 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Comment on “Lewis acidic ionic liquids of crown ether complex cations: preparation and applications in organic reactions” by Y. Liang, J. Wang, C. Cheng and H. Jing, RSC Adv., 2016, 6, 93546
Małgorzata Swadźba-Kwaśny
QUILL Research Centre, School of Chemistry and Chemical Engineering, Queen's University Belfast, UK. E-mail: m.swadzba-kwasny@qub.ac.uk
First published on 7th November 2017
Introduction
A paper published recently in RSC Advances reports on a new group of solvate ionic liquids,1 generated by the combination of a crown ether (18-crown-6), an alkali metal chloride (NaCl or KCl) and a halide-abstracting agent, which was also a metal chloride (AlCl3, FeCl3, or ZnCl2).2 The structures of synthesised solvate ionic liquids were postulated (Fig. 1, left). The anion structure was confirmed using Raman spectroscopy, with bands characteristic of M–Cl stretching frequencies reported, as shown in Fig. 1, right.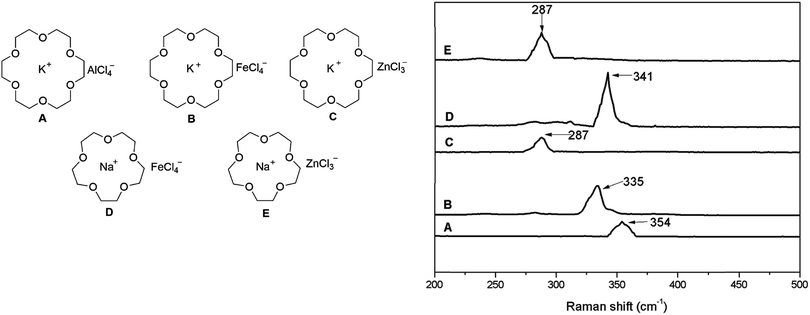 | ||
| Fig. 1 Left: postulated structures of Lewis acidic ionic liquids; right: fragments of Raman spectra, corresponding to M–Cl stretching frequencies. Adapted from ref. 2. | ||
The assignment of bands in spectrum A to [AlCl4]− and in spectra B and D to [FeCl4]− is consistent with all literature reports,3 and supported with appropriate citations. However, assignment of the band at 287 cm−1 (spectra C and E) to [ZnCl3]− is in stark contrast with the current knowledge.
Anionic speciation in chlorometallate ionic liquids has vital impact on their properties: viscosity of ionic liquids with doubly-charged anions is dramatically higher, conductivity is lower, and chemical behaviour differs, therefore it is of utmost importance to have them correctly understood.
Speciation of chlorozincate anions in ionic liquids – Raman spectroscopy and other techniques vs. mass spectrometry
The most common chlorozincate anion is [ZnCl4]2−.4 In the Cambridge Structural Database, there is a plethora of deposited single crystal structures with discrete tetrachlorozincate anions – the search for {ZnCl4} motif returns 849 hits, most of these featuring [ZnCl4]2−.5 In contrast, not a single structure with (presumably) trigonal planar [ZnCl3]− has been deposited to date. In reference books on vibrational spectroscopy, there is no mention of the [ZnCl3]− anion, but vibrational frequencies for [ZnCl4]2− are always reported – even in Nakamoto's book from 1963 (ν1 frequency at 282 cm−1).6Nevertheless, in publications on ionic liquids two opposing speciations are proposed: one assuming the existence of [ZnCl3]− and [Zn2Cl5]− anions,7,8 and another suggesting the formation of [ZnCl4]2− and [Zn2Cl6]2−.9 In 2011 we encountered this discrepancy in scientific literature, and decided to address it, as a part of our research on speciation studies of halometallate ionic liquids.3,10–14
A multi-technique, multi-phase study on chlorozincate(II) ionic liquids with 1-octyl-3-methylimidazolium, [C8mim]+ and 1-ethyl-3-methylimidazolium, [C2mim]+, cations was carried out.15
In order to study the crystalline phase, single crystals were grown from the [C2mim]Cl–ZnCl2 system at varying stoichiometries (defined in terms of molar fractions of ZnCl2, χZnCl2). The resulting structures were [C2mim]2[ZnCl4] for χZnCl2 = 0.33, and [C2mim]2[Zn2Cl6] for χZnCl2 = 0.50. Tricoordinate chlorozincate anions were not observed – neither in our study, nor in any other crystallographic work (as per CSD search).5 Raman vibrations assigned to the Zn–Cl vibrations in these solids were 280 cm−1 for [ZnCl4]2−, and 264 and 317 cm−1 for [Zn2Cl6]2−.
In Raman spectroscopic study of the liquid [C8mim]Cl–ZnCl2 system, corresponding species were detected, with the 275 cm−1 band for [ZnCl4]2− at χZnCl2 = 0.33, as well as 269 and 312 cm−1 bands for [Zn2Cl6]2− at χZnCl2 = 0.50. Thus, the presence of [ZnCl4]2− and [Zn2Cl6]2− anions was confirmed in both liquid and solid chlorozincate(II) ionic liquids.
Physico-chemical properties of the [C8mim]Cl–ZnCl2 system were also studied. Particularly telling was the study of Lewis acidity (Gutmann acceptor number approach, using triethylphosphine oxide as the 31P NMR spectroscopic probe). The most significant increase in Lewis acidity was noted at χZnCl2 = 0.33, that is when [ZnCl4]2− ions start being replaced by [Zn2Cl6]2− ions (Fig. 2). Simply based on stoichiometry, such pattern does not agree with the [ZnCl3]−/[Zn2Cl5]− speciation, for which there would be no discontinuity in properties at χZnCl2 = 0.33.
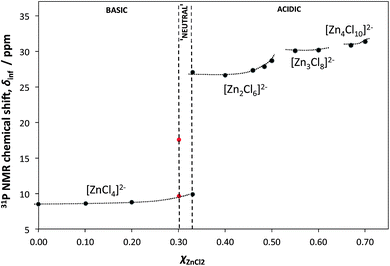 | ||
| Fig. 2 Plot of the 31P NMR shifts of triethylphosphine oxide (spectroscopic probe of Lewis acidity) dissolved in various compositions of the [C8mim]Cl–ZnCl2 system, and extrapolated to infinite dilution, δinf, as a function of composition (χZnCl2). The dotted lines are only visual guides. Adapted from ref. 15. | ||
Doubly-charged speciation was also inferred from the pseudo-phase diagram, generated from glass transition in the [C8mim]Cl–ZnCl2 system
Finally, ESI-MS spectra of several [C8mim]Cl–ZnCl2 samples were recorded – and, in contrast to all other multiple techniques, suggested the presence of [ZnCl3]− and [Zn2Cl5]−.In the literature, although ESI-MS was successfully used to study speciation in a wide variety of compounds, including some complex ionic liquids, e.g. polyiodides,16 numerous pitfalls are also reported. Notable are in-depth studies on perturbations in MS by Di Marco and co-workers,17,18 and a recent work by Clark and co-workers, entitled “Failure of ESI Spectra to Represent Metal-Complex Solution Composition: A Study of Lanthanide–Carboxylate Complexes”.19 The likely source of these discrepancies lays the fact that ions detected in MS are in fact in the gas phase, not in the liquid. The mechanisms leading to the formation of gas-phase ions from liquid-phase droplets are discussed expertly by Kebarle,20–22 with a recent tutorial review by Awad et al. offering a simple summary on the introductory level.23
In our chlorozincate study, under the ESI-MS conditions, the electrostatic factors most likely encouraged the separation of the doubly-negative charge in [Zn2Cl6]2− into two singly-charged [ZnCl3]− anions.15 Identical conclusions were reached in earlier work by Alves et al., published in Journal of Raman Spectroscopy, who stated “there is no experimental result that supports the formation of [ZnCl3]− or [Zn2Cl5]− species, but there are enough experimental data on the formation of [ZnCl4]2− and [Zn2Cl6]2− species in ZnCl2/LiCl melts (…). Therefore, we interpret the detection of [ZnCl3]− species by EMS as the result of the reaction [ZnCl4]2− → [ZnCl3]− + Cl−”.9 They also report the [ZnCl4]2− anion vibration at 276 cm−1, and list older studies on [ZnCl4]2− in various environments, with analogous vibrations at 275, 286 and 288 cm−1.
Subsequent investigations were carried out by the licence group using XPS24 and by the Abbott group using EXAFS.25 Both were consistent with the presence of [ZnCl4]2− and [Zn2Cl6]2− species.
Despite this breadth of work, Liang et al. assigned the Raman band at 287 cm−1 to [ZnCl3]−, citing neither of the above-mentioned articles, but a 1999 paper on aqueous chemistry of zinc chloride, published in Desalination, where the existence of such anion is indeed suggested, but with no further literature ref. 26.
Conclusion
Independent work carried out by several groups, using a very wide range of direct and indirect techniques, demonstrates explicitly two facts: (1) vibrational frequency at 287 cm−1 is in good agreement with a band corresponding to [ZnCl4]2−, and should be reported as such, and (2) the presence of [ZnCl3]− in chlorozincate(II) ionic liquids, albeit often cited, may be traced back to mass spectrometry studies, which return results contradictive to: Raman spectroscopy, XPS, EXAFS, and physico-chemical properties of chlorozincate(II) ionic liquids.Conflicts of interest
There are no conflicts to declare.Acknowledgements
I would like to thank Prof. Michael D. Ward for his very constructive remarks that contributed to the clarity of this manuscript.References
- T. Mandai, K. Yoshida, K. Ueno, K. Dokko and M. Watanabe, Phys. Chem. Chem. Phys., 2014, 16, 8761 RSC
.
- Y. Liang, J. Wang, C. Cheng and H. Jing, RSC Adv., 2016, 6, 93546 RSC
.
- J. Estager, J. D. Holbrey and M. Swadźba-Kwaśny, Chem. Soc. Rev., 2014, 43, 847 RSC
.
- J. Burgess and R. H. Prince, Zinc: Inorganic & Coordination Chemistry, John Wiley & Sons, Ltd, Chichester, UK, 31st edn, 2006, vol. 7 Search PubMed
.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2016, B72, 171 CrossRef PubMed
.
- A. Getsis, S. Tang and A.-V. Mudring, Eur. J. Inorg. Chem., 2010, 2010, 2172 CrossRef
.
- V. Lecocq, A. Graille, C. C. Santini, A. Baudouin, Y. Chauvin, J.-M. Basset, L. Arzel, D. Bouchu and B. Fenet, New J. Chem., 2005, 29, 700 RSC
.
- A. P. Abbott, G. Capper, D. L. Davies and R. Rasheed, Inorg. Chem., 2004, 43, 3447 CrossRef CAS PubMed
.
- M. B. Alves, V. C. D. Soares, P. A. Z. Suarez and J. C. Rubim, J. Raman Spectrosc., 2008, 39, 1388 CrossRef CAS
.
- D. C. Apperley, C. Hardacre, P. Licence, R. W. Murphy, N. V. Plechkova, K. R. Seddon, G. Srinivasan, M. Swadźba-Kwaśny and I. J. Villar-Garcia, Dalton Trans., 2010, 39, 8679 RSC
.
- C. Hardacre, R. W. Murphy, K. R. Seddon, G. Srinivasan and M. Swadźba-Kwaśny, Aust. J. Chem., 2010, 63, 845 CrossRef CAS
.
- J. Estager, A. A. Oliferenko, K. R. Seddon and M. Swadźba-Kwaśny, Dalton Trans., 2010, 39, 11375 RSC
.
- M. Currie, J. Estager, P. Licence, S. Men, P. Nockemann, K. R. Seddon, M. Swadźba-Kwaśny and C. Terrade, Inorg. Chem., 2013, 52, 1710 CrossRef CAS PubMed
.
- F. Coleman, G. Feng, R. W. Murphy, P. Nockemann, K. R. Seddon and M. Swadźba-Kwaśny, Dalton Trans., 2013, 42, 5025 RSC
.
- J. Estager, P. Nockemann, K. R. Seddon, M. Swadźba-Kwaśny and S. Tyrrell, Inorg. Chem., 2011, 50, 5258 CrossRef CAS PubMed
.
- M. Groessl, Z. Fei, P. J. Dyson, S. A. Katsyuba, K. L. Vikse and J. S. McIndoe, Inorg. Chem., 2011, 50, 9728 CrossRef CAS PubMed
.
- V. B. Di Marco, G. G. Bombi, S. Zambon and P. Traldi, J. Mass Spectrom., 2009, 44, 120 CrossRef CAS PubMed
.
- V. B. Di Marco, L. Raveane, A. Dean and P. Traldi, Rapid Commun. Mass Spectrom., 2010, 24, 868 CrossRef CAS PubMed
.
- L. W. McDonald IV, J. A. Campbell and S. B. Clark, Anal. Chem., 2014, 86, 1023 CrossRef PubMed
.
- P. Kebarle and L. Tang, Anal. Chem., 1993, 65, 972 Search PubMed
.
- P. Kebarle and U. H. Verkerk, Mass Spectrom. Rev., 2009, 28, 898 CrossRef CAS PubMed
.
- P. Kebarle, J. Mass Spectrom., 2000, 35, 804 CrossRef CAS
.
- H. Awad, M. M. Khamis and A. El-Aneed, Appl. Spectrosc. Rev., 2014, 50, 158 CrossRef
.
- A. W. Taylor, S. Men, C. J. Clarke and P. Licence, RSC Adv., 2013, 3, 9436 RSC
.
- A. P. Abbott, J. C. Barron, G. Frisch, S. Gurman, K. S. Ryder and A. Fernando Silva, Phys. Chem. Chem. Phys., 2011, 13, 10224 RSC
.
- F. Aouad, A. Lindheimer, M. Chaouki and C. Gavach, Desalination, 1999, 121, 13 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2017 |
