Open Access Article
Open Access ArticleHighly fouling-resistant brominated poly(phenylene oxide) membranes using surface grafted diethylenetriamine†
Muayad Al-Shaelia,
Stefan J. D. Smith ab,
Ezzatollah Shamsaeia,
Huanting Wang
ab,
Ezzatollah Shamsaeia,
Huanting Wang a,
Kaisong Zhangc and
Bradley P. Ladewig
a,
Kaisong Zhangc and
Bradley P. Ladewig *d
*d
aDepartment of Chemical Engineering, Monash University, VIC 3800, Australia
bCSIRO, Private Bag 10, Clayton South MDC, VIC 3169, Australia
cKey Laboratory of Urban Pollutant Conversion, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, China
dBarrer Centre, Department of Chemical Engineering, Imperial College London, Exhibition Road, SW7 2AZ, London, UK. E-mail: b.ladewig@imperial.ac.uk
First published on 27th July 2017
Abstract
Hydrophilic bromomethylated poly(phenylene oxide) (BPPO) ultrafiltration membranes were prepared via a single-step reaction with diethylenetriamine (DETA). The resulting DETA modified BPPO membranes are characterised using FTIR-ATR, SEM, fouling resistance, filtration test and contact angle measurements. Although permeation flux was adversely affected, the chemically bound DETA leads to a significant increase in surface hydrophilicity and anti-fouling properties of BPPO/DETA membranes. The composite BPPO/DETA membranes show a considerable reduction in membrane fouling and enhanced BSA rejection, with foulants easily removed by normal cleaning methods. Herein, a facile surface modification with DETA is shown to be an effective means of enhancing the flux recovery ratio and anti-fouling properties of BPPO membranes.
Introduction
Membranes have been widely applied in the field of water treatment, with extensive applications in water purification, desalination, and wastewater treatment.1,2 Membrane characteristics such as, surface hydrophilicity, porosity and anti-fouling properties are critically important in these separation processes. Ultrafiltration membranes (with pore sizes 2–100 nm) are widely used in different industrial applications, including applications of water and wastewater treatment; however, fouling remains a stubborn problem that restricts their widespread application.1,3–11 Most of the commercial UF membranes used in wastewater treatments are made from hydrophobic polymers such as, polyethersulfone (PES), poly(vinylidene fluoride) (PVDF), polysulfone (Psf) and polyacrylonitrile (PAN)12,13 which are particularly susceptible to membrane fouling. During filtration processes, deposition of foulants (e.g. proteins in solution) on membrane surfaces leads to drastic flux decline, shortened operational life, increased cleaning requirements and higher replacement and operational costs.14–16 Therefore, membranes with anti-fouling surfaces have considerable potential to improve the water permeation and performance of these processes. The hydrophilic modification of membrane surfaces is a an effective way to improve their permeability and anti-fouling properties as the hydrophilic groups can facilitates an enhancement in water flux, reduce the flow resistance and help prevents the adhesions of pollutants on the membrane surfaces.17–23Recently, a number of techniques have been investigated to improve the hydrophilicity and anti-fouling performance of membranes including grafting hydrophilic species to the top surface of pre-formed ultrafiltration membranes,24,25 hydrophilic polymer coatings,26–28 cold plasma treatment,29–33 covalent attachment of hydrophilic polymers,34,35 and incorporating hydrophilic fillers such as inorganic nanoparticles.36 Among these methods, grafting hydrophilic molecules to the top surface of membranes is a simple, practical and economical addition to existing membrane manufacturing processes, without requiring heat treatment or any other hazardous conditions.37
Brominated poly(phenylene oxide) (BPPO) is an outstanding ultrafiltration polymer with high glass transition temperature (210 °C), excellent membrane forming properties, good thermal and mechanical strength, and excellent chemical stability.38–40 It is commonly available and has been used to prepare ultrafiltration membranes with high permeation rate (high water flux).41 Despite these inherent material advantages, the hydrophobic nature of BPPO membranes makes them highly susceptible to fouling, which with their usually modest water permeability, proves a significant limitation in their application to water filtration.45
Compared to other UF polymers such as polyethersulfone (PES), poly(vinylidene fluoride) (PVDF), polysulfone (Psf) and polyacrylonitrile (PAN), BPPO contains a highly reactive groups (–CH2Br) group that can react directly with imidazole or amine species without the need to use a cross-linker or pre-treatment the membrane.42–44
This reactivity enables BPPO membranes to be readily chemically altered to induce controlled levels of hydrophilicity, excellent separation efficiency, and high rate of water permeation.18–20 Feng et al.37 modified the surface of BPPO UF membranes using chitosan as grafting agent. The modified BPPO membrane showed a slight decrease in water permeation after 6 h grafting duration. The chitosan-modified BPPO membranes showed an improved in anti-fouling performance with 85% flux recovery ratio. Using a more complicated approach, Lin et al.46 prepared a novel ultrafiltration BPPO/TPPOQP-Br via a phase inversion method and found that a membrane modified with TPPOQP-Br showed higher permeability (water flux) while maintaining higher rejection properties in the resulting ultrafiltration membranes. Similarly, Yang et al.47 incorporated polyethyleneimine factionalised graphene oxide (GO) into BPPO ultrafiltration membrane matrix by a covalent bond interaction (nucleophilic reaction) between PEI and benzyl bromide of BPPO. Then a crosslinking network of PEI-GO/BPPO was obtained to provide a route for the water passing rapidly through. The composite PEI-GO/BPPO membranes exhibited a highly improved in permeation rate (6 times higher) compared with the pristine BPPO membranes. In each case, the modified BPPO membranes showed higher porosity, surface hydrophilicity, and improved anti-fouling properties.
In this study, diethylenetriamine (DETA) chosen for the hydrophilic modification of BPPO UF membranes because of its (1) relatively low volatility, (2) reactive primary and secondary amine, (3) commercial availability, and (4) solubility in polar organic solvents. Previously, DETA has been used in various applications, including gas separations (H2/CO2), epoxy adhesives and in the petrochemical industry.48
In this work, DETA was grafted on the top surface of BPPO UF membranes using a direct single step reaction as depicted in Scheme 1.
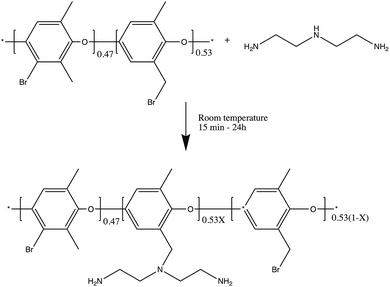 | ||
| Scheme 1 The chemical reaction between BPPO and DETA, where X represents the extent of reaction between BPPO and DETA. | ||
Experimental
Materials
Bromomethylated poly(phenylene oxide) (BPPO, MW = 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) with aryl substitution degree of 0.47 and benzyl substitution degree of 0.53 was purchased from Tianwei Membrane Corporation Ltd. (Shandong, China). NaOH (purity≥99%) was obtained from Merck KGaA Company (Germany). Diethylenetriamine (DETA) (purity, ≥99%), and 1-methyl-2-pyrrolidone (NMP, purity ≥99%) were obtained from Sigma-Aldrich, Australia. Poly(ethylene glycol) (PEG) with different molecular weights (35 kDa, 100 kDa, 200 kDa) was obtained from Sigma-Aldrich, Australia and used without purification. Bovine Serum Albumin (BSA) was obtained from Sigma-Aldrich Australia and used as model foulant. Milli-Q water was used in the experiments with a resistivity of 18.2 MΩ cm−1. Distilled water (DI) was provided a water distillation unit (Labglass Aqua III).
500) with aryl substitution degree of 0.47 and benzyl substitution degree of 0.53 was purchased from Tianwei Membrane Corporation Ltd. (Shandong, China). NaOH (purity≥99%) was obtained from Merck KGaA Company (Germany). Diethylenetriamine (DETA) (purity, ≥99%), and 1-methyl-2-pyrrolidone (NMP, purity ≥99%) were obtained from Sigma-Aldrich, Australia. Poly(ethylene glycol) (PEG) with different molecular weights (35 kDa, 100 kDa, 200 kDa) was obtained from Sigma-Aldrich, Australia and used without purification. Bovine Serum Albumin (BSA) was obtained from Sigma-Aldrich Australia and used as model foulant. Milli-Q water was used in the experiments with a resistivity of 18.2 MΩ cm−1. Distilled water (DI) was provided a water distillation unit (Labglass Aqua III).
Membrane preparation
BPPO UF membranes were prepared using a Phase inversion casting method. A 40 mL casting solution was prepared by stirring 20 wt% BPPO polymer in 80 wt% NMP solvent for 24 h using magnetic stirrer at 200 rpm. Afterward, the homogenous casting solution was left overnight in the fume cupboard to release air bubbles. The casting solution was then spread uniformly on a cleaned glass plate and cast using a casting knife (Paul Gardner CO., Inc. USA) with a 150 μm air gap. The membranes were then immersed in a coagulation bath of deionized water (DI). After peeling the membranes off from the glass plate, they were removed from the coagulation bath and washed three times with DDI and stored in fresh double deionized water (DDI) for at least one day before use.49,50To prepare BPPO/DETA composite membranes, the BPPO membrane was exposed to a 1 M solution of DETA in H2O by floating the BPPO membrane so only the top surface contacted the DETA solution. The reaction temperature was held constant at room temperature while the reaction time was varied from 15 min to 24 h. Afterward; the composite BPPO membranes were washed three times with DI water and then stored in fresh DI water for at least one day before use. The composite membranes were denoted according to the grafting period BPPO/DETA-15 min, BPPO/DETA-30 min, BPPO/DETA-1 h, BPPO/DETA-1.5 h, BPPO/DETA-2 h, BPPO/DETA-3 h, BPPO/DETA-4 h, BPPO/DETA-5 h, BPPO/DETA-6 h, BPPO/DETA-12 h, BPPO/DETA-24 h.
FTIR and contact angle measurements
The modified and unmodified BPPO membranes were characterised by Fourier Transform Infrared spectroscopy in the range of 4000–600 cm−1 at a resolution of 4 cm−1 and averaged from 64 scans. The surface morphology of BPPO and DETA/BPPO membranes were examined by scanning electron microscopy (SEM) (Magellan SEM, FEI Company, America). The hydrophilicity of BPPO membranes was determined with contact angle-measuring instrument (video based optical contact angle measuring instrument, OCA-15EC, Dataphysics, Germany) using 5 μL of water, dropped from the syringe onto the top surface of modified and unmodified BPPO membranes. Results have been averaged from, at least five contact angle measurements at different locations for each membrane. The porosity of membranes was calculated by mass difference between the membrane soaked in water for 3 h and after drying in vacuum oven at 60 °C. Membrane porosity was calculated according eqn (1):51,52
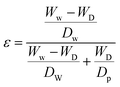 | (1) |
Permeation and molecular weight cut-off
The pure water flux (PWF) of the unmodified BPPO and BPPO/DETA composite membranes were measured using dead-end cell filtration unit (HP4750 stirred cell, Sterlitech, USA) with membrane samples cut to a diameter of 49 mm to effective membrane area of 14.6 cm2. The filtration cell was filled with 300 mL double deionized water (DDI) and then connected to 5 L dispensing vessel with compressed nitrogen gas used to control the feed pressure. The collected permeate was determined using an electronic balance with mass change recorded automatically with connected Labview software. During filtrations test, membrane samples are pre-compacted first at 150 kPa for at least 1 h to stabilise the flux, which is then recorded at a pressure of 100 kPa. Reported flux values are averaged from tests using at least five membrane samples.Molecular weight cut-off (MWCO) and solute rejection was determined using a series of 1 g L−1 of polyethylene glycol (PEG) solution, prepared by dissolving PEG of different molecular weights (35, 100, 200 kDa) in deionized water (DI). The PEG rejection rates were calculated by measuring the concentrations of PEG solution in the feed solution (Cf) and permeate water (CP) using a total organic carbon analyser (TOC-LCSH, Shimadzu, Japan) using eqn (2):53
| R = (1 − CP/Cf) × 100 | (2) |
The pore size of the membranes was then estimated based on the values of MWCO of the membrane according to the eqn (3):55
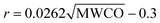 | (3) |
Fouling measurements
Constant flux fouling mode was used in this study to determine the fouling resistance of the membranes.56 The membrane samples were first pre-compacted by double deionised water at 150 kPa for at least 1 h. Then, DDI water was exchanged for a BSA protein solution (0.5 g L−1, prepared using phosphate buffer, pH = 7.4). During the fouling test, the flux was fixed at approximately 40 LMH for 2 h using a peristaltic pump (L/S Digital Drive, L/S Easy-Load 3 pump head, and peroxide-cured silicone tubing, L/S 13, Masterflex, USA). The transmembrane pressure (TMP) was recoded each 30 s using two pressure transducers. After testing, the fouled membranes were physically and chemically cleaned. For physical cleaning, the membranes in the filtration cell were rinsed twice with DDI for 20 min. The membrane flux after physical cleaning was recorded from the lab view software at 100 kPa.For chemical cleaning, 100 mL of NaOH solution (2 g L−1, pH = 12) was added to the filtration cell and stirred for 20 min before being rinsed three times with DDI to wash off the NaOH solution. After chemical cleaning step, the membrane flux was recorded at 100 kPa. Chemical cleaning performance was monitored over three fouling and cleaning cycles, with membrane flux recorded after each cycle.
To determine the performance of membranes, flux recovery ratio of membranes was calculated by comparing the flux after each cleaning cycle to the flux before fouling.
As a measure of fouling behaviour, membrane resistances were estimated using the following eqn (4–7):54
| Rt = Rm + Rir + Rr | (4) |
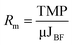 | (5) |
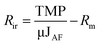 | (6) |
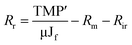 | (7) |
Static protein adsorption
Anti-fouling performance was examined using a Static protein adsorption experiments. Adsorption capacity of BSA macromolecules was evaluated by exposing the top surface of BPPO membranes (diameter 25 mm) to a BSA solution (0.5 mg L−1) in a stirring cell. The stirring cell was then incubated in a shaker (150 rpm agitation) at 30 °C for 24 h to reach the adsorption–desorption equilibrium. The amount of protein adsorbed on the top surface of membranes was calculated from the reduction concentration of BSA solution using the f eqn (8):55–57
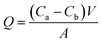 | (8) |
Results and discussion
The FTIR-ATR spectroscopy revealed the extent of chemical reaction between DETA and the BPPO UF membranes, as shown in Fig. 1.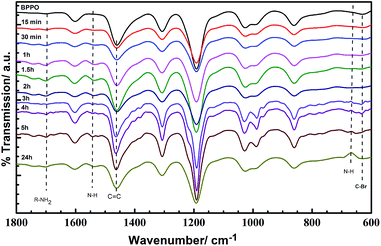 | ||
| Fig. 1 FTIR-ATR spectra for BPPO and DETA-modified membranes. The time indicated for each spectra is the duration of DETA-grafting. | ||
All the BPPO UF membranes showed the main characteristic peak at 1469 cm−1 which is attributed to stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of benzene.46 The C–Br stretching peak at 630 cm−1 displayed only minor changes after grafting,46 evidence that the reaction with DETA was limited to the top surface of BPPO UF membranes. After grafting, a series of new peaks emerged at 664 cm−1, 1646.5 cm−1, and 1535 cm−1 assigned to the N–H wagging, secondary amine (R–NH2) and R2NH deformation vibrations of DETA respectively. The results of FTIR-ATR spectra confirm that diethylenetriamine was successfully grafted to the surface of BPPO UF membranes and not reversibly adsorbed onto the membrane.
C of benzene.46 The C–Br stretching peak at 630 cm−1 displayed only minor changes after grafting,46 evidence that the reaction with DETA was limited to the top surface of BPPO UF membranes. After grafting, a series of new peaks emerged at 664 cm−1, 1646.5 cm−1, and 1535 cm−1 assigned to the N–H wagging, secondary amine (R–NH2) and R2NH deformation vibrations of DETA respectively. The results of FTIR-ATR spectra confirm that diethylenetriamine was successfully grafted to the surface of BPPO UF membranes and not reversibly adsorbed onto the membrane.
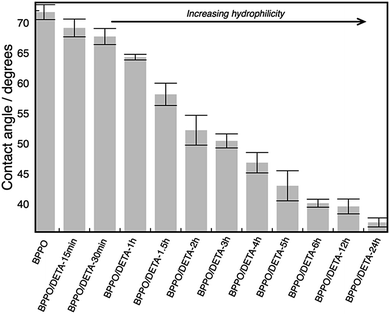
Surface hydrophilicity is very important factor in the anti-fouling properties and has a direct effect on the pure water flux (PWF) and anti-fouling performance of membranes. Reductions to the water contact angle of membranes are indicative of higher surface hydrophilicity and vice versa.58,59
Fig. 2 shows that the contact angle decreased significantly with DETA grafting period from the unmodified BPPO UF membranes contact angle 73°. These results indicate that the amount of DETA on the top surface of UF BPPO membranes increased with grafting period and that surface-attached DETA enhanced the hydrophilicity of BPPO membranes.
Pure water flux and rejection are two important parameters for applications of ultrafiltration membranes. Fig. 3 shows the measurements of pure water flux (PWF) for the unmodified and modified BPPO membranes. Pure water flux (PWF) for the unmodified BPPO membranes was 197 LMH. Each of the modified BPPO membranes displayed a notable decrease in flux, with the greatest reduction of 59% observed in the membrane grafted for 24 hours.
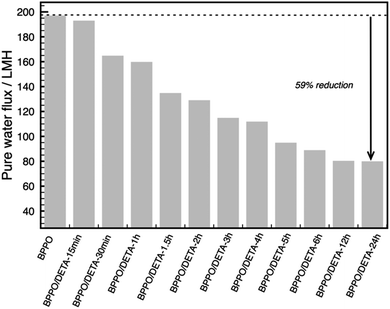 | ||
| Fig. 3 Pure water flux through BPPO and DETA-modified membranes. At the maximum grafting duration of 24 h, the pure water flux is reduced by 59% compared to the unmodified BPPO membrane. | ||
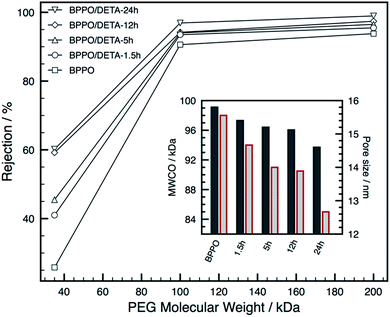
This can be explained by DETA molecules blocking or be covering the large pores within the membrane, leading to significant flux decline; a theory confirmed by the smaller pore size of the composite BPPO, as shown in Fig. 4.
The flux is generally determined by porosity of the entire membrane, pore size and thickness of membranes.60 As such, a lower pure water flux and higher retention rate are to be expected from the surface modification procedure.
SEM images of the membrane surfaces (Fig. S1† shows the top surface, Fig. S2† shows the bottom surface) do not show any apparent change in surface morphology, suggesting that the pore filling by DETA is mostly within the pore network, and is a coating of the internal pore surface (as opposed to complete pore blockage). Therefore, the pore wall of BPPO will be modified by DETA, leading to decrease the pore size and performance of membranes. This agrees with the observation that in the DETA-treated membranes the pure water flux decreases steadily as the treatment duration increases (Fig. 3).
Pore size and molecular weight cut-off for pristine and composite BPPO membranes was evaluated by the rejection of PEG. Molecular weight cut-off (MWCO) was calculated based on a polyethylene glycol rejection rate of 90%, which was then used to calculate the pore size of each membrane using eqn (2). The unmodified BPPO membrane showed a MWCO of 98 kDa, whilst the composite BPPO membranes showed a gradual decrease in MWCO that should be attributed to the decrease in the pore size of the active layer of membranes compared to the pristine BPPO membranes Table 1. Membrane resistances and recovery ratios measured and calculated from membrane fouling experiments for BPPO and BPPO/DETA composite membranes.
| Membrane | Porosity (%) | Pore size (nm) |
|---|---|---|
| BPPO | 70.6 | 15.8 |
| BPPO/DETA-15 min | 69.0 | 15.5 |
| BPPO/DETA-30 min | 65.8 | 15.1 |
| BPPO/DETA-1 h | 64.3 | 15.5 |
| BPPO/DETA-1.5 h | 63.0 | 15.4 |
| BPPO/DETA-5 h | 61.3 | 15.2 |
| BPPO/DETA-24 h | 55.6 | 14.6 |
The pore size of composite membranes could also be reduced by DETA cross-linking BPPO chains, which would decrease the number and the size of the pores in the top surface of the composite membranes. Consistent with our other observations, porosity calculated according to eqn (1), confirmed that surface coating with DETA decreased pore size and pore volume of BPPO membranes. Table 1 shows that membrane porosity decreased from 70.6% to 55.6% after a 24 h DETA treatment, indicative of a partial filling of the accessible pores with DETA.
To quantitatively examine the membrane fouling performance, total filtration resistance (Rt) is the sum of intrinsic membrane resistance (Rm) (related to the membrane properties), reversible resistance (Rr) (due to the external deposition of pollutants the membrane surface), irreversible resistance (Rir) (due to the strong adherence of pollutants on the membrane surface) were calculated using eqn (4)–(7) in the experimental section. The results of membrane resistances are presented in Table 2.
| Membrane | Rm × 109 | Rir × 109 | Rr × 109 | Rt × 109 | Rr/Rt | Rir/Rt |
|---|---|---|---|---|---|---|
| BPPO | 5.07 ± 0.63 | 4.12 ± 0.21 | 5.19 ± 0.31 | 14.39 | 0.36 | 0.28 |
| BPPO/DETA-15 min | 5.23 ± 0.57 | 2.38 ± 0.41 | 5.02 ± 0.61 | 12.63 | 0.39 | 0.18 |
| BPPO/DETA-30 min | 6.06 ± 0.23 | 2.43 ± 0.58 | 6.52 ± 0.99 | 15.01 | 0.43 | 0.16 |
| BPPO/DETA-1 h | 6.25 ± 0.55 | 2.08 ± 0.32 | 7.18 ± 1.1 | 15.51 | 0.46 | 0.134 |
| BPPO/DETA-1.5 h | 7.41 ± 0.41 | 1.52 ± 0.71 | 7.30 ± 1.67 | 16.23 | 0.45 | 0.09 |
| BPPO/DETA-2 h | 7.74 ± 0.62 | 1.38 ± 0.5 | 8.36 ± 0.88 | 17.48 | 0.48 | 0.07 |
| BPPO/DETA-3 h | 8.36 ± 0.11 | 0.77 ± 0.01 | 9.61 ± 0.97 | 18.70 | 0.51 | 0.04 |
| BPPO/DETA-4 h | 8.94 ± 0.23 | 0.34 ± 0.03 | 10.71 ± 0.3 | 19.90 | 0.53 | 0.017 |
| BPPO/DETA-5 h | 10.53 ± 0.2 | 0.28 ± 0.06 | 10.44 ± 1.54 | 21.25 | 0.49 | 0.013 |
| BPPO/DETA-6 h | 11.24 ± 0.33 | 0.17 ± 0.02 | 11.81 ± 0.81 | 23.22 | 0.50 | 0.007 |
| BPPO/DETA-12 h | 12.24 ± 0.92 | 0.1 ± 0.01 | 11.21 ± 2.1 | 23.50 | 0.47 | 0.004 |
| BPPO/DETA-24 h | 12.54 ± 0.51 | 0.11 ± 0.001 | 9.10 ± 0.59 | 21.75 | 0.42 | 0.005 |
Detailed analysis showed that the DETA-modified membranes exhibited significantly higher reversible fouling (Rr) compared to irreversible fouling (Rir). It is postulated that due to the smaller pore size of the DETA-modified membranes (consistent with the decreased pure water flux discussed earlier) the higher trans-membrane pressure (TMP) required to achieve constant flow during the fouling test, lead to greater irreversible fouling. However, a positive interpretation of this result is that the DETA-modified membranes can be readily and almost completely regenerated using physical and/or chemical cleaning. For all of the composite membranes, the (Rr/Rt) ratio is considerably higher than (Rir/Rt) as compared to the pristine BPPO membrane which that had a similar value. Hydrophilic surfaces usually resist protein adsorption and deposition by hydrophobic interaction, explaining the DETA modified BPPO higher anti-fouling properties. The results of intrinsic membrane resistance (Rm) were consistent with the results of pore size. This outcome may be attributed to the fact that the intrinsic membrane resistance (Rm) was relied highly on the pore sizes of membranes. If the pore size of membranes increases after modification, the Rm decreases.61
Likewise, static protein adsorption is one of the most important indicators for measuring the anti-fouling performance of membranes. The results of static protein adsorption are presented in Table 3 where it can be seen that the adsorption of protein decreased from 17.8 μg cm−2 for pristine BPPO to 17.1 μg cm−2 after treatment with DETA for 15 min; a reduction that continues monotonically to just 6.0 μg cm−2 BPPO/DETA-24 h. In a consistent manner, the flux recovery ratio (FRR) increases from 40.3%, for the untreated BPPO membrane, to 95.9% for the 24 h treated membrane. These results confirm the DETA-grafted BPPO membranes showed excellent anti-fouling properties, achieved through the facile through modification.
| Membrane | Static protein adsorption (μg cm−2) | Flux recovery ratio (%) |
|---|---|---|
| BPPO | 17.8 | 40.3 |
| BPPO/DETA-15 min | 17.1 | 48.3 |
| BPPO/DETA-30 min | 16.5 | 63.0 |
| BPPO/DETA-1 h | 15.3 | 70.8 |
| BPPO/DETA-1.5 h | 13.6 | 80.3 |
| BPPO/DETA-2 h | 11.7 | 81.3 |
| BPPO/DETA-3 h | 10.2 | 83.6 |
| BPPO/DETA-4 h | 9.3 | 86.4 |
| BPPO/DETA-5 h | 8.6 | 88.4 |
| BPPO/DETA-6 h | 7.7 | 90.6 |
| BPPO/DETA-12 h | 6.5 | 93.2 |
| BPPO/DETA-24 h | 6.0 | 95.9 |
Here, enhanced anti-fouling performance is generated by surface hydrophilicity of membrane surface.59 Hydrophilic surfaces can adsorb water molecules and form a hydrated layer and steric hindrance on the surface of membranes, which significantly prevents the adsorption of protein and other pollutants agents on the membrane surfaces.61
Consequently, the flux of the BPPO/DETA-24 h membrane can be almost completely recovered, unlike the unmodified BPPO, as adsorbed protein is loosely bound and more readily removed from the hydrophilic DETA surface of treated BPPO membranes.
Conclusions
In this article, we report the grafting of diethylenetriamine (DETA) to the top surface of BPPO membranes without pre-treatments or added cross-linking agent. Characterisation of the resulting BPPO/DETA membranes highlight that although DETA causes a reduction in the porosity and pure water flux, the rejection rate was improved. Further, as a result of introducing DETA's polar groups to the membrane surface, hydrophilicity of the modified membranes increased and BPPO's interaction with foulants at the membrane surface was reduced. These features result in the composite DETA/BPPO membranes having a much higher flux recovery ratio (almost 100%) than the pristine BPPO membranes. Although the flux is reduced by DETA modification, the improvement in anti-fouling performance promise to provide much greater operating lifetimes for BPPO membranes utilised for water and waste water treatment.Acknowledgements
Muayad al-Shaeli acknowledges scholarship support from the Iraqi Government. Bradley Ladewig acknowledges funding support from Imperial College London, including for open access publication charges.References
- W. Chen, Y. Su, J. Peng, X. Zhao, Z. Jiang, Y. Dong, Y. Zhang, Y. Liang and J. Liu, Environ. Sci. Technol., 2011, 45, 6545–6552 CrossRef CAS PubMed.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marĩas and A. M. Mayes, Nature, 2008, 452, 301–310 CrossRef CAS PubMed.
- W. Guo, H. H. Ngo and J. Li, Bioresour. Technol., 2012, 122, 27–34 CrossRef CAS PubMed.
- P. Le-Clech, V. Chen and T. A. G. Fane, J. Membr. Sci., 2006, 284, 17–53 CrossRef CAS.
- J. Mansouri, S. Harrisson and V. Chen, J. Mater. Chem., 2010, 20, 4567–4586 RSC.
- J. Peng, Y. Su, W. Chen, X. Zhao, Z. Jiang, Y. Dong, Y. Zhang, J. Liu and X. Fan, Ind. Eng. Chem. Res., 2013, 52, 13137–13145 CrossRef CAS.
- A. Ramesh, D. J. Lee, M. L. Wang, J. P. Hsu, R. S. Juang, K. J. Hwang, J. C. Liu and S. J. Tseng, Sep. Sci. Technol., 2006, 41, 1345–1370 CrossRef CAS.
- D. Rana and T. Matsuura, Chem. Rev., 2010, 110, 2448–2471 CrossRef CAS PubMed.
- Y. F. Yang, Y. Li, Q. L. Li, L. S. Wan and Z. K. Xu, J. Membr. Sci., 2010, 362, 255–264 CrossRef CAS.
- M. Yao, B. Ladewig and K. Zhang, Desalination, 2011, 278, 126–131 CrossRef CAS.
- M. Yao, K. Zhang and L. Cui, Desalination, 2010, 259, 11–16 CrossRef CAS.
- J. L. Acero, F. J. Benitez, F. J. Real and C. García, Sep. Purif. Technol., 2009, 65, 322–330 CrossRef CAS.
- K. Moons and B. Van der Bruggen, Desalination, 2006, 199, 245–247 CrossRef CAS.
- T. Barroso, M. Temtem, T. Casimiro and A. Aguiar-Ricardo, J. Supercrit. Fluids, 2011, 56, 312–321 CrossRef CAS.
- M. J. Han, G. N. B. Baroña and B. Jung, Desalination, 2011, 270, 76–83 CrossRef CAS.
- Z. Q. Tang, W. Li, J. Zhou, H. Y. Yu, L. Huang, M. G. Yan, J. S. Gu and X. W. Wei, Sep. Purif. Technol., 2009, 64, 332–336 CrossRef CAS.
- S. J. Lee, M. Dilaver, P. K. Park and J. H. Kim, J. Membr. Sci., 2013, 432, 97–105 CrossRef CAS.
- P. D. Peeva, T. Knoche, T. Pieper and M. Ulbricht, Ind. Eng. Chem. Res., 2012, 51, 7231–7241 CrossRef CAS.
- P. D. Peeva, N. Million and M. Ulbricht, J. Membr. Sci., 2012, 390–391, 99–112 CrossRef CAS.
- H. Susanto, A. Roihatin, N. Aryanti, D. D. Anggoro and M. Ulbricht, Mater. Sci. Eng., C, 2012, 32, 1759–1766 CrossRef CAS.
- Z. Wang, H. Yu, J. Xia, F. Zhang, F. Li, Y. Xia and Y. Li, Desalination, 2012, 299, 50–54 CrossRef CAS.
- F. Zhang, W. Zhang, Y. Yu, B. Deng, J. Li and J. Jin, J. Membr. Sci., 2013, 432, 25–32 CrossRef CAS.
- J. Zhang, Z. Xu, M. Shan, B. Zhou, Y. Li, B. Li, J. Niu and X. Qian, J. Membr. Sci., 2013, 448, 81–92 CrossRef CAS.
- G. Kang, M. Liu, B. Lin, Y. Cao and Q. Yuan, Polymer, 2007, 48, 1165–1170 CrossRef CAS.
- M. Ulbricht, H. Matuschewski, A. Oechel and H. G. Hicke, J. Membr. Sci., 1996, 115, 31–47 CrossRef CAS.
- L. E. S. Brink, S. J. G. Elbers, T. Robbertsen and P. Both, J. Membr. Sci., 1993, 76, 281–291 CrossRef CAS.
- Y. C. Chiag, Y. Chang, W. Y. Chen and R. C. Ruaan, Langmuir, 2012, 28, 1399–1407 CrossRef CAS PubMed.
- S. Yu, Z. Lü, Z. Chen, X. Liu, M. Liu and C. Gao, J. Membr. Sci., 2011, 371, 293–306 CrossRef CAS.
- S. Pal, S. K. Ghatak, S. De and S. DasGupta, J. Membr. Sci., 2008, 323, 1–10 CrossRef CAS.
- I. Sadeghi, A. Aroujalian, A. Raisi, B. Dabir and M. Fathizadeh, J. Membr. Sci., 2013, 430, 24–36 CrossRef CAS.
- M. L. Steen, L. Hymas, E. D. Havey, N. E. Capps, D. G. Castner and E. R. Fisher, J. Membr. Sci., 2001, 188, 97–114 CrossRef CAS.
- T. Vladkova, P. Atanasova, S. Petrov and P. Dineff, High Energy Chem., 2013, 47, 346–352 CrossRef CAS.
- H. Y. Yu, L. Q. Liu, Z. Q. Tang, M. G. Yan, J. S. Gu and X. W. Wei, J. Membr. Sci., 2008, 311, 216–224 CrossRef CAS.
- Y. Wang, J. H. Kim, K. H. Choo, Y. S. Lee and C. H. Lee, J. Membr. Sci., 2000, 169, 269–276 CrossRef CAS.
- L. P. Zhu, B. K. Zhu, L. Xu, Y. X. Feng, F. Liu and Y. Y. Xu, Appl. Surf. Sci., 2007, 253, 6052–6059 CrossRef CAS.
- J. Kim and B. Van Der Bruggen, Environ. Pollut., 2010, 158, 2335–2349 CrossRef CAS PubMed.
- Y. Feng, X. Lin, H. Li, L. He, T. Sridhar, A. K. Suresh, J. Bellare and H. Wang, Ind. Eng. Chem. Res., 2014, 53, 14974–14981 CrossRef CAS.
- L. Jiang, X. Lin, J. Ran, C. Li, L. Wu and T. Xu, Chin. J. Chem., 2012, 30, 2241–2246 CrossRef CAS.
- B. Tang, T. Xu, M. Gong and W. Yang, J. Membr. Sci., 2005, 248, 119–125 CrossRef CAS.
- T. Xu, D. Wu and L. Wu, Prog. Polym. Sci., 2008, 33, 894–915 CrossRef CAS.
- H. Wu, B. Tang and P. Wu, J. Membr. Sci., 2010, 362, 374–383 CrossRef CAS.
- X. Lin, X. Liang, S. D. Poynton, J. R. Varcoe, A. L. Ong, J. Ran, Y. Li, Q. Li and T. Xu, J. Membr. Sci., 2013, 443, 193–200 CrossRef CAS.
- X. Lin, J. R. Varcoe, S. D. Poynton, X. Liang, A. L. Ong, J. Ran, Y. Li and T. Xu, J. Mater. Chem. A, 2013, 1, 7262–7269 CAS.
- X. Lin, L. Wu, Y. Liu, A. L. Ong, S. D. Poynton, J. R. Varcoe and T. Xu, J. Power Sources, 2012, 217, 373–380 CrossRef CAS.
- J. Zheng, L. Li, H. K. Tsao, Y. J. Sheng, S. Chen and S. Jiang, Biophys. J., 2005, 89, 158–166 CrossRef CAS PubMed.
- X. Lin, K. Wang, Y. Feng, J. Z. Liu, X. Fang, T. Xu and H. Wang, J. Membr. Sci., 2015, 482, 67–75 CrossRef CAS.
- L. Yang, B. Tang and P. Wu, J. Mater. Chem. A, 2014, 2, 18562–18573 CAS.
- H. Wang, D. R. Paul and T. S. Chung, J. Membr. Sci., 2013, 430, 223–233 CrossRef CAS.
- S. P. Nunes, M. L. Sforça and K. V. Peinemann, J. Membr. Sci., 1995, 106, 49–56 CrossRef CAS.
- C. A. Smolders, A. J. Reuvers, R. M. Boom and I. M. Wienk, J. Membr. Sci., 1992, 73, 259–275 CrossRef CAS.
- C. Feng, R. Wang, B. Shi, G. Li and Y. Wu, J. Membr. Sci., 2006, 277, 55–64 CrossRef CAS.
- N. Li, C. Xiao, S. An and X. Hu, Desalination, 2010, 250, 530–537 CrossRef CAS.
- Y. Lu, S. L. Yu and L. L. Meng, J. Harbin Inst. Technol., 2009, 41, 64–69 CAS.
- A. Razmjou, J. Mansouri and V. Chen, J. Membr. Sci., 2011, 378, 73–84 CrossRef CAS.
- F. Li, J. Meng, J. Ye, B. Yang, Q. Tian and C. Deng, Desalination, 2014, 344, 422–430 CrossRef CAS.
- B. P. Tripathi, N. C. Dubey and M. Stamm, J. Membr. Sci., 2014, 453, 263–274 CrossRef CAS.
- R. Zhou, P. F. Ren, H. C. Yang and Z. K. Xu, J. Membr. Sci., 2014, 466, 18–25 CrossRef CAS.
- J. Hong and Y. He, Desalination, 2014, 332, 67–75 CrossRef CAS.
- C. Zhao, X. Xu, J. Chen and F. Yang, J. Environ. Chem. Eng., 2013, 1, 349–354 CrossRef CAS.
- S. J. Oh, N. Kim and Y. T. Lee, J. Membr. Sci., 2009, 345, 13–20 CrossRef CAS.
- C. Wang, R. Feng and F. Yang, J. Colloid Interface Sci., 2011, 357, 273–279 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra05524b |
| This journal is © The Royal Society of Chemistry 2017 |