
| 16 | 0.05 | Ph2SiH2 | EtOH (2 mL) | 80 | 86 |
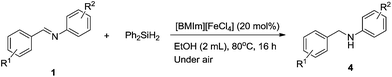
The scope and limitations of this first imidazolium-based-catalyzed hydrosilylation of imines with Ph2SiH2 were then explored using 20 mol% of [BMIm][FeCl4] to produce the secondary amines 4 at 80 °C under an air atmosphere (Table 1, entry 24). Various imines, 1, were applied to synthesize the secondary amines 4, as shown in Table 2.
| Entry | Product | Yield (%) | |
|---|---|---|---|
| a Typical conditions: [BMIm][FeCl4] (20 mol%), aldimine (0.5 mmol), Ph2SiH2 (0.75 equiv), EtOH (2 mL), 80 °C, 16 h. | |||
| 1 |  |
4a: R1 = H | 85 |
| 2 | 4b: R1 = p-OMe | 84 | |
| 3 | 4c: R1 = m-Me | 88 | |
| 4 | 4d: R1 = p-NO2 | 36 | |
| 5 | 4e: R1 = p-CN | 52 | |
| 6 |  |
4f: R1 = H | 94 |
| 7 | 4g: R1 = p-Br | 70 | |
| 8 | 4h: R1 = p-CO2Me | 75 | |
| 9 | 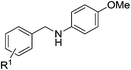 |
4i: R1 = H | 86 |
| 10 | 4j: R1 = p-Me | 90 | |
| 11 | 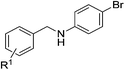 |
4k: R1 = H | 70 |
| 12 | 4l: R1 = p-Me | 72 | |
| 13 | 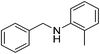 |
4m | 77 |
| 14 | 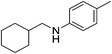 |
4n | 75 |
As shown in Table 2, secondary amine 4a, which was produced from imine 1a with a p-methyl group on the imine R2 aryl, was isolated in 85% yield (entry 1). The reaction could be carried out with p-OMe and m-Me substituents on the imines R1 aryl in 84% and 86% isolated yield, respectively (entries 2 and 3). However, the hydrosilylation was more difficult to perform with the nitro group and cyano group, where only 36% and 52% yield of the corresponding amines were obtained (entries 4 and 5), respectively. No dehalogenation occurred in this hydrosilylation of imine 1g and the corresponding amine 4g was generated in 70% yield (entry 7). More importantly, hydrosilylation tolerated the functional ester group of imine 1h, and the corresponding amine 4h (75%) was directly obtained without alteration of the carbonyl moieties (entry 8). The results of entries 9–12 indicated that the electronic effects of the donating group on the imine R2 aryl resulted in slightly better reactivity than those of the withdrawing group. The steric effect of the o-methyl group on the imine R2 aryl did not hamper the reaction (entry 13). Moreover, the hydrosilylation of the cyclohexane carboxaldehyde 1n successfully led to the corresponding amine 4n with 75% isolated yield (entry 14).
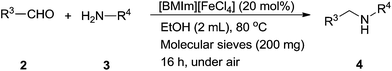
In addition, direct reductive amination from carbonyl derivatives in the presence of primary amines via in situ generated imine and catalytic reduction shows a more green process in the amine synthesis than reduction of imines, which eliminated one condensation and imine isolation step. The reductive amination of aldehydes 2 and anilines 3 with Ph2SiH2 was also explored using 20 mol% of [BMIm][FeCl4] under similar conditions, but with some molecular sieves (Table 3). Various aromatic aldehydes 2 successfully reacted with aniline 3a to produce the secondary amine 4 bearing the p-OMe, p-Me, m-Me, p-NO2, p-CN, and p-Br group on the amine R1 aryl in moderate to good yields (48–86%) (entries 1–7). Several anilines bearing a functional group, such as p-OMe, p-Cl, and o-F, were tested under similar conditions, leading to the corresponding amines 4i, 4q, and 4r in 78%, 75%, and 88% isolated yields, respectively (entries 8–10). Additionally, the aliphatic aldehyde could also be applied to synthesize a secondary amine under these hydrosilylation conditions (entry 11). The reductive product dibenzylamine 4t obtained from the reaction of benzylamine with benzaldehyde was produced and isolated in 76% yield.
| Entry | Product | Yield (%) | |
|---|---|---|---|
| a Typical conditions: [BMIm][FeCl4] (20 mol%), aldehydes (0.6 mmol), aniline (0.5 mmol), Ph2SiH2 (0.75 equiv.), 4 Å molecular sieves (200 mg), EtOH (2 mL), 80 °C, 16 h. | |||
| 1 | 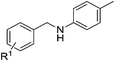 |
4a: R1 = H | 80 |
| 2 | 4b: R1 = p-OMe | 83 | |
| 3 | 4o: R1 = p-Me | 86 | |
| 4 | 4c: R1 = m-Me | 85 | |
| 5 | 4d: R1 = p-NO2 | 48 | |
| 6 | 4e: R1 = p-CN | 55 | |
| 7 | 4p: R1 = p-Br | 82 | |
| 8 | 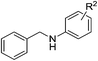 |
4i: R2 = p-OMe | 78 |
| 9 | 4q: R2 = p-Cl | 75 | |
| 10 | 4r: R2 = o-F | 88 | |
| 11 | 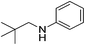 |
4s | 80 |
| 12 | 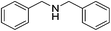 |
4t | 76 |
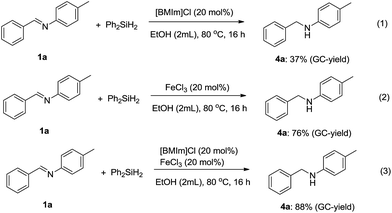
For the imidazolium-based catalytic hydrosilylation, Wang and co-workers reported that imidazolium preferred to activate the Si–H bond of hydrosilane as the first step, as obtained from the calculated results.23 Moreover, the Liu's group also demonstrated that the ionic liquid [BMIm]Cl favored the activation of the Si–H bond of phenylsilane then proceeded for the next transformation.21 To obtain more clear information about the mechanism of this catalytic system, some control experiments were performed (Scheme 3). When [BMIm]Cl instead of [BMIm][FeCl4] was used as a catalyst, the secondary amine 4a was also obtained, but with 37% GC-yield under the same conditions. Moreover, 76% GC-yield of secondary amine 4a was detected using only FeCl3 as a catalyst. However, while mixing both [BMIm]Cl and FeCl3, the GC-yield of the secondary amine 4a was increased to 88%. These results indicate that imidazolium and FeCl3 interact to effect the transformation.
Based on the previous works21,23 and the abovementioned control experiments, we proposed a mechanism (Scheme 4) for the [BMIm][FeCl4]-catalyzed reductive amination. First, [BMIm][FeCl4] interacts with silane silicone, making the Si–H bond of diphenylsilane insert into the in situ generated imine much easier, resulting in the formation of the N-silylamine; the resulting product is then generated via hydrolysis with H2O or EtOH.
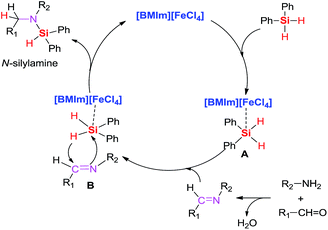 | ||
| Scheme 4 Proposed catalytic cycle for the [BMIm][FeCl4]-catalyzed direct reductive amination of aldehyde and aniline. | ||
In summary, we developed the first imidazolium-based ionic liquid-catalyzed hydrosilylation of imine using catalytic amount of 1-butyl-3-methylimidazolium tetrachloride iron [BMIm][FeCl4]. Moreover, this catalytic system was also applied in the reductive amination of aldehydes to access secondary amines with diphenylsilane. Furthermore, many functional groups, such as nitro, cyano, halide, and ester, were tolerated under this catalytic process. A possible mechanism for the [BMIm][FeCl4]/Ph2SiH2 catalytic system has been demonstrated.
Acknowledgements
We thank the Support received from the Natural Science Foundation of Guangdong Province (No.: 2014A030310211), the Foundation of the Department of Education of Guangdong Province (No.: YQ2015162), SRF for ROCS, SEM (No.: [2015]311), and the Science Foundation for Young Teachers of Wuyi University (No.: 2014zk04, No.: 2015td01 and 2016zk03).Notes and references
- Selected representative books: (a) R. C. Larock, in Comprehensive Organic Transformations: a Guide to Functional Group Preparation, Wiley-VHC, New York, 1989 Search PubMed; (b) A. Ricci, in Modern Amination Method, Wiley-VCH, New York, 2000 Search PubMed; (c) S. A. Lawrence, in Amines: Synthesis, Properties and Applications, Cambridge University Press, Cambridge, 2004 Search PubMed; (d) A. Ricci, in Amino Group Chemistry. From Synthesis to the Life Sciences, ed. A. Ricci, Wiley-VCH, Weinheim, 2008 Search PubMed.
- For reviews see: (a) F. Spindler and H.-U. Blaser, in Transition Metals for Organic Synthesis, ed. M. Beller and C. Bolm, Wiley-VCH, Weinheim, 2nd edn, 2004, vol. 2, p. 113 Search PubMed; (b) H.-U. Blaser and F. Spindler, in Handbook of Homogeneous Hydrogenation, ed. J. G. de Vries and C. J. Elsevier, Wiley-VCH, Weinheim, 2007, vol. 3, p. 1193 Search PubMed; (c) H.-U. Blaser, C. Malan, B. Pugin, F. Spinder, H. Steiner and M. Studer, Adv. Synth. Catal., 2003, 345, 103 CrossRef CAS; (d) W. Tang and X. Zhang, Chem. Rev., 2003, 103, 3029 CrossRef CAS PubMed; (e) T. C. Nugent and M. El-Shazly, Adv. Synth. Catal., 2010, 352, 753 CrossRef CAS; (f) M. Rüping, E. Sugiono and F. R. Schoepke, Synlett, 2010, 852 CrossRef.
- (a) J.-H. Xie, S.-F. Zhu and Q.-L. Zhou, Chem. Rev., 2011, 111, 1713 CrossRef CAS PubMed; (b) N. Fleury-Brégeot, V. de la Fuente, S. Castillon and C. Claver, ChemCatChem, 2010, 2, 1346 CrossRef; (c) Transition Metals for Organic Synthesis, ed. M. Beller and C. Bolm, Wiley-VCH, Weinheim, 2nd edn, 2004, Search PubMed; (d) A. Fabrello, A. Bachelier, M. Urrutigoïty and P. Kalck, Coord. Chem. Rev., 2010, 254, 273 CrossRef CAS.
- (a) S. Gladiali and E. Alberico, in Transition Metals for Organic Synthesis, ed. M. Beller and C. Bolm, Wiley-VCH, Weinheim, 2004, vol. 2, p. 145 Search PubMed; (b) S. E. Clapham, A. Hadzovic and R. H. Morris, Coord. Chem. Rev., 2004, 248, 2201 CrossRef CAS; (c) J. S. M. Samec and J.-E. Bäckvall, Chem.–Eur. J., 2002, 8, 2955 CrossRef CAS PubMed; (d) C. P. Casey and J. B. Johnson, J. Org. Chem., 2003, 68, 1998 CrossRef CAS PubMed; (e) C. Zhu, K. Saito, M. Yamanaka and T. Akiyama, Acc. Chem. Res., 2015, 48, 388 CrossRef CAS PubMed.
- (a) B. Xiong, S. Zhang, H. Jiang and M. Zhang, Org. Lett., 2016, 18, 724 CrossRef CAS PubMed; (b) B. Xiong, Y. Li, W. Lv, Z. Tan, H. Jiang and M. Zhang, Org. Lett., 2015, 17, 4054 CrossRef CAS PubMed; (c) F. Xie, M. Zhang, H. Jiang, M. Chen, W. Lv, A. Zheng and X. Jian, Green Chem., 2015, 17, 279 RSC.
- Selected representative books: (a) I. Ojima, in The Chemistry of Organic Silicon Compounds, ed. S. Patai and Z. Rappoport, Wiley, Chichester, 1989, vol. 1 Search PubMed; (b) V. B. Pukhnarevich, E. Lukevics, L. T. Kopylova and M. G. Voronkov, in Perspectives of Hydrosilylation, ed. E. Lukevics, Institute of Organic Synthesis, Riga, 1992 Search PubMed; (c) M. A. Brook, in Silicon in Organic, Organometallic, and Polymer Chemistry, Wiley, New York, 2000 Search PubMed; (d) H. Nishiyama, in Transition Metals For Organic Synthesis, ed. M. Beller and C. Bolm, 2nd edn, 2004, vol. 2, p. 182 Search PubMed; (e) B. Marciniec, Coord. Chem. Rev., 2005, 249, 2374 CrossRef CAS; (f) O. Riant, in: Modern Reduction Methods, ed. P. G. Anderson and I. J. Munslow, 2008, p. 321 Search PubMed; (g) C. G. Arena, Mini Rev. Org. Chem., 2009, 6, 159 CrossRef CAS; (h) H. Nishiyama, in Comprehensive Chirality, ed. E. M. Carreira and H. Yamamoto, 2012, vol. 5, p. 31:8 Search PubMed.
- Representative examples: (a) J. J. Kennedy-Smith, K. A. Nolin, H. P. Gunterman and F. D. Toste, J. Am. Chem. Soc., 2003, 125, 4056 CrossRef CAS PubMed; (b) K. A. Nolin, R. W. Ahn and F. D. Toste, J. Am. Chem. Soc., 2005, 127, 12462 CrossRef CAS PubMed; (c) S. Abbina, S. Bian, C. Oian and G. Du, ACS Catal., 2013, 3, 678 CrossRef CAS; (d) S. Zhou, K. Junge, D. Addis, S. Das and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 9507 CrossRef CAS PubMed; (e) L. C. Misal Castro, J.-B. Sortais and C. Darcel, Chem. Commun., 2012, 48, 151 RSC; (f) B. H. Lipshutz and H. Shimizu, Angew. Chem., Int. Ed., 2004, 43, 2228 CrossRef CAS PubMed; (g) A. Volkov, F. Tinnis, T. Slagbrand, I. Pershagen and H. Adolfsson, Chem. Commun., 2014, 50, 14508 RSC; (h) Y. Sunada, H. Kawakami, T. Imaoka, Y. Motoyama and H. Nagashima, Angew. Chem., Int. Ed., 2009, 48, 9511 CrossRef CAS PubMed; (i) S. Das, B. Wendt, K. Möller, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2012, 51, 1662 CrossRef CAS PubMed.
- (a) X. Verdaguer, U. E. W. Lange, M. T. Reding and S. L. Buchwald, J. Am. Chem. Soc., 1996, 118, 6784 CrossRef CAS; (b) X. Verdaguer, U. E. W. Lange and S. L. Buchwald, Angew. Chem., Int. Ed., 1998, 37, 1103 CrossRef CAS; (c) H. Gruber-Woelfler, J. G. Khinast, M. Flock, R. C. Fischer, J. Sassmannshausen, T. Stanoeva and G. Gescheidt, Organometallics, 2009, 28, 2546 CrossRef CAS.
- (a) Y. Nishibayashi, I. Takei, S. Uemura and M. Hidai, Organometallics, 1998, 17, 3420 CrossRef CAS; (b) B. Li, J.-B. Sortais, C. Darcel and P. H. Dixneuf, ChemSusChem, 2012, 5, 396 CrossRef CAS PubMed; (c) B. Li, C. B. Bheeter, C. Darcel and P. H. Dixneuf, ACS Catal., 2011, 1, 1221 CrossRef CAS; (d) B. Li, C. B. Bheeter, C. Darcel and P. H. Dixneuf, Top. Catal., 2014, 57, 833 CrossRef CAS.
- (a) L. D. Field, B. A. Messerle and S. L. Rumble, Eur. J. Org. Chem., 2005, 2881 CrossRef CAS; (b) Y. Corre, W. Iali, M. Hamdaoui, X. Trivelli, J.-P. Djukic, F. Agbossou-Niedercorn and C. Michon, Catal. Sci. Technol., 2015, 5, 1452 RSC.
- (a) A. H. Vetter and A. Berkessel, Synthesis, 1995, 419 CrossRef CAS; (b) L. P. Bheeter, M. Henrion, M. J. Chetcuti, C. Darcel, V. Ritleng and J.-B. Sortais, Catal. Sci. Technol., 2013, 3, 3111 RSC; (c) H. Tofazolian and J. A. R. Schmidt, Catal. Sci. Technol., 2016, 6, 685 RSC.
- (a) J. Gajewy, J. Gawronski and M. Kwit, Org. Biomol. Chem., 2011, 9, 3863 RSC; (b) A. Adamkiewicz and J. Mlynarski, Eur. J. Org. Chem., 2016, 1060 CrossRef CAS.
- T. Mizuta, S. Sakaguchi and Y. Ishii, J. Org. Chem., 2005, 70, 2195 CrossRef CAS PubMed.
- (a) S. C. A. Sousa and A. C. Fernandes, Adv. Synth. Catal., 2009, 352, 2218 CrossRef; (b) J. R. Bernardo, S. C. A. Sousa, P. R. Florindo, M. Wolff, B. Machura and A. C. Fernandes, Tetrahedron, 2013, 69, 9145 CrossRef CAS; (c) B. G. Das and P. Ghorai, Org. Biomol. Chem., 2013, 11, 4379 RSC.
- (a) J. Zheng, T. Roisnel, C. Darcel and J.-B. Sortais, ChemCatChem, 2013, 5, 2729 CrossRef; (b) B. Li, J.-B. Sortais and C. Darcel, RSC Adv., 2016, 6, 57603 RSC.
- S. Enthaler, Catal. Lett., 2011, 141, 55 CrossRef CAS.
- (a) S. Enthaler, ChemCatChem, 2010, 2, 1411 CrossRef CAS; (b) H. Jaafar, H. Li, L. C. Misal Castro, J. Zheng, T. Roisnel, V. Dorcet, J.-B. Sortais and C. Darcel, Eur. J. Inorg. Chem., 2012, 3546 CrossRef CAS.
- (a) P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772 CrossRef CAS; (b) B. Singh and S. S. Sekhon, Chem. Phys. Lett., 2005, 414, 34 CrossRef CAS; (c) A. Noda and M. Watanabe, Electrochim. Acta, 2000, 45, 1265 CrossRef CAS; (d) A. Lewandowski and A. Swiderska, Solid State Ionics, 2004, 169, 21 CrossRef CAS.
- (a) Z. F. Zhang, Y. Xie, W. J. Li, S. Q. Hu, J. L. Song, T. Jiang and B. X. Han, Angew. Chem., Int. Ed., 2008, 47, 1127 CrossRef CAS PubMed; (b) F. Shi, Y. Q. Deng, T. L. SiMa, J. J. Peng, Y. L. Gu and B. T. Qiao, Angew. Chem., Int. Ed., 2003, 42, 3257 CrossRef CAS PubMed; (c) L. P. Wu, Q. Liu, I. Fleischer, R. Jackstell and M. Beller, Nat. Commun., 2014, 5, 3091 Search PubMed; (d) Y. F. Zhao, B. Yu, Z. Z. Yang, H. Y. Zhang, L. D. Hao, X. Gao and Z. M. Liu, Angew. Chem., Int. Ed., 2014, 53, 5922 CrossRef CAS PubMed; (e) W. J. Lu, J. Ma, J. Y. Hu, J. L. Song, Z. F. Zhang, G. Y. Yang and B. X. Han, Green Chem., 2014, 16, 221 RSC; (f) J. J. Peng and Y. Q. Deng, New J. Chem., 2001, 25, 639 RSC.
- (a) L. F. Zhang, X. L. Fu and G. H. Gao, ChemCatChem, 2011, 3, 1359 CrossRef CAS; (b) A. L. Girard, N. Simon, M. Zanatta, S. Marmitt, P. Goncalves and J. Dupont, Green Chem., 2014, 16, 2815 RSC; (c) M. Zanatta, A. L. Girard, N. M. Simon, G. Ebeling, H. K. Stassen, P. R. Livotto, F. P. dos Santos and J. Dupont, Angew. Chem., Int. Ed., 2014, 53, 12817 CrossRef CAS PubMed; (d) M. C. Corvo, J. Sardinha, S. C. Menezes, S. Einloft, M. Seferin, J. Dupont, T. Casimiro and E. J. Cabrita, Angew. Chem., Int. Ed., 2013, 52, 13024 CrossRef CAS PubMed.
- L. Hao, Y. Zhao, B. Yu, Z. Yang, H. Zhang, B. Han, X. Gao and Z. Liu, ACS Catal., 2015, 5, 4989 CrossRef CAS.
- (a) B. Li, J. Yu, C. Li, Y. Li, J. Luo and Y. Shao, Tetrahedron Lett., 2017, 58, 137 CrossRef CAS; (b) B. Li, J. Zheng, W. Zeng, Y. Li and L. Chen, Synthesis, 2017, 49, 1349 CrossRef CAS.
- F. Huang, G. Lu, L. L. Zhao, H. X. Li and Z. X. Wang, J. Am. Chem. Soc., 2010, 132, 12388 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra04245k |
| This journal is © The Royal Society of Chemistry 2017 |