Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A highly effective Ag–RANEY® nickel hybrid catalyst for reduction of nitrofurazone and aromatic nitro compounds in aqueous solution
Alireza Khorshidi * and
Bahareh Ghorbannezhad
* and
Bahareh Ghorbannezhad
Department of Chemistry, Faculty of Sciences, University of Guilan, P. O. Box: 41335-1914, Iran. E-mail: khorshidi@guilan.ac.ir; Fax: +98 13 33 33 32 62; Tel: +98 911 339 7159
First published on 9th June 2017
Abstract
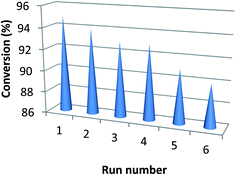
RANEY® nickel reduced Ag+ ions to form ultrafine spherical silver nanoparticles over itself, and the obtained hybrid material was used as catalyst for efficient and selective reduction of aromatic nitro compounds, and nitrofurazone as a non-aromatic example, in aqueous solution by using NaBH4 as reducing agent. Other silver nanostructures with different morphologies such as silver nano-flowers were also prepared and their efficiency in the reduction process was evaluated. Ag–RANEY® nickel catalyst however, had superior advantages including mild reaction conditions, higher conversion yield, and reduction in aqueous solution at near ambient temperature. The catalyst was also recoverable and showed 5% decrease in efficiency after six successive runs.
Introduction
Nitroaromatic compounds are generally prepared via nitration of simple aromatic compounds1 and are extensively being used in various industries such as plastics, dyeing, agriculture, etc. As a result, nitroaromatic compounds are usually present in industrial effluents and agricultural waste water.2 Presence of these compounds in this waste, even in small quantities, is highly undesirable and has drastic adverse effects on the environment and human beings. Hence, there is an ongoing demand for new methods of removal or conversion of these compounds to less harmful products. Anilines are an important class of precursors for the synthesis of pharmaceuticals, dyes, and other biologically active compounds such as Glu-P-1 and Trp-P-2,3–5 and can be produced by selective reduction of nitroaromatic compounds. While traditional methods such as reduction with iron powder in presence of hydrochloric or acetic acid6 are gradually being eliminated due to environmental issues, catalytic hydrogenation processes have emerged as more effective alternatives. Some of the recent reports in this context include application of silver nanoparticles stabilized by polyaminocyclodextrin,7 Fe(II) complexes and nano zero-valent iron,8 gold nanoparticles,9 NaBH4/RANEY® nickel,10 Ag nanoparticles supported on cellulosic fibers,11 palladium nanoparticles,12 and platinum single-atom catalysts.13 Other reagents such as hydrazine/RANEY® nickel,14 Na2S/NEt4Br,15 Sm(0),16 H2/RANEY® nickel,17 metal carbonyls such as Mo(CO)6,18 and Pd/Fe bimetal nanoparticles embedded in P-doped mesoporous carbons,19 have also been reported. However, limitations associated with these methods, such as long reaction time, high temperature or pressure, use of harmful organic solvents, need of special apparatus and expensive reagents or catalysts, prevent their practical application. RANEY® nickel, on the other hand, is an outstanding catalyst in the field of transfer hydrogenation.20–23 Its performance in the catalytic hydrogenation of nitroaromatic compounds however, still needs to be improved. Hereby, we report a combination of silver nanoparticles and RANEY® nickel as an effective catalyst in the reduction of a variety of nitroaromatic compounds as well as nitrofurazone, as a non-aromatic example, by NaBH4 (Scheme 1).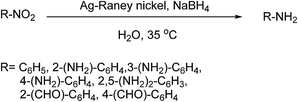 | ||
| Scheme 1 Reduction of various nitro compounds in water at near ambient temperature catalyzed by Ag–RANEY® nickel. | ||
Experimental
Materials and methods
RANEY®-nickel was purchased from W. R. Grace and Co. All of the other chemicals were purchased from Sigma-Aldrich and used without further purification. Flower-like silver nanoparticles were prepared according to the literature.24 Silver nanoparticles deposited over the surface of RANEY® nickel were prepared via two different methods. For the reduction of Ag+ ions with NaBH4, the method of Mirkin,25 without using hydrogen peroxide and PVP was used. For the reduction of Ag+ ions with RANEY® nickel itself as a reducing agent, the following method was used. RANEY® nickel (100 mg) was dispersed in 500 mL of double distilled water for 15 min in a round-bottom flask located in an ultrasonic bath. Then the mixture was simultaneously stirred by means of a mechanical stirrer rotating at 1500 rpm and 100 mL of 0.5 mM AgNO3 solution was injected at once. The trend of reduction was monitored by sodium chloride test. After 30 min, the product (Ag–RANEY® nickel) was separated by centrifugation at 2000 rpm, washed with acetone and dried under reduced pressure.Instrumentation
UV-Vis spectra were recorded on a Perkin Elmer LAMBDA 25 spectrophotometer. XRD measurements were performed by using a Philips X'pert diffractometer with mono chromatized Cu Kα radiation at 40 kV and 20 mA (Ni filter, 2θ 10 to 70° with a step size of 0.05° and a count time of 1 s). Scanning electron micrographs were obtained on a Jeol JSM 6400. TEM images were obtained on a transmission electron microscope (TEM-PHILIPS MC 10) with an acceleration voltage of 80 kV. ICP-OES analysis was performed on a PerkinElmer Optima 8300 ICP-OES spectrometer.Results and discussion
Characterization of the catalysts
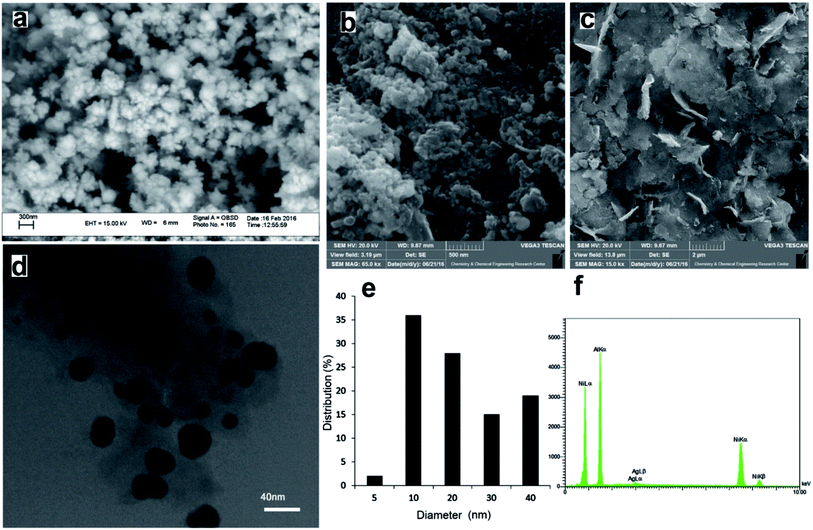
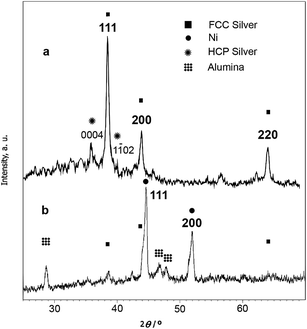
Silver nanostructures with a drastically different morphology were prepared and their efficiency in reduction of nitroaromatic compounds was evaluated. Low-magnification SEM micrographs of the flower-like silver nanoparticles, Ag nanoparticles prepared by reduction with NaBH4, and those prepared by reduction with RANEY® nickel are shown in Fig. 1a–c. From Fig. 1a, it is clear that the obtained nanoflowers have rod-like tips of about 60–90 nm in diameter. Reduction of Ag+ with NaBH4 on the other hand, resulted in aggregates of disordered silver nanoparticles (Fig. 1b) over the surface of RANEY® nickel. Reduction with RANEY® nickel however, resulted in aggregate free surfaces (Fig. 1c), and a closer look by a transmission electron microscope revealed the formation of ultrafine spherical silver nanoparticles ranging from 10 to 40 nm throughout the entire surface (Fig. 1d) based on the corresponding size distribution chart (Fig. 1e). Energy dispersive X-ray analysis also, revealed the simultaneous presence of Ni, Al and Ag (Fig. 1f).In the XRD diffractogram of the flower-like silver nanoparticles (Fig. 2a), three peaks at 38°, 44° and 74° correspond to 111, 200 and 220 planes of face-centered-cubic (FCC) silver on the basis of standard values in the card (JCPDS file no. 04-783). Moreover, formation of some hcp phase, based on the reflections at 36° and 40° indexed to (0004) and (1102) planes of hcp silver is evident. In the XRD pattern of the Ag nanoparticles prepared by reduction with RANEY® nickel (Ag–RANEY® nickel, Fig. 2b), in addition to the (111) and (200) reflections of Ni at 44.5° and 52°, corresponding reflections of fcc silver were also present. Other peaks at 28.5°, 46.5° and 47.5° correspond to alumina trihydrates. Generally, RANEY® nickel consists of metallic nickel, and aluminum as either metal or Al2O3·3H2O. The alumina trihydrates were identified as components of the RANEY® nickel and it has been shown that their crystalline structure does not dehydrate considerably during evacuation at elevated temperature.26 It should also be noted that the Ag content of the Ag–RANEY® nickel catalyst was determined by ICP-OES method and the result was similar to that obtained by EDX analysis (0.83 w% vs. 0.71 w%).
Taking into account the requirements of sustainable and green chemistry, including mild reaction conditions, using non-toxic solvents and in situ preparation of reactive components such as hydrogen, reduction of nitrobenzene in water was selected as a model reaction to determine the optimum conditions such as catalyst type, catalyst loading, pH, and temperature. NaBH4 was used as the source of hydrogen because it is fairly an inexpensive reagent. Control experiments showed that the reduction efficiency depends on the catalyst type. From Table 1 it is clear that under the same conditions, Ag–RANEY® nickel resulted in the best reduction efficiency in terms of the apparent rate constant being calculated as ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C/C0 = −kt (where C is the residual and C0 is the initial concentration of the nitrobenzene, measured at 269 nm at room temperature in pH = 7.0). Higher activity of Ag–RANEY® nickel may be attributed to the uniform distribution of ultrafine silver nanoparticles which provides higher surface area for these nanoparticles, and more active sites would be available for the catalytic reduction.
C/C0 = −kt (where C is the residual and C0 is the initial concentration of the nitrobenzene, measured at 269 nm at room temperature in pH = 7.0). Higher activity of Ag–RANEY® nickel may be attributed to the uniform distribution of ultrafine silver nanoparticles which provides higher surface area for these nanoparticles, and more active sites would be available for the catalytic reduction.
| Entrya | Catalyst type | Catalyst loading (mg) | Apparent rate constant, k (min−1) |
|---|---|---|---|
| a All of the reactions were carried out according to the general experimental procedure at room temperature. | |||
| 1 | None | — | 0.001 |
| 2 | Flower-like Ag nanoparticles | 5 | 0.159 |
| 3 | RANEY® nickel | 5 | 0.254 |
| 4 | Ag aggregates on RANEY® nickel | 5 | 0.342 |
| 5 | Ag–RANEY® nickel | 5 | 0.435 |
It was also found that reduction efficiency depends on the catalyst loading. Fig. 3 shows that increase in the catalyst loading from 3.0 to 10.0 mg results in a non-linear increase in the reduction efficiency. Based on these data, 5.0 mg of the Ag–RANEY® nickel catalyst was selected as the optimum amount.
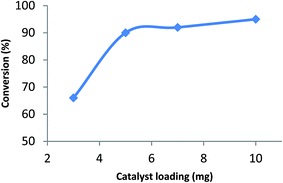 | ||
| Fig. 3 Reduction efficiency in terms of conversion (%) vs. catalyst loading, after 5 min under general experimental conditions. | ||
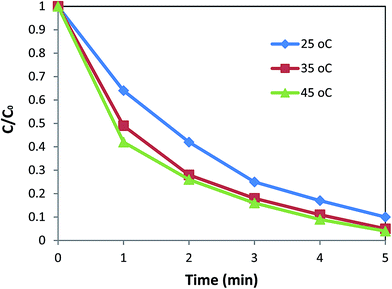
In order to investigate the effect of temperature on the reduction of nitrobenzene, the reaction was carried out at different temperatures of 25, 35, and 45 °C under an optimized catalyst loading of 5.0 mg, and C/C0 vs. time was plotted. From Fig. 4, it is clear that an increase in the temperature results in slightly better efficiency.
It was also found that the reduction of nitrobenzene follows a pseudo first order kinetic with the apparent rate constant being calculated as ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C/C0 = −kt. The value of k for 5.0 mg of the catalyst was found to be 0.457, 0.568, and 0.605 min−1 at 25, 35, and 45 °C, respectively (Fig. 5). Based on these data, 35 °C was selected as the optimum temperature for reduction of nitroaromatic compounds, which in turn, has the advantage of proximity to the ambient temperature.
C/C0 = −kt. The value of k for 5.0 mg of the catalyst was found to be 0.457, 0.568, and 0.605 min−1 at 25, 35, and 45 °C, respectively (Fig. 5). Based on these data, 35 °C was selected as the optimum temperature for reduction of nitroaromatic compounds, which in turn, has the advantage of proximity to the ambient temperature.
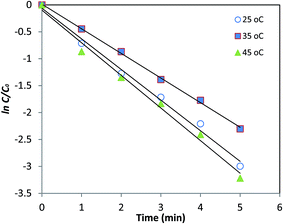 | ||
Fig. 5 Plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C/C0 vs. time at 25, 35, and 45 °C for reduction of nitrobenzene in presence of 5.0 mg of the catalyst. C/C0 vs. time at 25, 35, and 45 °C for reduction of nitrobenzene in presence of 5.0 mg of the catalyst. | ||
With the optimized conditions in hand, a variety of nitroaromatic compounds were used to evaluate generality and scope of this reductive protocol, and typical results are shown in Table 2.
| Entrya | Nitroaromatic compound | Productb | Apparent rate constant, k (min−1) |
|---|---|---|---|
| a All of the reactions were carried out according to the general experimental procedure.b Products were identified from their MS spectra in comparison with authentic samples. | |||
| 1 | 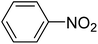 |
 |
0.568 |
| 2 | 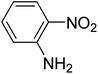 |
 |
0.386 |
| 3 |  |
 |
0.229 |
| 4 |  |
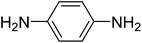 |
0.528 |
| 5 | 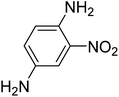 |
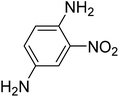 |
0.124 |
| 6 | 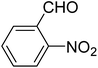 |
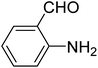 |
0.139 |
| 7 | 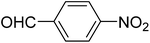 |
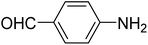 |
0.061 |
| 8 | 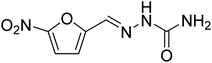 |
 |
0.035 |
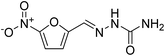
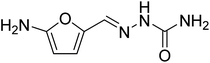
In order to extend applicability of the Ag–RANEY® nickel/NaBH4 protocol to non-aromatic nitro compounds, nitrofurazone (Fig. 6) was selected due to its contribution in carcinogenesis.
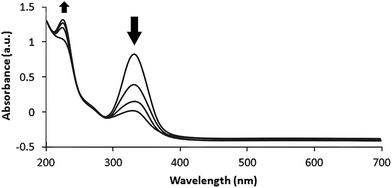
Nitrofurazone is a broad-spectrum antibiotic used in livestock, and has been detected in tissues and milk of animals.27,28 Significant correlation between nitrofurazone reduction and cellular DNA damage has been reported.29 Thus reduction of nitrofurazone residues may prevent release of this drug into the animal food and subsequent risk to human health. When 100 mL of a 0.2 mM solution of nitrofurazone in water was treated with 5 mg of Ag–RANEY® nickel catalyst and 1.0 mL of freshly prepared 0.04 M NaBH4 at 45 °C, a continuous change in the UV-Vis spectrum of the solution was observed. Fig. 7 shows the recorded spectra after 15, 30, 45 and 60 min of stirring of the reaction mixture. Clearly, the band at 324 nm originating from the extended conjugated structure of nitrofurazone tends to disappear, while a distinct evolution in the band at 220 nm, corresponding to n → π* transitions of NH2 groups, was observed. These changes, coincides with the color change of the solution from yellow to colorless. Under these circumstances, 89% of the starting nitrofurazone was reduced based on the measurement of the absorbance of the solution at 324 nm.
In order to evaluate reusability of the catalyst, reduction of nitrobenzene was carried out in presence of the recycled catalyst. Fig. 8 shows the reduction efficiency against successive reaction runs. As it is clear, after six runs, only 5% decrease in efficiency in terms of conversion percent was observed.
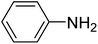
To get an insight into the reaction mechanism of reduction of nitrobenzene, we assumed that if the reaction proceeds via polar intermediates such as nitrosobenzene, phenylhydroxylamine and hydrazine, then each of these intermediates must follow the same path, leading to the same product. Formation of such intermediates is often observed with many other reducing agents.16,30 When nitrosobenzene or phenylhydroxylamine was treated with Ag–RANEY® nickel and NaBH4 in aqueous solution at 35 °C, the final product was aniline and it can be concluded that the following reaction path rationalized the overall reduction.
| Ph-NO2 → Ph-NO → Ph-NHOH → Ph-NH2 |
Conclusions
In conclusion, we have devised a new catalyst based on silver nanoparticles distributed over the surface of RANEY® nickel for efficient reduction of nitro compounds in aqueous solution under mild conditions. Highlights of the present work include near ambient reaction temperature, use of water as a green solvent, in situ production of the reducing agent from readily available NaBH4, and above all, reusability which promises minimization of the catalyst waste.Acknowledgements
The authors are grateful to the Research Council of University of Guilan for the partial support of this study.References
- J. P. Adams and J. R. Paterson, J. Chem. Soc., Perkin Trans. 1, 2000, 3695–3705 RSC.
- H.-Y. Lee and M. An, Bull. Korean Chem. Soc., 2004, 25, 1717–1719 CrossRef CAS.
- H.-U. Blaser, Science, 2006, 313, 312–313 CrossRef CAS PubMed.
- A. Corma, P. Concepción and P. Serna, Angew. Chem., 2007, 119, 7404–7407 CrossRef.
- T. Sugimura, K. Wakabayashi, H. Nakagama and M. Nagao, Cancer Sci., 2004, 95, 290–299 CrossRef CAS PubMed.
- C. A. Merlic, S. Motamed and B. Quinn, J. Org. Chem., 1995, 60, 3365–3369 CrossRef CAS.
- M. Russo, F. Armetta, S. Riela, D. C. Martino, P. L. Meo and R. Noto, J. Mol. Catal. A: Chem., 2015, 408, 250–261 CrossRef CAS.
- S. He, H. Niu, T. Zeng, S. Wang and Y. Cai, ChemistrySelect, 2016, 1, 2821–2825 CrossRef CAS.
- H. Zhu, X. Ke, X. Yang, S. Sarina and H. Liu, Angew. Chem., 2010, 122, 9851–9855 CrossRef.
- I. Pogorelić, M. Filipan-Litvić, S. Merkaš, G. Ljubić, I. Cepanec and M. Litvić, J. Mol. Catal. A: Chem., 2007, 274, 202–207 CrossRef.
- F. Torkamani and S. Azizian, J. Mol. Liq., 2016, 214, 270–275 CrossRef CAS.
- N. Mei and B. Liu, Int. J. Hydrogen Energy, 2016, 41, 17960–17966 CrossRef CAS.
- H. Wei, X. Liu, A. Wang, L. Zhang, B. Qiao, X. Yang, Y. Huang, S. Miao, J. Liu and T. Zhang, Nat. Commun., 2014, 5, 5634 CrossRef CAS PubMed.
- F. Yuste, M. Saldaña and F. Walls, Tetrahedron Lett., 1982, 23, 147–148 CrossRef CAS.
- G. D. Yadav and S. V. Lande, Adv. Synth. Catal., 2005, 347, 1235–1241 CrossRef CAS.
- C. Yu, B. Liu and L. Hu, J. Org. Chem., 2001, 66, 919–924 CrossRef CAS PubMed.
- M. Viktor, D. Ilavsky and J. Salon, Molecules, 1997, 2, M13 CrossRef CAS.
- S. Iyer and G. M. Kulkarni, Synth. Commun., 2004, 34, 721–725 CrossRef CAS.
- Y. Zhou, L. Tang, G. Yang, G. Zeng, Y. Deng, B. Huang, Y. Cai, J. Tang, J. Wang and Y. Wu, Catal. Sci. Technol., 2016, 6, 1930–1939 CAS.
- H. D. Burge, D. J. Collins and B. H. Davis, Ind. Eng. Chem. Prod. Res. Dev., 1980, 19, 389–391 CrossRef CAS.
- P. Gallezot, P. Cerino, B. Blanc, G. Fleche and P. Fuertes, J. Catal., 1994, 146, 93–102 CrossRef CAS.
- H. Adkins and H. R. Billica, J. Am. Chem. Soc., 1948, 70, 695–698 CrossRef CAS.
- P. Fouilloux, Appl. Catal., 1983, 8, 1–42 CrossRef CAS.
- T. Liu, D. Li, D. Yang and M. Jiang, Langmuir, 2011, 27, 6211–6217 CrossRef CAS PubMed.
- G. S. Métraux and C. A. Mirkin, Adv. Mater., 2005, 17, 412–415 CrossRef.
- S. Robertson and R. Anderson, J. Catal., 1971, 23, 286–294 CrossRef CAS.
- E. Sugden, A. Macintosh and A. Vilim, J. - Assoc. Off. Anal. Chem., 1983, 66, 874–880 CAS.
- A. Vilim and A. MacIntosh, J. - Assoc. Off. Anal. Chem., 1979, 62, 19–22 CAS.
- P. L. Olive, Chem.-Biol. Interact., 1978, 20, 323–331 CrossRef CAS PubMed.
- Q. X. Shi, R. W. Lu, K. Jin, Z. X. Zhang and D. F. Zhao, Chem. Lett., 2006, 35, 226–227 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |