Open Access Article
Open Access ArticleIncorporating a TiOx shell in single-walled carbon nanotube/fullerodendron coaxial nanowires: increasing the photocatalytic evolution of H2 from water under irradiation with visible light†
K. Kurniawana,
T. Tajima *a,
Y. Kuboa,
H. Miyake
*a,
Y. Kuboa,
H. Miyake b,
W. Kurashigec,
Y. Negishi
b,
W. Kurashigec,
Y. Negishi c and
Y. Takaguchi
c and
Y. Takaguchi *a
*a
aGraduate School of Environmental and Life Science, Okayama University, 3-1-1 Tsushima-Naka, Kita-ku, Okayama, 700-8530, Japan. E-mail: tajimat@cc.okayama-u.ac.jp; yutaka@cc.okayama-u.ac.jp
bGraduate School of Sciences and Technology for Innovation, Yamaguchi University, 2-16-1 Tokiwadai, Ube, Yamaguchi 755-8611, Japan
cDepartment of Applied Chemistry, Faculty of Science Division I, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan
First published on 21st June 2017
Abstract
A custom-tailored single-walled carbon nanotube (SWCNT) photocatalyst with an electron-extracting TiOx shell, i.e., a SWCNT/fullerodendron/TiOx coaxial nanowire, has been fabricated. Due to the presence of the TiOx shell, the SWCNT/fullerodendron/TiOx coaxial nanowire shows an enhanced photocatalytic activity (Φ = 0.47) for the evolution of hydrogen from water under irradiation with visible light (λ = 450 nm).
The interfaces between metal oxides and organic compounds play an important role in the field of organic electronics, which include organic light-emitting devices (OLEDs),1,2 organic photovoltaic cells (OPVs),1,3 dye-sensitized solar cells (DSSCs),1c and transistors.4 To improve the device performance, these interfaces can often be modified by insertion of a functional interfacial layer. For example, transparent titanium oxides (TiOx) exhibit electronic levels that match the LUMOs of the C60-derivatives used in OPVs well, which renders TiOx a promising candidate for electron-transport materials.5,6 Waldauf and co-workers have prepared OPVs with an ITO/TiOx/RR-P3HT:PCBM/PEDOT:PSS/Au structure using coating techniques, and demonstrated that these OPVs exhibit improved fill factors (FF).5 Kuwabara et al. have reported efficient inverted BHJ solar cells that contain TiOx as the electron-extraction layer,6 which resulted in an improved short-circuit photocurrent (Jsc), open-circuit voltage (Voc), FF, and power-conversion efficiency (η).
Meanwhile, coaxial nanowires with a donor–acceptor heterojunction have shown great potential for applications in innovative photofunctional materials. Resent theoretical7 and experimental8 studies have indicated that coaxial nanowire structures could potentially improve the carrier collection and overall efficiency relative to bulk semiconductors of the same materials. We have reported the fabrication and efficient photo-induced electron-transfer processes of single-walled carbon nanotube (SWCNT)/anthryl dendron9,10 and SWCNT/fullerodendron supramolecular nanocomposites,9,11 wherein a coaxial nanowire structure provides a donor–acceptor heterojunction between the SWCNT-core and the C60-based fullerodendrons. We have also shown that SWCNT/fullerodendron (Φ = 0.28) and SWCNT/fullerodendron/SiO2 (Φ = 0.31) coaxial nanohybrid materials can be used as effective photosensitizers for the catalytic evolution of H2 from water under irradiation with visible light (λ = 450 nm).12 Moreover, we have reported the direct incorporation of a co-catalyst into the shell of SWCNT/fullerodendron supramolecular nano-composites.13 Upon chirality-selective photo-excitation using monochromatic light (λ = 680 nm), which is suitable for the E22 absorption of (8,3) SWCNTs, we observed the first example for the evolution of H2 (Φ = 0.015) photosensitized by SWCNTs.14 However, further improvements of the quantum yield of the SWCNT photocatalyst are necessary in order to further develop this technology.
These results prompt us to explore a new coaxial photosensitizer with a TiOx shell as an electron-extraction layer that covers a photo-functional SWCNTs/C60 interface. Here we describe the fabrication of SWCNT/fullerodendron/TiOx coaxial nano-hybrids that can be used for the effective photo-catalytical evolution of H2, which shows the highest AQYs (Φ = 0.47) under irradiation with visible light (λ = 450 nm).
The molecular structure of the fullerodendron used in the present study is shown in Fig. 1. SWCNT/fullerodendron/TiOx nano-hybrids were fabricated by a polycondensation reaction of titanium tetra isopropoxide (TTIP)15 using SWCNT/fullerodendron supramolecular nano-composites (Fig. 1) as catalytic scaffolds according to previous reports on SWCNT/fullerodendron/SiO2 nano-hybrids.12 In a typical run, an aqueous solution of SWCNT/fullerodendron nanocomposites (250 μL: the content of SWCNT is 0.025 mg, 2.0 μmol as a C atom) was diluted with water (750 μL). The pH was adjusted to pH 3 with HCl (1.0 N, 2.8 μL). After the stirring for 1 h, an EtOH solution of titanium tetra isopropoxide (3.51 mM, 20 μL, 7.4 × 10−8 mol) was added to the solution at 0 °C and stirred for 48 h to obtain a dispersion of SWCNT/fullerodendron/TiOx coaxial nanohybrids.
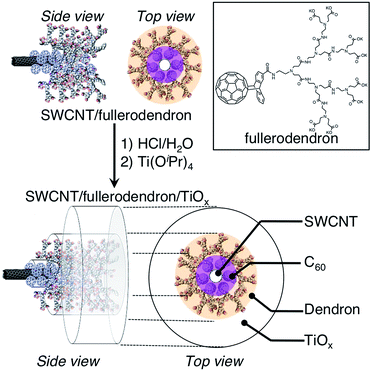 | ||
| Fig. 1 Schematic illustration of the fabrication of SWCNT/fullerodendron/TiOx coaxial hybrid nano-wires. | ||
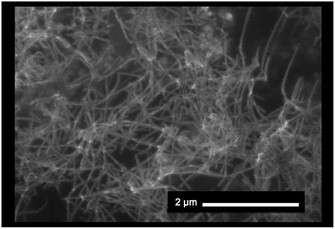
The morphology of the SWCNT/fullerodendron/TiOx nano-hybrids was examined by scanning electron microscopy (SEM). The SEM images exhibited SWCNT/fullerodendron/TiOx nanowires that are similar to SWCNT/fullerodendron/SiO2 nanowires (Fig. 2).12 This structural observation is consistent with the results of transmission electron microscopy (TEM; Fig. S1†) and atomic force microscopy (AFM) measurements (Fig. S2†). The TEM analysis indicated that a uniformly thick shell composed of nano-sized TiOx covered the SWCNT/fullerodendron nano-wire. The height profiles in the AFM analysis revealed that the thickness of SWCNT/fullerodendron/TiOx coaxial nano-hybrids (∼15 nm) is higher than that of the SWCNT/fullerodendron supramolecular nano-composite (2–3 nm).13 Based on these results, the thickness of the TiOx layer was estimated to be 6–7 nm. These observations are consistent with the SWCNT/fullerodendron/TiOx coaxial nano-hybrid structure, the SWCNT-core surrounded by the fullerodendrons, coated by the outer TiOx shell.
A Raman spectrum of the SWCNT/fullerodendron/TiOx coaxial nano-hybrids showed typical scattering of the disorder-induced mode (D) and the tangential displacement mode (TDM; also called the G band), which were observed at 1339 and 1610 cm−1, respectively (Fig. S3†). The very small intensity of the D-band indicates that the SWCNTs within the composites did not sustain any substantial damage. Broad peaks at 610 and 425 cm−1 were ascribed to TiOx. To obtain a better understanding of the fullerodendron/TiOx junction, FT-IR spectroscopic measurements of the SWCNT/fullerodendron/TiOx nano-hybrid were conducted (Fig. S4†). The IR spectra of the SWCNT/fullerodendron/TiOx nano-hybrids exhibit the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching modes at 1725 cm−1, which is shifted toward higher frequencies than those of the SWCNT/fullerodendron supramolecular nano-composites (1680 cm−1). This result indicates the formation of Ti–OCOR bonds16 at the termini of the fullerodendrons. The sharp absorption band observed at 635 cm−1 was ascribed to the Ti–O–Ti moieties, while the strong absorption band at 1055 cm−1 was attributed to the Ti–O–C stretching mode. Based on these observations, we concluded that the TiOx layer is attached onto the surface of the SWCNT/fullerodendron supramolecular nano-composites without significant damage to the SWCNTs.
O stretching modes at 1725 cm−1, which is shifted toward higher frequencies than those of the SWCNT/fullerodendron supramolecular nano-composites (1680 cm−1). This result indicates the formation of Ti–OCOR bonds16 at the termini of the fullerodendrons. The sharp absorption band observed at 635 cm−1 was ascribed to the Ti–O–Ti moieties, while the strong absorption band at 1055 cm−1 was attributed to the Ti–O–C stretching mode. Based on these observations, we concluded that the TiOx layer is attached onto the surface of the SWCNT/fullerodendron supramolecular nano-composites without significant damage to the SWCNTs.
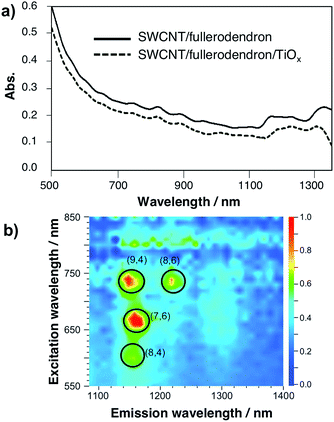
The TiOx layer of the SWCNT/fullerodendron/TiOx nano-hybrids shows high optical transparency in the visible and near infrared (NIR) region. Fig. 3a and S5† show the absorption spectra of the SWCNT/fullerodendron supramolecular nano-composites and the SWCNT/fullerodendron/TiOx coaxial nano-hybrids. The absorption of the SWCNT/fullerodendron/TiOx coaxial nano-hybrids is smaller than that of the SWCNT/fullerodendron composites, as the concentration of the SWCNT/fullerodendron/TiOx coaxial nano-hybrids was lowered during the sol–gel condensation process. However, the absorption and/or scattering due to the TiOx layer on the shell should be negligible on account of the nano-sized thickness of the TiOx layer. The coaxial nanowire structure with isolated SWCNTs was confirmed by three-dimensional photoluminescence (PL) intensity mapping in D2O (Fig. 3b), which allowed assigning four intense peaks with reasonable certainty to the (9,4), (8,6), (7,6), and (8,4) SWCNTs. It should be noted that the coaxial structure is maintained, i.e., the formation of bundles and/or aggregation of the SWCNTs after the formation of the SWCNT/fullerodendron/TiOx coaxial nano-hybrids was not observed.
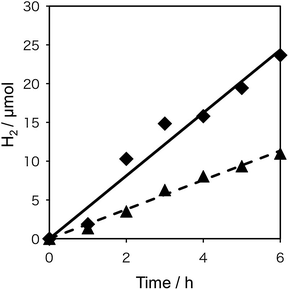
To probe the beneficial aspects of the incorporation of the transparent electron-extraction layer into an SWCNT/fullerodendron coaxial photosensitizer, we explored the photocatalytic evolution of H2 from water using a system based on the SWCNT/fullerodendron/TiOx supramolecular nano-composites coupled with colloidal poly(vinylpyrrolidone)–platinum (PVP–Pt).17 Typically, 150 mL of an aqueous solution, consisting of SWCNT/fullerodendron/TiOx nano-composites (1 mL), Tris–HCl buffer (3.5 mL in H2O, pH = 7.5, 5 mM), methyl viologen dichloride (MV; 92.4 mg, 359 μmol), 1-benzyl-1,4-dihydronicotinamide (BNAH; 38.6 mg, 180 μmol), and a colloidal solution of PVP–Pt (15 mL in H2O; 512 μmol of Pt), was vigorously stirred at 25 °C while being exposed to light (λ > 422 nm) from a 300 W Xe arc lamp. After the designated period, the gas phase above the solution was analyzed by gas chromatography. Fig. 4 shows the plots of the total amount of H2 produced as a function of time using either SWCNT/fullerodendron/TiOx coaxial nano-hybrids (♦) or SWCNT/fullerodendron composites (▲). The generation of H2 (4.0 μmol h−1) proceeded steadily and an induction period or decreasing activity was not observed during 6 h of irradiation.
Although the SWCNT/fullerodendron supramolecular nano-composites also worked as a photosensitizer for the evolution of H2, the reaction rate of the H2 generation (1.9 μmol h−1; Fig. 3) is lower than that of SWCNT/fullerodendron/TiOx nano-hybrids (4.0 μmol h−1). Given that the absorbance of the SWCNT/fullerodendron/TiOx nano-hybrids is lower than that of the SWCNT/fullerodendron supramolecular nano-composites, the photocatalytic activity of the SWCNT/fullerodendron/TiOx nano-hybrids must be higher than that of the SWCNT/fullerodendron supramolecular nano-composites. In order to compare the efficiency of the photocatalytic evolution of H2 between the SWCNT/fullerodendron supramolecular nano-composite and the SWCNT/fullerodendron/TiOx nano-hybrid photocatalysts, we evaluated their quantum yields by using monochromic light (λ = 450 ± 2 nm), which revealed apparent quantum yields (AQYs) for the evolution of H2 (2 × number of molecules of H2 generated/number of photons absorbed) of 0.47 (SWCNT/fullerodendron/TiOx) and 0.12 (SWCNT/fullerodendron). By changing the reaction time of sol–gel condensation, it seems that we can control the thickness of TiOx layer on the SWCNT/fullerodendron supramolecular nano-composites as judged by the SEM images (Fig. S6†). The photocatalytic activity of SWCNT/fullerodendron/TiOx nano-hybrids is affected by the reaction time of sol–gel condensation. The reaction rate of the H2 generation were 0.1 μmol h−1 (for 1 day condensation), 4.0 μmol h−1 (for 2 day condensation), and 0.1 μmol h−1 (for 3 day condensation), respectively. The SWCNT/fullerodendron/TiOx nano-hybrids maintained its photocatalytic activity for at least 12 h. However, the H2 production rate gradually decreases along with irradiation. The result can be attributed to the decrease of BNAH (sacrificial electron donor) concentration. It is notable that 81% of H2-evolving activity was remained for the second use of a spray-coated film of SWCNT/fullerodendron/TiOx on a FTO plate after the 6 hour H2 evolution reaction upon illumination followed by the rinsing and drying.
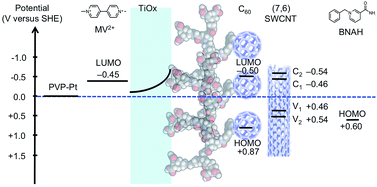
An energy-level diagram of the conduction (C1 and C2) and valence bands (V1 and V2) of (7,6) SWCNT,18 which is one of the most commonly encountered types of CNTs in HiPco, together with the LUMO levels of C60,19 MV2+,20 BNAH,21 and the energy level of Pt and TiOx is shown in Fig. 5. A distinctive advantage of the coaxial architecture is that the carrier separation occurs in the shorter radial direction as opposed to the longer axial direction. Moreover, the energy-level diagram indicates that the electron-extraction layer of TiOx not only decelerates the electron back-transfer, but also accelerates the electron forward-transfer from the conduction band of TiOx to the LUMO of MV2+, thus leading to an efficient electron-transfer pathway.
A bespoke SWCNT photocatalyst with an electron-extracting TiOx shell was fabricated. On account of the presence of the TiOx shell, the SWCNT/fullerodendron/TiOx coaxial nanowire exhibits a high activity in the catalytic evolution of H2 from water under irradiation with visible light, because the electron-extracting TiOx layer accelerates the electron forward-transfer under concomitant deceleration of the undesirable electron back-transfer. Even though various kinds of organic and inorganic coaxial nanowires have been developed so far, coaxial nanowire photocatalysts consisting of (p-type semiconductor)/(n-type semiconductor)/(electron-extraction layer) are still in the early stages of development, mostly due to their relatively complicated fabrication procedures. Further studies on the applications of such SWCNT photocatalysts with coaxial nanowire structures are currently in progress, and will be reported in due course.
Acknowledgements
This work was partially supported by JSPS KAKENHI grants 15H03519 (Y. T.) and 16K05895 (T. T.).References
- For reviews, see: (a) J. Meyer, S. Hamwi, M. Kröger, W. Kowalsky, T. Riedl and A. Kahn, Adv. Mater., 2012, 24, 5408 CrossRef CAS PubMed; (b) K. Zilberberg, J. Meyer and T. Riedl, J. Mater. Chem. C, 2013, 1, 4796 RSC; (c) E. L. Ratcliff, B. Zacher and N. R. Armsrong, J. Phys. Chem. Lett., 2011, 2, 1337 CrossRef CAS PubMed.
- (a) S. Tokito, K. Noda and Y. Taga, J. Phys. D: Appl. Phys., 1996, 29, 2750 CrossRef CAS; (b) H. Kanno, R. J. Holmes, Y. Sun, S. Kena-Cohen and S. R. Forrest, Adv. Mater., 2006, 18, 339 CrossRef CAS; (c) K. J. Reynold, J. A. Barker, N. C. Greenham, R. H. Friend and G. L. Frey, J. Appl. Phys., 2002, 92, 7556 CrossRef; (d) C.-W. Chu, C.-W. Chen, S.-H. Li, E. H. E. Wu and Y. Yang, Appl. Phys. Lett., 2005, 86, 253503 CrossRef; (e) H. You, Y. Dai, Z. Zhang and D. Ma, J. Appl. Phys., 2007, 101, 026105 CrossRefJ. Meyer, S. Hamwi, M. Kröger, W. Kowalsky, T. Riedl and A. Kahn, Adv. Mater., 2012, 24, 5408 CrossRef CAS PubMed; (f) H. J. Bolink, E. Coronado, D. Repetto, M. Sessolo, E. M. Barea, J. Bisquert, G. Garcia-Belmonte, J. Prochazka and L. Kavan, Adv. Funct. Mater., 2008, 18, 145 CrossRef CAS.
- For reviews, see: (a) F. Wang, Z. Tan and Y. Li, Energy Environ. Sci., 2015, 8, 1059 RSC; (b) S. Chen, J. R. Manders, S.-W. Tsang and F. So, J. Mater. Chem., 2012, 22, 24202 RSC.
- S. Cho, J. H. Seo, K. Lee and A. J. Heeger, Adv. Funct. Mater., 2009, 19, 1459 CrossRef CAS.
- C. Waldauf, M. Morana, P. Denk, P. Schilinsky, K. Coakley, S. A. Choulis and C. J. Brabec, Appl. Phys. Lett., 2006, 89, 233517 CrossRef.
- T. Kuwabara, T. Nakayama, K. Uozumi, T. Yamaguchi and K. Takahashi, Sol. Energy Mater. Sol. Cells, 2008, 92, 1476 CrossRef CAS.
- (a) B. M. Kayes, H. A. Atwater and N. S. Lewis, J. Appl. Phys., 2005, 97, 114302 CrossRef; (b) Y. Zhang, L. W. Wang and A. Mascarenhas, Nano Lett., 2007, 7, 1264 CrossRef CAS PubMed.
- (a) B. Tian, X. Zheng, T. J. Kempa, Y. Fang, N. Yu, G. Yu, J. Huang and C. M. Lieber, Nature, 2007, 449, 885 CrossRef CAS PubMed; (b) Y. Dong, B. Tian, T. J. Kempa and C. M. Lieber, Nano Lett., 2009, 9, 2183 CrossRef CAS PubMed; (c) K.-Q. Peng and S.-T. Lee, Adv. Mater., 2011, 23, 198 CrossRef CAS PubMed; (d) T. J. Kempa, R. W. Day, S.-K. Kim, H.-G. Park and C. M. Lieber, Energy Environ. Sci., 2013, 6, 719 RSC; (e) N. Zhang, M.-Q. Yang, Y. Sun and Y.-J. Zu, Chem. Rev., 2015, 115, 10307 CrossRef CAS PubMed; (f) S. Liu, C. Han, Z.-R. Tang and Y.-J. Xu, Mater. Horiz., 2016, 3, 270 RSC; (g) L. Yuan, C. Han, M.-Q. Yang and Y.-J. Xu, Int. Rev. Phys. Chem., 2016, 35, 1 CrossRef CAS; (h) C. Han, Z. N. Xhang and Y.-J. Zu, Nano Today, 2016, 11, 351 CrossRef CAS.
- Y. Takaguchi, M. Tamura, Y. Sako, Y. Yanagimoto, S. Tsuboi, T. Uchida, K. Shimamura, S. Kimura, T. Wakahara, Y. Maeda and T. Akasaka, Chem. Lett., 2005, 34, 1608 CrossRef CAS.
- A. S. D. Sandanayaka, Y. Takaguchi, T. Uchida, Y. Sako, Y. Morimoto, Y. Araki and O. Ito, Chem. Lett., 2006, 35, 1188 CrossRef CAS.
- A. S. D. Sandanayaka, Y. Takaguchi, Y. Sako, M. Tamura and O. Ito, Adv. Sci. Lett., 2010, 3, 353 CrossRef CAS.
- T. Tajima, W. Sakata, T. Wada, A. Tsutsui, S. Nishimoto, M. Miyake and Y. Takaguchi, Adv. Mater., 2011, 23, 5750 CrossRef CAS PubMed.
- Y. Sasada, T. Tajima, T. Wada, T. Uchida, M. Nishi, T. Ohkubo and Y. Takaguchi, New J. Chem., 2013, 37, 4214 RSC.
- N. Murakami, Y. Tango, H. Miyake, T. Tajima, Y. Nishina, W. Kurashige, Y. Negishi and Y. Takaguchi, Sci. Rep., 2017, 7, 43445 CrossRef PubMed.
- R. M. Pasquarelli, D. S. Ginley and R. O'Hayre, Chem. Soc. Rev., 2011, 40, 5406 RSC.
- F. X. Perrin, V. Nguyen and J. L. Vernet, J. Sol-Gel Sci. Technol., 2003, 28, 205 CrossRef CAS.
- H. Hirai, Y. Nakao and N. Toshima, J. Macromol. Sci., Chem., 1979, 13, 1979 Search PubMed.
- Y. Tanaka, Y. Hirana, Y. Niidome, K. Kato, S. Saito and N. Nakashima, Angew. Chem., Int. Ed., 2009, 48, 7655 CrossRef CAS PubMed.
- Y. Takaguchi, Y. Sako, Y. Yanagimoto, S. Tsuboi, J. Motoyoshiya, H. Aoyama, T. Wakahara and T. Akasaka, Tetrahedron Lett., 2003, 44, 5777 CrossRef CAS.
- M. Ito and T. Kuwana, Electroanal. Chem., 1971, 32, 415 CrossRef CAS.
- X. Q. Zhu, M. T. Zhang, A. Yu, C. H. Wang and J. P. Cheng, J. Am. Chem. Soc., 2008, 130, 2501 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, Fig. S1–S7. See DOI: 10.1039/c7ra05412b |
| This journal is © The Royal Society of Chemistry 2017 |