Open Access Article
Open Access ArticleMillifluidic synthesis of cadmium sulfide nanoparticles and their application in bioimaging
Liying Hong†
 ab,
Tai-Lok Cheung†c,
Nanxi Raoc,
Qingling Ouyangad,
Yue Wange,
Shuwen Zengad,
Chengbin Yangab,
Dang Cuonga,
Peter Han Joo Chong*f,
Liwei Liu
ab,
Tai-Lok Cheung†c,
Nanxi Raoc,
Qingling Ouyangad,
Yue Wange,
Shuwen Zengad,
Chengbin Yangab,
Dang Cuonga,
Peter Han Joo Chong*f,
Liwei Liu *e,
Wing-Cheung Law*c and
Ken-Tye Yong*ab
*e,
Wing-Cheung Law*c and
Ken-Tye Yong*ab
aSchool of Electrical and Electronic Engineering, Nanyang Technological University, 50 Nanyang Avenue, 639798, Singapore. E-mail: ktyong@ntu.edu.sg
bNanyang Environment and Water Research Institute, Nanyang Technological University, Singapore
cDepartment of Industrial and Systems Engineering, The Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong SAR, P. R. China. E-mail: roy.law@polyu.edu.hk
dCINTRA CNRS/NTU/THALES, UMI 3288, Research Techno Plaza, 50 Nanyang Drive, Border X Block, Singapore
eSchool of Science, Changchun University of Science and Technology, International Joint Research Center for Nanophotonics and Biophotonics, Changchun 130000, P. R. China. E-mail: liulw@cust.edu.cn
fDepartment of Electrical and Electronic Engineering, Auckland University of Technology, New Zealand. E-mail: peter.chong@aut.ac.nz
First published on 25th July 2017
Abstract
In this paper, a miniature fluidic synthesis platform utilizing millimeter dimension channels for the synthesis of cadmium sulfide (CdS) quantum dots and nanocrystals is demonstrated. Traditional nanoparticle synthesis techniques involve macroscopic flasks where reaction conditions may vary at different positions inside the vessel. Therefore challenges in terms of batch reproducibility for large scale production are of great concern. Here, we show that it is possible to replicate reaction conditions so as to produce nanoparticles with similar optical characteristics across different batches using the same reaction parameters. Particle size control was established by varying the flow rate of the precursors, yielding gradually increasing nanocrystal sizes from 2.4 nm to 3.7 nm with increasing residence time. The as-synthesized CdS nanoparticles exhibited tunable photoluminescence by adjusting the molar ratio of the cadmium and sulfur precursors, giving rise to greenish-blue and orange-red emissions under ultraviolet light illumination. The particles were then studied and characterized using transmission electron microscopy (TEM), ultraviolet-visible (UV-Vis) absorbance, photoluminescence, X-ray diffraction (XRD) and selected area electron diffraction (SAED) techniques. Lastly, bioimaging of RAW264.7 mice macrophage cells using ligand exchanged CdS nanoparticles is presented.
Introduction
Quantum dots (QDs) are semiconductor nanoparticles whose sizes in all three dimensions are around or less than the exciton Bohr radius of the bulk material. For the case of cadmium sulfide (CdS), the exciton Bohr radius is 2.8 nm,1 which is much smaller than other semiconductor cadmium chalcogenides like cadmium selenide (CdSe – 5.6 nm)2 and cadmium telluride (CdTe – 7.3 nm).3 Therefore, the synthesis of such small nanoparticles is challenging using bulk synthesis methods. Studies on colloidal CdS began as early as 1981 by Grätzel et al.4 when they investigated the electrolysis of water driven by irradiation of light on CdS nanoparticles with ruthenium(IV) oxide catalyst. Following which many preparation methods such as hot-injection,5,6 solvothermal,7,8 non-injection9,10 and microwave-assisted synthesis11,12 emerged over time. However, some of these techniques involve the use of elevated temperatures, pressures, highly explosive organometallic compounds, and long reaction durations, resulting in differences in terms of particle size, morphology and crystallinity of the CdS nanoparticles. Therefore, we believe that miniature reaction vessels can help to improve the quality, reproducibility and ease of production of luminescent CdS nanocrystals (NCs).CdS semiconductor nanoparticles are of interest in various applications such as optoelectronics particularly in photovoltaics and photoactivation,13–16 bioimaging17 and biosensing.18 At the nanoscale where quantum confinement effects come into play, these nanoparticles can exhibit very different optical and chemical properties from its bulk material. CdS nanocrystals exhibit rich morphologies, for example spheres, nanorods, nanocubes, tripods, tetrapods, candy corn, and arrow-shaped.5,19 Therefore, understanding and controlling of the shapes, sizes and surface characteristics of CdS nanoparticles are crucial for developing devices with unique properties like quantum dot-sensitized solar cells with higher stability than thin-film based solar cells made using the same materials.20
Miniature reaction chambers provide the advantages of a well-controlled environment by means of reproducible mixing conditions enabled by manipulation of laminar fluid flow in engineered channel designs or active micromixers,28,29 excellent heat and mass transfer due to increased surface area to volume ratio30 provided by the miniature channel dimensions. In addition, on-chip synthesis provides a spatial-to-temporal mapping of the reaction dynamics.31 Traditional macroscale QD fabrication methods such as organometallic synthesis involve the usage of highly toxic precursors and generation of considerable chemical waste like solvents, surfactants, unreacted precursors and unwanted reaction by-products. On the other hand, microfluidic and millifluidic synthesis methods are greener approaches as the quantity of required chemicals are much reduced and the homogenous reaction conditions result in lesser waste products due to more efficient reaction kinetics.32
Due to the potential advantages of miniature systems, many groups have also explored CdS nanoparticle synthesis using miniature reaction vessels such as capillary tubes, microreactors, microfluidic chips, and even microorganisms like bacteria (Table 2). Although there is great promise in these minuscule technologies, they are still under development and have different limitations which need to be tackled. For example, microscale systems involving capillary tubes offer simplicity in terms of implementation and adaptation to macroscale injection methods, but are constrained by limited geometric designs and relatively larger footprints.33 Biosynthesis methods, on the other hand, despite enabling the fabrication of biocompatible nanoparticles using green chemistry at room temperatures, suffers from low throughput, long synthesis durations,27 and requires further investigation of the nanoparticle purification processes as biomolecules might still be attached to the nanoparticle surfaces. Miniature fluidic channels or lab-on-chip (LoC) devices, in addition to the advantages offered by capillary tubes, provide additional flexibility for various designs to generate reproducible mixing within the channel. However, most of the existing on-chip synthesis of CdS nanoparticles are based on aqueous solvents with reaction temperatures around 25–30 °C, yielding poor crystalline nanoparticles.
In this work, controlling the size and morphology of CdS nanocrystals (NCs) in a millifluidic chip was demonstrated. By varying the injection flow rate and precursor ratio, spheres, nanorods and multipods with sizes ranging from 2.6 nm to 6.7 nm were obtained. The NCs synthesized in less than 3.5 min exhibited good crystallinity as substantiated by the high resolution transmission electron microscope (HRTEM) images as well as the powder X-ray diffraction (XRD) patterns. Size variations along with changes in the optical properties of these as-synthesized CdS NCs were also achieved by varying the residence time, and molar ratios of the precursors. Here, we employed oleylamine (OAm) as both the surfactant and coordinating solvent for the reaction. Oleylamine has been successfully proven to control shape and size of nanocrystals in traditional benchtop methods as demonstrated by Yong et al.5 and Joo et al.,19 where elemental sulfur was added to metal–oleylamine complexes to form semiconducting metal sulfide nanoparticles. Therefore, we believe that there is great potential in extending their use in millifluidic synthesis methods. We first discuss the effects of chip residence time on the NCs formed, followed by examining the effect of varying the molar ratios of the Cd and S precursors. Lastly, the millifluidic chip synthesized CdS NCs were employed in a proof of concept demonstration for in vitro bioimaging using RAW264.7 mice macrophage cells.
Experimental
Materials
Cadmium chloride and sulfur (Sigma Aldrich), oleylamine (>50.0%, Tokyo Chemical Industry Co., Ltd.). Toluene (>99.5%, Chemicals Testing and Laboratory, Singapore), ethanol (95%, Aik Moh Pte Ltd, Singapore) were used in the synthesis of cadmium sulfide nanoparticles. The 184 Sylgard silicone elastomer kit from Dow Corning was used to fabricate the polydimethylsiloxane (PDMS) millifluidic chip. RAW264.7 mice macrophage cell line (ATCC, Manassas, VA, USA), Dulbecco's Modified Eagle's Medium ([DMEM], Hyclone), fetal bovine serum ([FBS], Hyclone), penicillin-streptomycin (Gibco, Life Technologies, SG, Singapore) were used for the cell culture. All the above chemicals were used as purchased without further purification. Deionized (DI) water mentioned in the experiments was purified by a Milli-Q water purification system.Fabrication of millifluidic chip
The millifluidic channels were designed using the free software SketchUp and the mould was printed in acrylonitrile butadiene styrene (ABS) material using the UP Plus 2 3D printer (PP3DP). The Sylgard 184 Elastomer Kit was used for the polydimethylsiloxane (PDMS) mixture based on a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the base resin to the curing agent. The base and curing agent were then mixed homogeneously before degassing the mixture in a dessicator to remove the bubbles. Following that, the PDMS mixture was poured into the mould, degassed and heat cured on a hotplate at 70 °C for at least 4 hours. Subsequently, the PDMS was carefully removed from the mould and bonded to a glass slide. Holes were then made at the inlets and outlet of the chip and polytetrafluoroethylene (PTFE) tubes used to deliver the precursors and nanocrystal product were inserted and sealed to the chip.
1 ratio of the base resin to the curing agent. The base and curing agent were then mixed homogeneously before degassing the mixture in a dessicator to remove the bubbles. Following that, the PDMS mixture was poured into the mould, degassed and heat cured on a hotplate at 70 °C for at least 4 hours. Subsequently, the PDMS was carefully removed from the mould and bonded to a glass slide. Holes were then made at the inlets and outlet of the chip and polytetrafluoroethylene (PTFE) tubes used to deliver the precursors and nanocrystal product were inserted and sealed to the chip.
Synthesis of CdS nanocrystals in millifluidic chip
The synthesis protocol used is similar to ref. 5 and 19. Briefly, 6 mmol of sulfur powder was dissolved in 10 ml of oleylamine and stirred at room temperature in a flask until all the powder has completely dissolved. In another three neck flask, 1 mmol of cadmium chloride (CdCl2) was dissolved in 10 ml of oleylamine, heated and stirred at 170 °C under nitrogen (N2) flow for 20 minutes, before the temperature was raised further to 200 °C for another 10 minutes. Both the sulfur-oleylamine (S-OAm) and the cadmium-oleylamine (Cd-OAm) mixtures were then withdrawn using separate syringes and injected into the PDMS millifluidic chip.The precursors were delivered into the millifluidic chip via the syringe pump (KD Scientific), where the injection flow rates of the precursors were set at 5000 μl min−1, 3000 μl min−1, 1500 μl min−1, 750 μl min−1 and 375 μl min−1 and the products of the reaction was collected at the outlet of the chip. In addition, different molar ratios of the precursors were also used. The molar ratios of cadmium is to sulfur (Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S) were varied from 1
S) were varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (1 mmol of CdCl2 and 3 mmol of S), 1
3 (1 mmol of CdCl2 and 3 mmol of S), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, and 1
6, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 (1 mmol of CdCl2 and 12 mmol of S). Toluene was added external to the chip in the collecting vial to quench the reaction and stop further growth of the nanocrystals. To purify the CdS NCs, 2 parts of ethanol were added to 1 part of the output solution and mixed before centrifuging at 8000 rpm for 3 minutes. The supernatant was decanted and the pellet was redispersed in toluene for subsequent storage of the NCs.
12 (1 mmol of CdCl2 and 12 mmol of S). Toluene was added external to the chip in the collecting vial to quench the reaction and stop further growth of the nanocrystals. To purify the CdS NCs, 2 parts of ethanol were added to 1 part of the output solution and mixed before centrifuging at 8000 rpm for 3 minutes. The supernatant was decanted and the pellet was redispersed in toluene for subsequent storage of the NCs.
Characterization of CdS nanocrystals
The ultraviolet-visible (UV-Vis) absorption spectrum of the CdS nanocrystals was measured using a Shimadzu UV-2450 spectrophotometer, while the photoluminescence emission spectrum was collected using a HORIBA Jobin Yvon Fluorolog-3 Fluorometer. Transmission electron microscope (TEM) images were obtained using a JEOL JEM-2011 TEM. The Rigaku SmartLab powder X-ray diffractometer was used to carry out the powdered X-ray diffraction (XRD) measurements to determine the crystallographic structures present.Ligand exchange of CdS nanocrystals with mercaptosuccinic acid
For 5 mg of CdS NCs with oleylamine ligands, 150 mg of mercaptosuccinic acid (MSA) should be used. The MSA was dissolved in 3 ml of chloroform with stirring, then the as-synthesized CdS with oleylamine ligands from the previous section was added. Subsequently, ammonium hydroxide (NH3·H2O) and deionized water (DI H2O) were added to the mixture in a volumetric ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 and stirred vigorously overnight. When the ligand exchange process is completed, the mixture should exhibit two distinct layers with the yellow CdS NCs as the top layer and a clear bottom layer. Extract the top layer carefully and flocculate the NCs using ethanol, decant the supernatant and dissolve the pellet in deionized water for further use.
7 and stirred vigorously overnight. When the ligand exchange process is completed, the mixture should exhibit two distinct layers with the yellow CdS NCs as the top layer and a clear bottom layer. Extract the top layer carefully and flocculate the NCs using ethanol, decant the supernatant and dissolve the pellet in deionized water for further use.
Cell culture and bioimaging of macrophage cells using millifluidic CdS nanocrystals
RAW264.7 mice macrophage cells (American Type Culture Collection) were cultured with Dulbecco's modified eagle's medium (DMEM, Hyclone), supplemented with 10% FBS, penicillin (100 μg ml−1, Gibco) and streptomycin (100 μg ml−1, Gibco) in an incubator at 37 °C and 5% CO2. The cells were seeded at a density of 1 × 105 cells per well with DMEM culture medium. 3.8 mg ml−1 of CdS-MSA were added to the macrophage cells and incubated for 4 hours. Then, the treated cells were rinsed thrice with PBS buffer (pH = 7.2) and fixed with 4% formaldehyde. In vitro microscope images were obtained using a fluorescence microscope (Eclipse Ti–U Inverted Microscope System, Nikon) and a filter cube with 350 nm excitation wavelength and a red emission filter with a centre wavelength of 630 nm.Results & discussion
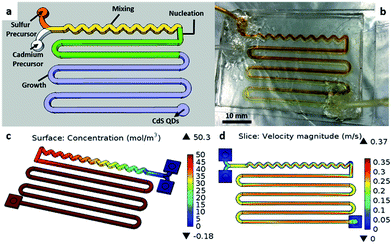
The dimensions of the channel on the miniature chip is 1.5 mm (width) by 1.5 mm (height), spanning a total length of approximately 545 mm and capable of carrying a total fluid capacity of 1227 mm3 or 1227 μl. Due to the millimeter scaled channel, we term the chip as a millifluidic chip. There are five sections constituting this chip. Two inlets for the precursor solutions and one outlet are depicted in Fig. 1b. The sulfur and cadmium precursors are injected into the chip via separate inlets before the two streams meet at the Y-shaped region. Next, the streams encounter a winding section, which folds the fluids and promotes mixing34 as illustrated by the flow velocity profile in Fig. 1d. After mixing, the solution then flows into the straight regions where nucleation and growth take place to form the CdS NCs indicated by the increase in CdS concentration simulated in Fig. 1c. Finally, the reaction is quenched as it flows out of the chip outlet into the cold toluene solution.The base of the millifluidic chip is bonded to a glass slide to allow uniform heating of the entire chip and reduce temperature fluctuations of particles at different regions of the chip. Our continuous flow fluidic chip boasts a simple passive mixing environment providing stable conditions for nanoparticle incubation. Unlike traditional flask synthesis techniques for CdS nanoparticles, where it is necessary to flush the reaction vessel with nitrogen gas5,19 to avoid oxidation of the nanoparticles, this millifluidic synthesis platform enables mixing of the precursors within the chip without the need to purge the channels with inert gases.35 The inert environment is only required in the precursor preparation stage, prior to mixing of both precursors.
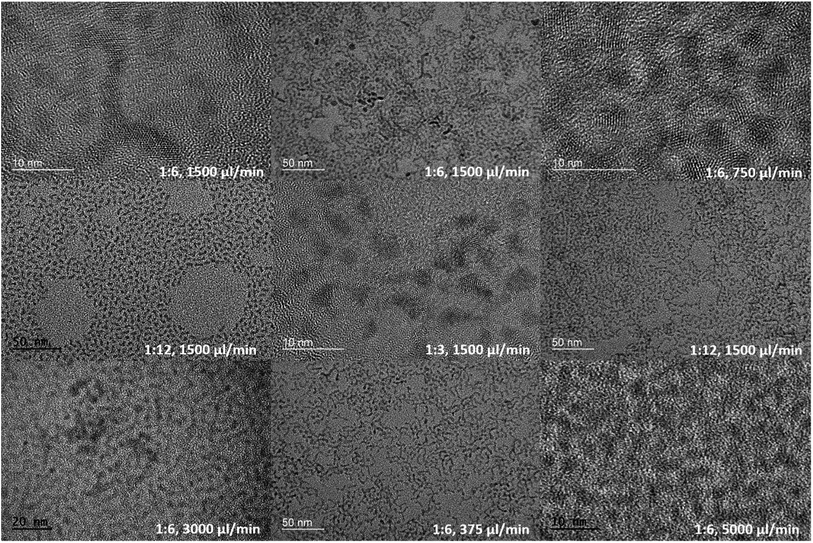
Examples of CdS NCs with different morphologies and sizes synthesized using our millifluidic chip are shown in Fig. 2. By varying parameters such as the injection flow rate, dominantly spherical particles could be obtained with sizes ranging from 2.4–5.8 nm. In addition, morphologies such as tripods and bipods were also obtained by changing the flow rate and the Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S ratios.
S ratios.
Effect of injection flow rate/residence time
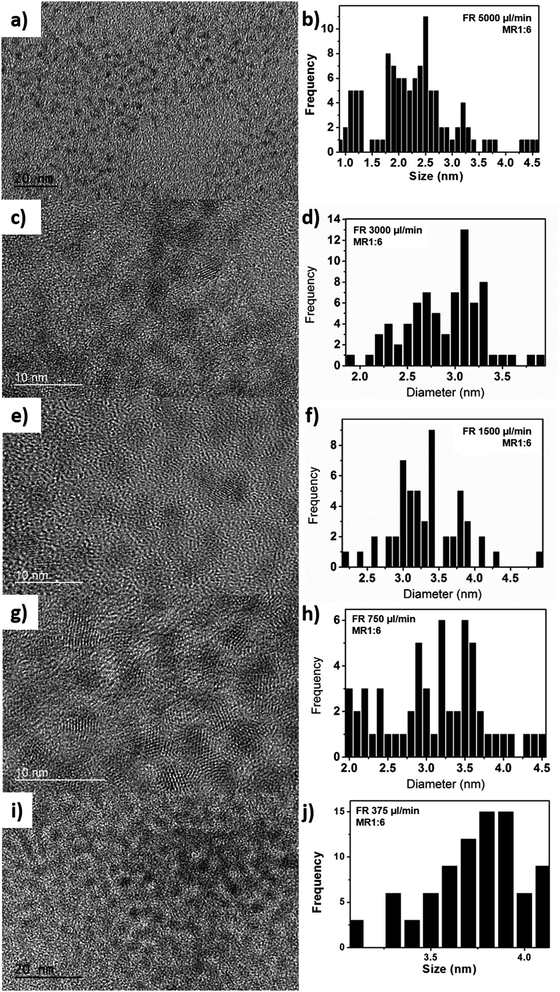
Different injection flow rates were studied to examine their effects on the formation of CdS NCs. The injection flow rate determines the residence time (τR) of the fluid in the chip. Because the reaction time in bulk macroscale synthesis methods cannot be directly converted into the residence time in the chip,36 studies involving short reactions times can be made possible by utilizing miniature reactors. Compared to conventional bench top synthesis using the same reactants,5,19 our homemade millifluidic chip was set with flow rates of 5000 μl min−1 and 3000 μl min−1, corresponding to a residence time of about 15 s and 25 s respectively, produced quantum-confined NCs or quantum dots with an average size of 2.4 ± 0.4 nm and 2.8 ± 0.4 nm (Fig. 6a–d), bordering the exciton Bohr radius of CdS. Upon decreasing the flow rate to 1500 μl min−1, 750 μl min−1, and 375 μl min−1, larger nanocrystals were obtained with average diameters of 3.3 ± 0.5 nm (Fig. 6e and f), 3.5 ± 0.5 nm (Fig. 6g and h), and 3.7 ± 0.3 nm (Fig. 6i and j) respectively. Based on the TEM images in Fig. 2, the shape of the CdS NCs were varied (i.e. spherical, bipod and tripod) by altering the injection flow rate.The ultraviolet-visible (UV-Vis) absorption curves in Fig. 3a show the first excitonic peaks for the CdS NCs synthesized at different flow rates. Based on empirical studies by Yu et al.,37 the size of the NCs can be estimated from the position of the excitonic peaks. The absorption spectra in Fig. 3a further corroborates the particle sizes determined from the TEM images with peak values at 385 nm, 401 nm, 410 nm, 419 nm, and 426 nm corresponding to flow rates of 5000 μl min−1, 3000 μl min−1, 1500 μl min−1, 750 μl min−1, and 375 μl min−1 respectively. The size of these NCs determined from our TEM images (Table 1) agree largely with the empirical estimation derived by Yu et al.,37 varying on an average of 0.5 nm. The longer residence time due to slower injection flow rates enabled the particles to increase in size as a result of the longer growth period.
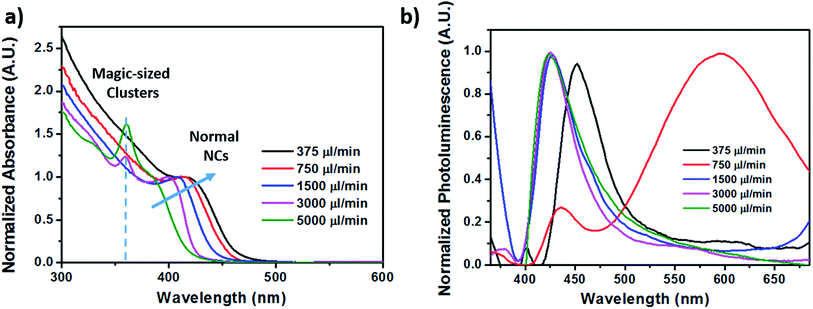 | ||
Fig. 3 (a) Absorption curves and (b) photoluminescence spectra of CdS NCs formed at different flow rates with Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S molar ratio fixed at 1 S molar ratio fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. 6. | ||
| Synthesis method | Reaction condition/vessel | τR | Absorption excitonic peak | Photoluminescence (Y/N) | Size and shape+, D: diameter, L: length, W: width | Crystal phase | Year and ref |
|---|---|---|---|---|---|---|---|
| a τR – residence/reaction time. Photoluminescence (Y/N) – resence of photoluminescence (yes/no). MSC – magic-size cluster. + – sizes listed are the diameters of the nanoparticles unless otherwise stated. * – size calculated from excitonic peak of absorption spectrum according to ref. 24. ‡ – not stated explicitly, inferred from X-ray diffraction (XRD) pattern. † – stated that particles synthesized at room temp were not very crystalline, hence was not characterized. N. A. – not applicable (due to lack of data/description). | |||||||
| Microfluidics-chip based droplet segmented flow (aqueous) | Microfluidic channels, reaction at room temp | 75 ms | 290–370 nm | Y, 360 nm, only description, no other details | N. A.†, D: 1.32 nm, 1.64 nm, 2.51 nm* | N. A.†, particles synthesized at room temp were not very crystalline | 2004 (ref. 21) |
| Adjust relative inlet flow rates to control droplet ratio | N. A. | 427–477 nm | Y, 618 nm | Spherical, D: 4.2–8.2 nm | N. A. | 2005 (ref. 22) | |
| Microfluidic-capillary microreaction system (organic) | Capillary microreactor with heater at 260–285 °C | 20 s | 411–459 nm | Y, ∼420–460 nm, noticeable trap state emission | Spherical, D: 3.74–5.66 nm* | Zinc blende‡ | 2009 (ref. 23) |
| Microfluidic-continuous flow capillary microreactor (organic) | Capillary microreactor in oil bath at 180–260 °C | 68 s | 389–453 nm HWHM ∼12 nm, MSC peak at 320 nm | Y, 391–463 nm. Weak PL due to high conc of surface defects | Spherical, D: 3.0–5.4 nm* | Zinc blende | 2010 (ref. 24) |
| Millifluidic reactor (organic) | Reactants mixed prior to entering the stainless steel coil maintained at 270 °C | 3 min | 425 nm | Y, 443 nm | N. A., D: 4.25 nm* | N. A. | 2015 (ref. 25) |
| Biosynthesis-extracellular microbial synthesis (aqueous) | White rot fungus at 37 °C | 12 h | ∼450 nm | Y, 458 nm, FWHM < 30 nm | Spherical, D: 1.5–2.0 nm (TEM), 2.56 nm average (from XRD Scherer's eqn) | Cubic zinc blende | 2014 (ref. 26) |
| Bacteria from living abalone at 30 °C | 7 days | 375 nm (broad band-edge absorption) | Y, 590 nm and 610 nm | Spherical, D: 50–100 nm, (∼90 nm average) | Cubic zinc blende | 2016 (ref. 27) | |
| Millifluidic-continuous flow millifluidic chip (organic) | Millimeter dimensioned channels, reaction at 170 °C | 25–98 s | 393–426 nm MSC peak at 361 nm | Y, band-edge ∼430 nm, defect states: ∼530–390 nm | Spherical, D: 2.4–5.8 nm, bipods and multipods, L: 3.5–6.7 nm, W: 2.6–5.6 nm | Mixed phases of wurtzite and zinc blende | 2017, our work |
| Flow rate (μl min−1) | Molar ratio (Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S) S) |
Precursor conc. (Cd, S) (mol l−1) | Shape | Average dimensions* (nm) | Excitonic peak (nm) | PL peak and FWHM (nm) | τR (s) | Crystal structure |
|---|---|---|---|---|---|---|---|---|
| a Conc. – concentration, * – determined using TEM images, MSC - magic-sized clusters, FWHM – full wave half maximum, PL – photoluminescence, τR – residence time. | ||||||||
| 5000 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
0.10, 0.6 | Spherical | 2.4 ± 0.4 | 361 (MSC), 385 | 425 ± 27 | 15 | Greenockite (wurtzite) dominant |
| 3000 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
0.10, 0.6 | Spherical | 2.8 ± 0.4 | 361 (MSC), 401 | 427 ± 21 | 25 | Greenockite (wurtzite) dominant |
| 1500 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
0.10, 0.6 | Spherical, some bipods and tripods | 3.3 ± 0.5 | 410 | 426 ± 23 | 49 | Mixed phases |
| 750 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
0.10, 0.6 | Spherical, some bipods and tripods | 3.5 ± 0.5 | 419 | 434 ± 19, and 594 ± 75 (bimodal) | 98 | Greenockite (wurtzite) dominant |
| 375 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
0.10, 0.6 | Spherical | 3.7 ± 0.3 | 426 | 449 ± 25 | 196 | Mixed phases |
| 1500 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
0.10, 1.2 | Spherical, some bipods and tripods | 5.8 ± 0.9, 5.6 ± 0.1 | 409 | 425 ± 27 | 49 | Greenockite (wurtzite) dominant |
| 1500 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.10, 0.3 | Spherical, some bipods | 6.7 ± 2, 2.6 ± 0.6 | 361 (MSC), 393 | 427 ± 21 | 49 | Mixed phases |
Besides that, a consistent additional peak was observed at 361 nm for the absorption spectra of CdS NCs synthesized at fast flow rates of 5000 μl min−1 and 3000 μl min−1, exhibiting linewidths as narrow as 14 nm. The presence of this discrete peak indicates the formation of a magic-size cluster (MSC), which degrades in intensity over time before spontaneously red-shifting to another distinct wavelength instead of gradually red-shifting over time.38 This is in stark contrast to the red-shifted peak at the longer wavelength region, which progressively shifted from 385 nm to 426 nm as the residence time increased from 15 s to 196 s, while the MSC peak remained constant at 361 nm as indicated in Fig. 3a. In addition, we demonstrated the high reproducibility for synthesizing highly monodispersed CdS NCs under the flow rate of 5000 μl min−1 by showing that the same MSC peak at 361.7 ± 0.5 nm (Fig. 4a) could be replicated. Fig. 4 also shows other synthesis parameters which exhibited almost identical absorption spectra, providing compelling evidence for the reliability and stability of the millifluidic chip platform as a miniature reaction vessel.
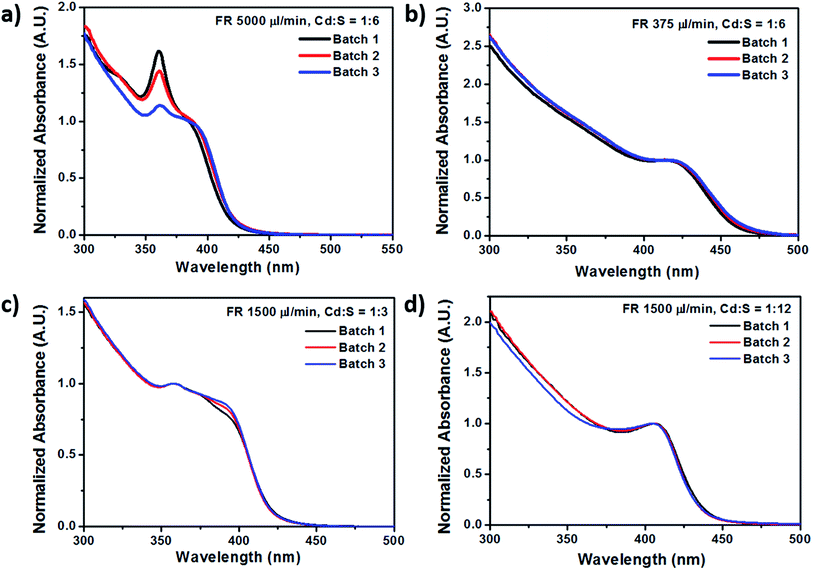 | ||
| Fig. 4 Absorption curves of selected batches of CdS NCs showing the high reproducibility of nanoparticle fabrication using these millifluidic chip reactors. | ||
These as-synthesized CdS NCs emitted visible light when irradiated with ultraviolet (UV) light with an excitation wavelength of 350 nm. From Fig. 3b, NCs produced using the slower flow rate of 750 μl min−1 exhibited a bimodal emission with a longer wavelength peak at around 594 nm, along with a shorter wavelength peak corresponding to the band edge emission at 434 nm. The intensity of the intensity of the longer wavelength peak was much higher than that of the shorter wavelength peak. Also, the 594 nm peak had a broader linewidth compared to the 434 nm peak. Meanwhile, particles fabricated using the high flow rates of 1500 μl min−1 and above had a dominant peak at the band edge (∼427 nm) with a trailing edge spreading over to the longer wavelength region.
For this set of CdS NCs (i.e. with varying flow rates), the molar ratio of Cd and S precursors were fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, with S being in excess. The red-shifted broadband photoluminescence emission depicted in Fig. 5 is attributed to the sulfur defect states.39 Incomplete passivation of the CdS nanocrystals with oleylamine ligands resulted in dangling bonds at the nanocrystal surface and thus caused surface trap states to exist within the nanocrystal band gap. These surface states then affect the relaxation process of the carriers, resulting in non-radiative pathways which reduce the energy of the emitted photon upon carrier recombination, thereby causing the red shift and broadband spectral features of the photoluminescence. This broadband emission has been commonly observed for such small sized CdS quantum dots and nanocrystals.7,22,23,39,40
6, with S being in excess. The red-shifted broadband photoluminescence emission depicted in Fig. 5 is attributed to the sulfur defect states.39 Incomplete passivation of the CdS nanocrystals with oleylamine ligands resulted in dangling bonds at the nanocrystal surface and thus caused surface trap states to exist within the nanocrystal band gap. These surface states then affect the relaxation process of the carriers, resulting in non-radiative pathways which reduce the energy of the emitted photon upon carrier recombination, thereby causing the red shift and broadband spectral features of the photoluminescence. This broadband emission has been commonly observed for such small sized CdS quantum dots and nanocrystals.7,22,23,39,40
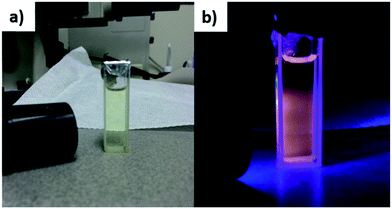 | ||
| Fig. 5 Millifluidic (mF) CdS NCs under (a) ambient light or (b) ultraviolet (UV) irradiation, with the reddish glow of mF CdS defect state emission in the dark. | ||
Effect of different Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) S molar ratios
S molar ratios
In addition to the initial Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S precursor molar ratio of 1
S precursor molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, the influence of the relative ratios of cadmium and sulfur on the size and morphology of the NCs was also studied. Two additional Cd
6, the influence of the relative ratios of cadmium and sulfur on the size and morphology of the NCs was also studied. Two additional Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S precursor molar ratios were employed, making the range of molar ratios investigated – 1
S precursor molar ratios were employed, making the range of molar ratios investigated – 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and 1
6 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12. In all three cases, the ratio of 1 corresponded to 0.1 M of the cadmium precursor. Fig. 7 depicts the TEM images of the NCs formed at a fixed flow rate of 1500 μl min−1 but with different molar ratios of 1
12. In all three cases, the ratio of 1 corresponded to 0.1 M of the cadmium precursor. Fig. 7 depicts the TEM images of the NCs formed at a fixed flow rate of 1500 μl min−1 but with different molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fig. 7a–c), 1
3 (Fig. 7a–c), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (Fig. 7d–f), 1
6 (Fig. 7d–f), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
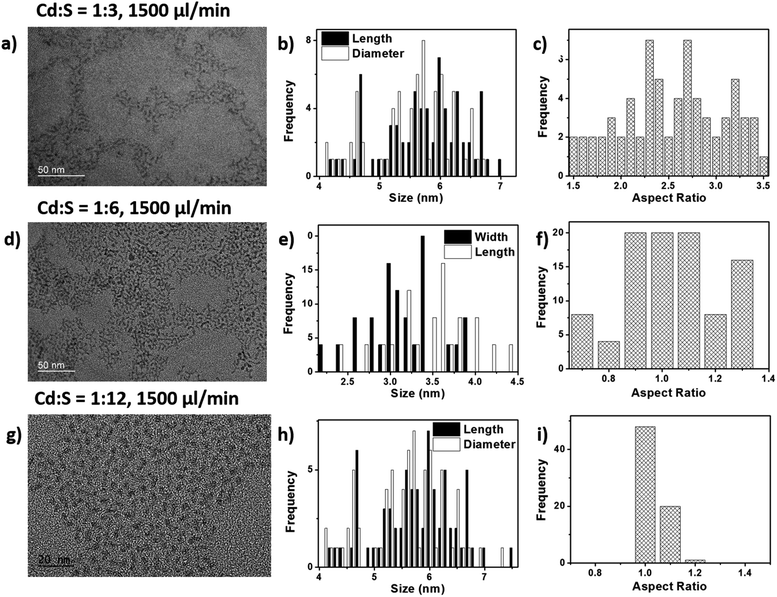
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 (Fig. 7g–i) together with their size distributions. Attempts were made to put the ratio of the Cd precursor in excess, however they were unsuccessful as the precursors precipitated very quickly, causing blockages in the tubes and channels. Therefore, the change in molar ratio was only confined to increasing the amount of sulfur used in the reaction.
12 (Fig. 7g–i) together with their size distributions. Attempts were made to put the ratio of the Cd precursor in excess, however they were unsuccessful as the precursors precipitated very quickly, causing blockages in the tubes and channels. Therefore, the change in molar ratio was only confined to increasing the amount of sulfur used in the reaction.
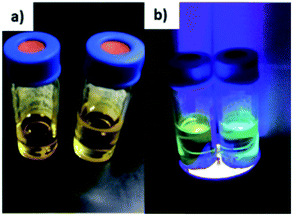 | ||
| Fig. 8 Millifluidic chip synthesized CdS NCs seen under (a) ambient light and (b) upon illumination with ultraviolet (UV) light and emitting greenish-blue light due to band edge emissions. | ||
Based on the TEM images in Fig. 7, it was observed that the molar ratio of Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S (1
S (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) gave rise to substantial amounts of tripod structures having average arm lengths of 6.7 ± 2 nm and diameters of 2.6 ± 0.6 nm and aspect ratios of 2.58 ± 0.6. The molar ratio of 1
3) gave rise to substantial amounts of tripod structures having average arm lengths of 6.7 ± 2 nm and diameters of 2.6 ± 0.6 nm and aspect ratios of 2.58 ± 0.6. The molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 yielded a mixture of spherical and multipod structures. For the extreme case of Cd
6 yielded a mixture of spherical and multipod structures. For the extreme case of Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 1
S = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, large particles with bipod shapes were observed. The absorption and photoluminescence spectra of the CdS NCs synthesized using the Cd
12, large particles with bipod shapes were observed. The absorption and photoluminescence spectra of the CdS NCs synthesized using the Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S molar ratio of 1
S molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 showed that these NCs exhibited different optical characteristics from that of the 1
3 showed that these NCs exhibited different optical characteristics from that of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and 1
6 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 cases. Along with larger particle sizes when Cd
12 cases. Along with larger particle sizes when Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 1
S = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, a bimodal fluorescence peak consisting of the narrow band edge emission at 434 nm and a broadband defect emission at the longer wavelength of 594 nm (Fig. 9) was also obtained.
3, a bimodal fluorescence peak consisting of the narrow band edge emission at 434 nm and a broadband defect emission at the longer wavelength of 594 nm (Fig. 9) was also obtained.
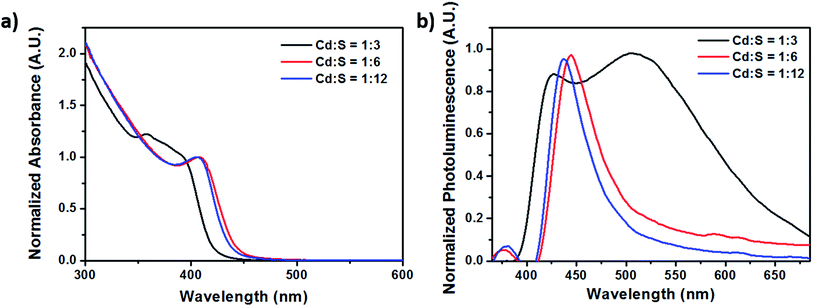 | ||
| Fig. 9 (a) Absorption and (b) photoluminescence spectra of CdS NCs synthesized using different molar ratios. The flow rate was kept constant at 1500 μl min−1. | ||
Fig. 8b shows the greenish-blue emissions of the millifluidic prepared CdS NCs under UV light illumination, which is different from the orange-red emission (due to surface defect states) in Fig. 5. This greenish-blue photoluminescence (PL) is due to the band edge emission of the CdS NCs. Comparing the photoluminescence spectrum in Fig. 3b and 9b, both show the presence of bimodal fluorescence peaks. However, when the molar ratio (MR) of Cd and S was varied such that Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 1
S = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fig. 9b), the relative PL intensity of the narrow linewidth band edge emission was almost comparable to that of the broadband surface defects emission, while for the case of sulfur being in larger excess with MR of 1
3 (Fig. 9b), the relative PL intensity of the narrow linewidth band edge emission was almost comparable to that of the broadband surface defects emission, while for the case of sulfur being in larger excess with MR of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (Cd
6 (Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S), the band edge emission was much weaker compared to the emission due to surface defect states (Fig. 3b). In a study by Wei et al.,39 they showed that when the CdS QDs were terminated with sulfur, the band edge photoluminescence was quenched, giving rise to little or no emission, while cadmium terminated CdS QDs exhibited higher photoluminescence and appeared brighter. Therefore, due to the excess sulfur precursors employed for MR 1
S), the band edge emission was much weaker compared to the emission due to surface defect states (Fig. 3b). In a study by Wei et al.,39 they showed that when the CdS QDs were terminated with sulfur, the band edge photoluminescence was quenched, giving rise to little or no emission, while cadmium terminated CdS QDs exhibited higher photoluminescence and appeared brighter. Therefore, due to the excess sulfur precursors employed for MR 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, it was likely that the band edge emission was significantly quenched, resulting in dominant long wavelength trap states in the emission spectrum. Consequently, with the reduction of the sulfur precursor to 1
6, it was likely that the band edge emission was significantly quenched, resulting in dominant long wavelength trap states in the emission spectrum. Consequently, with the reduction of the sulfur precursor to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, less quenching occurred causing the intensity of the band edge emission to increase, thereby exhibiting equal dominance with the surface defect state emissions.
3, less quenching occurred causing the intensity of the band edge emission to increase, thereby exhibiting equal dominance with the surface defect state emissions.
The photoluminescence of the CdS NCs could be further enhanced by coating the NCs with zinc sulfide (ZnS) shells. ZnS is a wide band gap material which confines the exciton to the core of the NCs, thus improving the quantum yield of the NCs. Techniques such as successive ionic layer adsorption and reaction (SILAR) and thermal cycling with a single precursor (TC-SP) have been demonstrated to improve the quantum yield of the core–shell CdS/ZnS NCs from around 6% to 30% (SILAR)41,42 and 50% (TC-SP).41
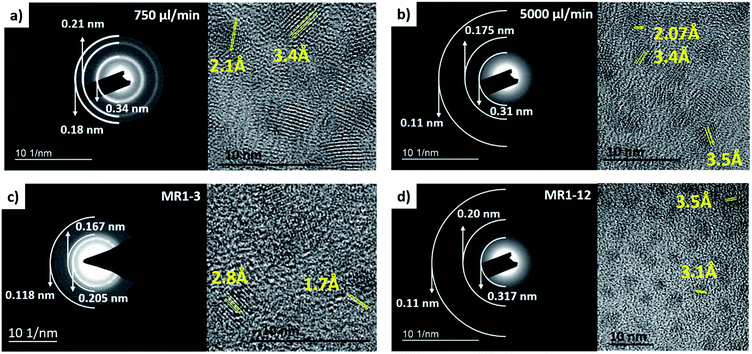
Although the synthesis of CdS in miniature reaction vessels have been studied by many groups as shown earlier in Table 1, some have reported that the particles exhibited poor crystal quality, while others mostly reported cubic zinc blende structures. Based on the XRD measurements depicted in Fig. 10, in terms of the flow rate, we observed that at 5000 μl min−1 where the residence time is only 15 s, some signal can still be captured. The presence of crystal lattice fringes in the high resolution TEM (HRTEM) and diffraction rings in the selected area electron diffraction (SAED) pattern for the NCs synthesized at 5000 μl min−1 as presented in Fig. 11b confirmed the existence of crystalline nanoparticles with interplanar lattice spacings of 2.07 Å matching the d spacings of the 〈1 1 0〉 wurtzite planes and (2 2 0) zinc blende planes, and 3.4 Å tallying with the 〈1 0 0〉 wurtzite (d = 3.58 Å) and (1 1 1) zinc blende (d = 3.35 Å) planes. The SAED pattern also revealed lattice plane distances of 1.1 Å and 1.75 Å, which pointed to the wurtzite 〈3 0 2〉 (d = 1.13 Å) and zinc blende (5 1 1) (d = 1.12 Å) planes as well as the 〈1 1 2〉 (d = 1.76 Å) and (3 1 1) (d = 1.75 Å) planes. However, there are unique signatures such as the 2.07 Å lattice spacing indicating the 〈1 1 0〉 wurtzite structure and the diffraction ring for the 3.1 Å 〈1 0 1〉 plane. This indicated the possibility of mixed phases being present in the CdS NCs with a dominant wurtzite crystal structure.
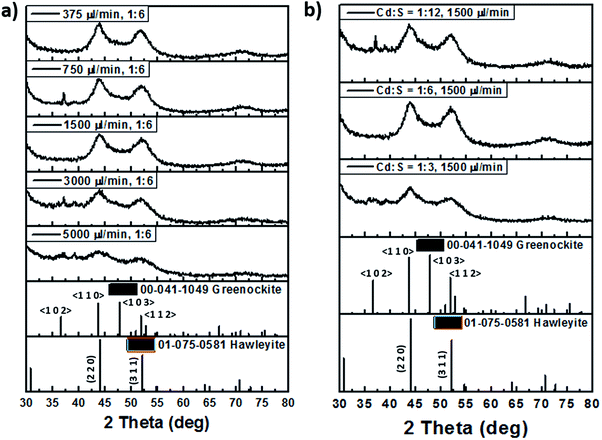 | ||
| Fig. 10 Powered X-ray diffraction (XRD) patterns of millifluidic chip synthesized CdS at (a) different flow rates and (b) molar ratios. | ||
Subsequently as the flow rate increased, there were two main peaks at 44° and 52°, both of which could be contributed by either the hexagonal wurtzite Greenockite (43.7° and 51.9° corresponding to 〈1 1 0〉 and 〈1 1 2〉 planes) or cubic zinc blende Hawleyite (44.0° and 52.2° corresponding to (2 2 0) and (3 1 1) planes) structures. The subsequent emergence of a tiny peak at 36° matching the 〈1 0 2〉 plane of wurtzite evident in the XRD patterns for CdS NCs fabricated at flow rates of 750 μl min−1 and 3000 μl min−1 in Fig. 10a and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 Cd
12 Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S molar ratio in Fig. 10b provided compelling substantiation for the dominant wurtzite structure for these NCs.
S molar ratio in Fig. 10b provided compelling substantiation for the dominant wurtzite structure for these NCs.
Bioimaging with macrophage cells
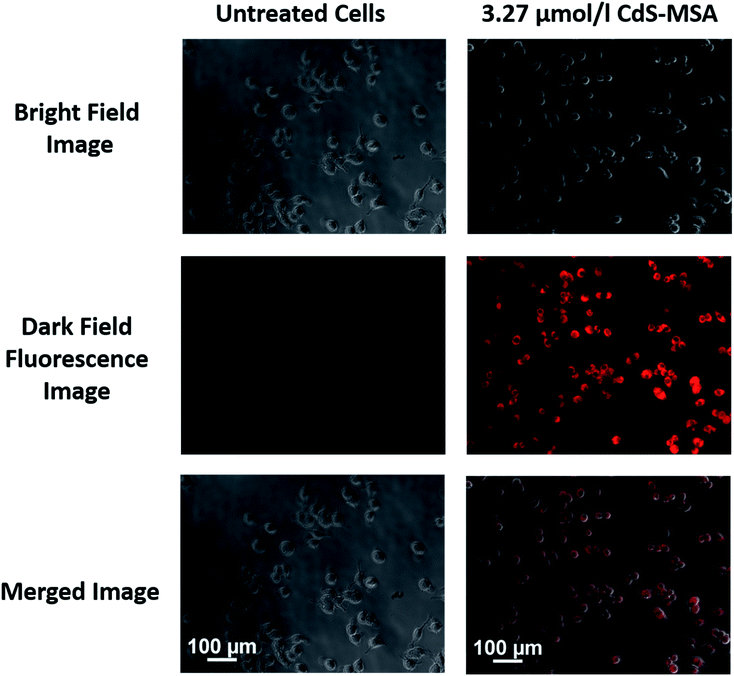
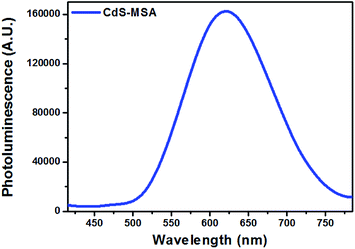
QDs have been used for biological imaging as optical imaging probes,43 and near infrared imaging probes44,45 due to properties such as their wide absorption spectrum, narrow emission spectrum, and improved photostability compared to organic dyes.46 However, concerns involving the use of QDs include photoblinking and heavy metal toxicity.47 Here, we demonstrate fluorescence imaging of the RAW264.7 mice macrophage cell line with CdS NCs which have been ligand exchanged with mercaptosuccinic acid (MSA) for solubility in aqueous conditions. In this work, the CdS nanoparticles used for cellular imaging were not coated with any particular protein or biomolecules, only the mercaptosuccinic acid (MSA) ligand to make the nanoparticles biocompatible and facilitate cell uptake. For selective endocytosis, these nanoparticles can be further engineered by attaching antibodies and targeting moieties to the nanoparticle surface. These CdS-MSA NCs can be used as optical probes to monitor the activity of the nanoparticles and investigate the breakdown of CdS in the cells. We believe that the small dimensions of the millifluidic chip synthesized CdS NCs would serve as an excellent model to study the excretion pathway of the nanoparticles.3.27 μM CdS-MSA NCs were added to RAW264.7 mice macrophage cells and incubated for 24 hours before imaging under ultraviolet (UV) light. The NCs were readily taken up by the macrophages as indicated by the numerous red regions observed from the dark field images depicted in Fig. 12. The photoluminescence spectrum of the CdS-MSA NCs in Fig. 13 showed a peak at around 620 nm. Therefore, the red fluorescence is indicative of the presence of CdS-MSA NCs ingested by the macrophages, while the control experiment shown in Fig. 12 exhibited no fluorescence for cells which were not treated (i.e. untreated) with the NCs.
Nanoparticles have large surface area to volume ratios, thus quantum confinement effects such as size, shape, surface charge, and surface passivation contribute significantly to the toxicity of the nanoparticles. Therefore, we believe that our millifluidic chip serves as an excellent platform to synthesize highly reproducible nanoparticles with good monodispersity, which would then serve as models for detailed studies on the effects of NC size, morphologies and surface modifications on nanoparticle toxicity.
A few strategies can be employed to reduce the toxicity of the NCs, one of which being coating the bare NCs with a zinc sulfide (ZnS) layer41,42 or encapsulation of these NCs48 to reduce leakage of free Cd2+ ions from the NC surface into the cellular environment. Free Cd2+ ions are highly toxic as they can generate reactive oxygen species (ROS), resulting in oxidative stress and cell apoptosis. Therefore, containment of the Cd core is crucial for biological applications of such cadmium based quantum dots.
Conclusion
In this work, we have demonstrated that CdS nanoparticles can be fabricated with ease and high reproducibility using a simple millifluidic chip. Changing the residence time (or injection flow rate) of the reactants in the millifluidic chip enabled precise control over nucleation and growth conditions and facilitated the synthesis of spherical CdS nanocrystals from as small as 2.4 ± 0.4 nm to 5.8 ± 0.9 nm to multipod structures with arms spanning 6.7 ± 2 nm long and 2.6 ± 0.6 nm wide. The novelty in this work is the synthesis of highly reproducible, luminescent CdS nanocrystals with the presence of magic sized clusters. In addition, multipod structures were also observed in short reaction durations of less than 2 minutes. Lastly, we also showed that these luminescent nanocrystals can be employed as fluorescent probes for cell imaging studies. Therefore, we believe that there is great potential for millifluidic chip devices in the area of nanoparticle synthesis as they allow design flexibility to promote efficient mixing and provide stable environments suitable for nucleation and growth of nanoparticles.Acknowledgements
This work was supported by the Singapore Ministry of Education (Grants Tier 2 MOE2010-T2-2-010 (M4020020.040 ARC2/11) and Tier 1 M4010360.040 RG29/10), NTU-NHG Innovation Collaboration Grant (No. M4061202.040), A*STAR Science and Engineering Research Council (No. M4070176.040), NEWRI seed funding (Grant No. NEWRI SF20140901) and School of Electrical and Electronic Engineering at NTU, The Hong Kong Polytechnic University (Project Grant: 1-ZE3A), the Research Grants Council of the Hong Kong Special Administrative Region, China (PolyU 25200914) and National Natural Science Foundation of China (61405169). The authors would like to thank Mr Jayaram s/o Raju for his assistance with this project.References
- J. Z. Zhang, J. Phys. Chem. B, 2000, 104, 7239–7253 CrossRef CAS
.
- R. W. Meulenberg, J. R. I. Lee, A. Wolcott, J. Z. Zhang, L. J. Terminello and T. van Buuren, ACS Nano, 2009, 3, 325–330 CrossRef CAS PubMed
.
- R. Luque and R. S. Varma, Sustainable Preparation of Metal Nanoparticles: Methods and Applications, The Royal Society of Chemistry, 2012 Search PubMed
.
- K. Kalyanasundaram, E. Borgarello, D. Duonghong and M. Grätzel, Angew. Chem., Int. Ed. Engl., 1981, 20, 987–988 CrossRef
.
- K.-T. Yong, Y. Sahoo, M. T. Swihart and P. N. Prasad, J. Phys. Chem. C, 2007, 111, 2447–2458 CAS
.
- Z. A. Peng and X. Peng, J. Am. Chem. Soc., 2001, 123, 183–184 CrossRef CAS PubMed
.
- X. Wang, Z. Feng, D. Fan, F. Fan and C. Li, Cryst. Growth Des., 2010, 10, 5312–5318 CAS
.
- S. Kar, S. K. Panda, B. Satpati, P. V. Satyam and S. Chaudhuri, J. Nanosci. Nanotechnol., 2006, 6, 771–776 CrossRef CAS PubMed
.
- Y. Zou, D. Li and D. Yang, Nanoscale Res. Lett., 2011, 6, 1–6 Search PubMed
.
- Y. C. Cao and J. Wang, J. Am. Chem. Soc., 2004, 126, 14336–14337 CrossRef CAS PubMed
.
- Y. Wada, H. Kuramoto, J. Anand, T. Kitamura, T. Sakata, H. Mori and S. Yanagida, J. Mater. Chem., 2001, 11, 1936–1940 RSC
.
- H. Yang, C. Huang, X. Li, R. Shi and K. Zhang, Mater. Chem. Phys., 2005, 90, 155–158 CrossRef CAS
.
- V. Smyntyna, B. Semenenko, V. Skobeeva and N. Malushin, Beilstein J. Nanotechnol., 2014, 5, 355–359 CrossRef PubMed
.
- A. Giberti, B. Fabbri, A. Gaiardo, V. Guidi and C. Malagù, Appl. Phys. Lett., 2014, 104, 222102 CrossRef
.
- C. A. Rodríguez-Castañeda, P. M. Moreno-Romero, C. Martínez-Alonso and H. Hu, J. Nanomater., 2015, 2015, 10 Search PubMed
.
- R. Sharma, S. Bhalerao and D. Gupta, Org. Electron., 2016, 33, 274–280 CrossRef CAS
.
- N. Ma, J. Yang, K. M. Stewart and S. O. Kelley, Langmuir, 2007, 23, 12783–12787 CrossRef CAS PubMed
.
- A. Gaiardo, B. Fabbri, V. Guidi, P. Bellutti, A. Giberti, S. Gherardi, L. Vanzetti, C. Malagù and G. Zonta, Sensors, 2016, 16, 296 CrossRef PubMed
.
- J. Joo, H. B. Na, T. Yu, J. H. Yu, Y. W. Kim, F. Wu, J. Z. Zhang and T. Hyeon, J. Am. Chem. Soc., 2003, 125, 11100–11105 CrossRef CAS PubMed
.
- Y. Wu, C. Wadia, W. Ma, B. Sadtler and A. P. Alivisatos, Nano Lett., 2008, 8, 2551–2555 CrossRef CAS PubMed
.
- I. Shestopalov, J. D. Tice and R. F. Ismagilov, Lab Chip, 2004, 4, 316–321 RSC
.
- L.-H. Hung, K. M. Choi, W.-Y. Tseng, Y.-C. Tan, K. J. Shea and A. P. Lee, Lab Chip, 2006, 6, 174–178 RSC
.
- H. Yang, W. Luan, Z. Wan, S.-t. Tu, W.-K. Yuan and Z. M. Wang, Cryst. Growth Des., 2009, 9, 4807–4813 CAS
.
- Z. Wan, H. Yang, W. Luan, S.-t. Tu and X. Zhou, Nanoscale Res. Lett., 2009, 5, 130–137 CrossRef PubMed
.
- M. S. Naughton, V. Kumar, Y. Bonita, K. Deshpande and P. J. A. Kenis, Nanoscale, 2015, 7, 15895–15903 RSC
.
- G. Chen, B. Yi, G. Zeng, Q. Niu, M. Yan, A. Chen, J. Du, J. Huang and Q. Zhang, Colloids Surf., B, 2014, 117, 199–205 CrossRef CAS PubMed
.
- X. Zhu, D. Kumari, M. Huang and V. Achal, Mater. Des., 2016, 98, 209–214 CrossRef CAS
.
- A. J. de Mello, Nature, 2006, 442, 394–402 CrossRef CAS PubMed
.
- V. Hessel, H. Löwe and F. Schönfeld, Chem. Eng. Sci., 2005, 60, 2479–2501 CrossRef CAS
.
- S. Pennathur, C. D. Meinhart and H. T. Soh, Lab Chip, 2008, 8, 20–22 RSC
.
- K. Sai Krishna, C. V. Navin, S. Biswas, V. Singh, K. Ham, G. L. Bovenkamp, C. S. Theegala, J. T. Miller, J. J. Spivey and C. S. S. R. Kumar, J. Am. Chem. Soc., 2013, 135, 5450–5456 CrossRef CAS PubMed
.
- J.-I. Yoshida, H. Kim and A. Nagaki, ChemSusChem, 2011, 4, 331–340 CrossRef CAS PubMed
.
- S. E. Lohse, J. R. Eller, S. T. Sivapalan, M. R. Plews and C. J. Murphy, ACS Nano, 2013, 7, 4135–4150 CrossRef CAS PubMed
.
- V. Mengeaud, J. Josserand and H. H. Girault, Anal. Chem., 2002, 74, 4279–4286 CrossRef CAS PubMed
.
- T.-L. Cheung, L. Hong, N. Rao, C. Yang, L. Wang, W. J. Lai, P. H. J. Chong, W.-C. Law and K.-T. Yong, Nanoscale, 2016, 8, 6609–6622 RSC
.
- J.-I. Yoshida, Basics of Flow Microreactor Synthesis, Springer, 2015 Search PubMed
.
- W. W. Yu, L. Qu, W. Guo and X. Peng, Chem. Mater., 2003, 15, 2854–2860 CrossRef CAS
.
- S. M. Harrell, J. R. McBride and S. J. Rosenthal, Chem. Mater., 2013, 25, 1199–1210 CrossRef CAS
.
- H. H.-Y. Wei, C. M. Evans, B. D. Swartz, A. J. Neukirch, J. Young, O. V. Prezhdo and T. D. Krauss, Nano Lett., 2012, 12, 4465–4471 CrossRef CAS PubMed
.
- A. Veamatahau, B. Jiang, T. Seifert, S. Makuta, K. Latham, M. Kanehara, T. Teranishi and Y. Tachibana, Phys. Chem. Chem. Phys., 2015, 17, 2850–2858 RSC
.
- D. Chen, F. Zhao, H. Qi, M. Rutherford and X. Peng, Chem. Mater., 2010, 22, 1437–1444 CrossRef CAS
.
- J. S. Steckel, J. P. Zimmer, S. Coe-Sullivan, N. E. Stott, V. Bulović and M. G. Bawendi, Angew. Chem., Int. Ed., 2004, 43, 2154–2158 CrossRef CAS PubMed
.
- J. Li and J.-J. Zhu, Analyst, 2013, 138, 2506–2515 RSC
.
- T. Zako, M. Yoshimoto, H. Hyodo, H. Kishimoto, M. Ito, K. Kaneko, K. Soga and M. Maeda, Biomater. Sci., 2015, 3, 59–64 RSC
.
- E. İ. Altınoğlu and J. H. Adair, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2010, 2, 461–477 CrossRef PubMed
.
- U. Resch-Genger, M. Grabolle, S. Cavaliere-Jaricot, R. Nitschke and T. Nann, Nat. Methods, 2008, 5, 763–775 CrossRef CAS PubMed
.
- L. Yildirimer, N. T. K. Thanh, M. Loizidou and A. M. Seifalian, Nano Today, 2011, 6, 585–607 CrossRef CAS PubMed
.
- S. Veeranarayanan, A. C. Poulose, M. S. Mohamed, Y. Nagaoka, S. Iwai, Y. Nakagame, S. Kashiwada, Y. Yoshida, T. Maekawa and D. S. Kumar, Int. J. Nanomed., 2012, 7, 3769–3786 CAS
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |