Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Notable in situ surface transformation of Cu2O nanomaterials leads to dramatic activity enhancement for CO oxidation
Dan Zhao *,
Chang-Man Tu,
Xue-Jing Hu and
Ning Zhang*
*,
Chang-Man Tu,
Xue-Jing Hu and
Ning Zhang*
Institute of Applied Chemistry, College of Chemistry, Nanchang University, Nanchang, Jiangxi 330031, China. E-mail: zhaodan@ncu.edu.cn; nzhang.ncu@163.com; Tel: +86-0791-83969332
First published on 28th July 2017
Abstract
Cu2O is an important compound for many promising applications; however, its real state under serious application conditions, such as redox atmosphere of catalysis reactions, has seldom been investigated. In this study, a dramatic and sustainable activity enhancement for CO oxidation on catalytically used Cu2O nanoporous sphere samples was discovered as compared to that on their fresh counterparts. To illustrate the phenomenon, comprehensive characterizations such as XRD, H2-TPR, TEM, XPS, and CO-TPD were performed on fresh and used Cu2O samples. It was found that after one or several catalytic runs, the main phase among the used samples retained the crystalline feature of Cu2O. However, the surface would transform into a stable multivalent composite interface with almost unchanged Cu(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(I) ratio (around 4.0) and active oxygen distribution, even after only one catalytic run for CO oxidation; this indicated a notable reaction-induced or in situ surface restructuring effect of the Cu2O substrates. Compared to that of pure Cu2O, CuO, and reported CuxO samples, the active chemisorbed oxygen on these in situ restructured samples was observed in particularly higher surface composition (with the ratio of chemisorbed oxygen to lattice oxygen being around 5.5); this was further demonstrated to be highly efficient for the oxidization of CO in a relatively low temperature range even in the absence of O2 in the atmosphere and could be the main contributor to speed up CO oxidation in these samples. A similar enhancement in the activity on the used samples for CO oxidation as compared to that on the fresh samples was further testified in Cu2O nanomaterials with different morphologies such as cubic, octahedral, and 18-facet polyhedral nanoparticles; this implied that the surface restructuring effect under a redox reaction atmosphere was a common feature of the Cu2O-based materials, and the transformed surface could act as a superior and stable interface for heterogeneous catalysis. These findings might help in recognizing the real state of Cu2O-containing materials under serious application conditions or designing highly efficient Cu2O-based materials via convenient atmosphere-controlled manipulation for advanced applications.
Cu(I) ratio (around 4.0) and active oxygen distribution, even after only one catalytic run for CO oxidation; this indicated a notable reaction-induced or in situ surface restructuring effect of the Cu2O substrates. Compared to that of pure Cu2O, CuO, and reported CuxO samples, the active chemisorbed oxygen on these in situ restructured samples was observed in particularly higher surface composition (with the ratio of chemisorbed oxygen to lattice oxygen being around 5.5); this was further demonstrated to be highly efficient for the oxidization of CO in a relatively low temperature range even in the absence of O2 in the atmosphere and could be the main contributor to speed up CO oxidation in these samples. A similar enhancement in the activity on the used samples for CO oxidation as compared to that on the fresh samples was further testified in Cu2O nanomaterials with different morphologies such as cubic, octahedral, and 18-facet polyhedral nanoparticles; this implied that the surface restructuring effect under a redox reaction atmosphere was a common feature of the Cu2O-based materials, and the transformed surface could act as a superior and stable interface for heterogeneous catalysis. These findings might help in recognizing the real state of Cu2O-containing materials under serious application conditions or designing highly efficient Cu2O-based materials via convenient atmosphere-controlled manipulation for advanced applications.
1. Introduction
As one of the earliest used metals in human history, copper is basically featured by its stable existent forms, such as Cu, Cu2O, and CuO, with multiple oxidation states under ambient conditions, which offers wide applications ranging from roofing and plumbing material to electrical transmission and power generation. Among the popular Cu compounds, cuprous oxide (Cu2O) is extraordinarily attractive due to its non-stoichiometric p-type semiconductor property1,2 and its prospective applications in gas sensing,3,4 CO oxidation,5,6 photocatalysis,7,8 photocurrent generation,9,10 and organic synthesis.11,12 It is believed that the physical or chemical properties involved in the surface or interface structure of Cu2O-based materials directly govern their performance. For instance, it had been reported that when Cu2O nanoparticles in different shapes were employed, the crystalline Cu2O surface plays an important role in CO oxidation13,14 and photocatalytic decomposition of dyes.15,16 In addition, the strategy of combining Cu2O on ZnO or TiO2 substrates to form a composite interface was also functional to enhance the efficiency of the solar cells17 or the photocatalytic activity for CO2 reduction to methanol.18 These studies focused on deliberate surface manipulations made the main contribution to the current innovation of Cu2O-based materials.19However, it should be noted that the possible deviation of Cu2O from its metastable property and the corresponding influences have seldom been considered; this can lead to misinterpretation of functions of its real state for practical processes, especially under some special application conditions. For instance, Cu2O has been directly employed or composited with other components for some important thermal catalysis reactions,11–14,20 such as CO oxidation, in which high temperature and oxygen are always required.13,20 In terms of thermodynamics, Cu2O can be easily oxidized under an oxidizing environment due to its very negative ΔrGØm values (standard Gibbs free energy for reaction) even at room temperature (the value is calculated from the data of ref. 21 at 25 °C) from reaction (1). The endothermic feature of the reaction implies that the spontaneous change can be intensified when Cu2O is exposed to the real environment of the abovementioned catalysis reactions. However, to the best of our knowledge, the transformations of Cu2O materials under these serious conditions and the derived influences have seldom been considered or systematically investigated.
| 2Cu2O + O2 → 4CuO, ΔrGØm = −226.8 kJ mol−1 | (1) |
In this study, a dramatic and sustainable enhancement in the activity on a catalytically used Cu2O nanoporous sphere sample for CO oxidation was discovered as compared to that on its fresh counterpart; the reason for this observation was investigated via a series of comprehensive characterizations among fresh and reused samples (the samples after one or more catalytic runs). It was observed that the surface of the Cu2O substrates would spontaneously transform into a multivalent composite interface with a certain and steady ratio of Cu(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(I) after just one or more catalytic runs. The particularly higher composition of active chemisorbed oxygen on this reaction-induced or in situ restructured composite surface was determined and demonstrated to be the main contributor to speed up CO oxidation in a relatively low temperature range. These findings suggested that the real state derived from serious application conditions for Cu2O-containing materials should be noted, which could serve as clues to understand or design the functional surface structure for advanced applications of the Cu2O-based materials.
Cu(I) after just one or more catalytic runs. The particularly higher composition of active chemisorbed oxygen on this reaction-induced or in situ restructured composite surface was determined and demonstrated to be the main contributor to speed up CO oxidation in a relatively low temperature range. These findings suggested that the real state derived from serious application conditions for Cu2O-containing materials should be noted, which could serve as clues to understand or design the functional surface structure for advanced applications of the Cu2O-based materials.
2. Experiments
2.1 Preparation of the Cu2O nanoporous sphere and reference samples
All chemicals were analytical grade reagents and used as received without further purification. Copper acetate monohydrate (Cu(CH3COO)2·H2O, AR, ∼99.0%) was obtained from Aladdin. Diethylene glycol (DEG, GC, ≥ 99.0%) and D-(+)-glucose were obtained from Sigma-Aldrich. Polyvinylpyrrolidone (PVP, Mw: ∼55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was a product of Aldrich.
000) was a product of Aldrich.
Briefly, 1.6 g PVP was dissolved in 25 mL DEG under magnetic stirring at 110 °C. Moreover, 0.60 g Cu(CH3COO)2·H2O and 1.3 g glucose were individually dissolved in 15 mL DEG, and then, the two solutions were simultaneously injected into the hot PVP solution; after about 2 h, the mixture turned yellow, suggesting the formation of the Cu2O particles. The mixture was cooled down to room temperature and centrifuged at 9000 rpm for 8 min to obtain the precipitate, which was washed with ethanol for at least four times and dried at 60 °C for 12 h in vacuum to obtain the Cu2O nanoporous sphere sample.
For reference, a Cu2O sample was calcined in a muffle furnace at 400 °C for 4 h to obtain the CuO sample. Other Cu2O nanomaterials with different morphologies such as cubic, octahedral, and 18-facet polyhedral particles were synthesized following the procedures described in ref. 22 and 23.
2.2 Characterization
X-ray diffraction (XRD) patterns of the samples were obtained using a Persee XD-3 X-ray diffractometer at a scan rate of 2° min−1 in the angle (2θ) range from 10° to 90°. The wavelength of the incident radiation was 1.5406 Å (Cu Kα).X-ray photoelectron spectroscopy (XPS) measurements were performed using an Axis Ultra DLD apparatus with monochromatized Al Kα (hν = 1486.6 eV) as the excitation source. XPS data were charge corrected using the adventitious carbon C 1s peak at 285.0 eV as a reference.
The morphology of the samples was characterized by transmission electron microscopy (TEM) using a JEOL JEM-2100 microscope operating at 200 kV. The samples were prepared by placing a drop of the ethanol suspension containing the sample powder on a carbon film-coated Cu grid (3 mm, 400 mesh), followed by drying under ambient conditions.
The scanning electron microscopy (SEM) measurements were performed using an FEI Quanta 200F microscope operating at 20.0 kV.
2.3 Chemical measurements
The catalytic performances of the samples for CO oxidation were measured via a continuous-flow fixed-bed quartz reactor using 60 mg sample. Prior to the test, the sample was purged with high purity Ar at 30 mL min−1 for 20 min, and then, a reactant gas at the flow rate of 18 mL min−1 was supplied into the reactor. The reactant gas flow was composed of 1 vol% CO and 1 vol% O2 and balanced by N2. The reaction temperature was increased from room temperature at a step of 20 °C until 100% CO conversion was obtained. The reaction system was maintained at each temperature for 40 min to obtain experimental values at the steady state. The outlet gas was analyzed on-line using a GC-7890 gas chromatograph equipped with a thermal conductivity detector (TCD).Temperature-programmed reduction by hydrogen (H2-TPR) and temperature-programmed desorption of carbon monoxide (CO-TPD) were performed on samples using a Micromeritics AutoChem II 2920 Chemisorption Analyzer. For the H2-TPR measurement, the degassed sample was exposed to a flow of 5.0 vol% hydrogen in Ar at the speed of 30 mL min−1 and heated up to 400 °C at the rate of 10 °C min−1 to obtain the TCD signal; the TCD apparatus was calibrated by quantitative reduction of a given quantity of CuO to metallic copper. For the TPD measurement, the degassed sample was exposed to 30 mL min−1 Ar flow and heated up to 150 °C for 2 h; then, the system was cooled down to room temperature with Ar as the protector gas, and CO-contained flow was switched into the system for 1 h. After blowing Ar for 30 min, the sample was exposed to 30 mL min−1 He flow and heated up to 600 °C at the rate of 10 °C min−1 to obtain the TCD signals.
3. Results and discussion
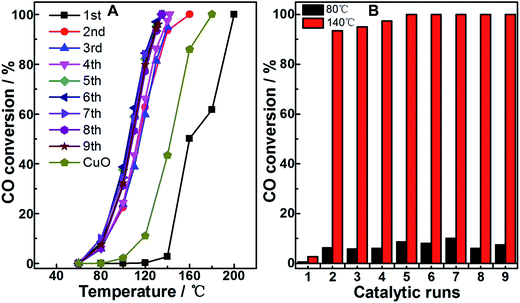
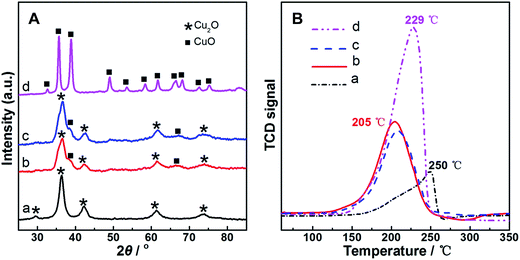
The CO oxidation performance on the as-prepared (fresh) Cu2O particles was obtained as the change of CO conversion with temperature, as shown in Fig. 1A and denoted by the curve 1st. The reaction temperature window (RTW) on the sample was between 110 and 200 °C, which was close to the reported reaction temperature range for CO oxidation on Cu2O nanoparticles in the irregular shape.13,20 One of the samples after the examination was not immediately removed from the reactor; after the temperature of the system naturally cooled down to room temperature by blowing pure N2, the examination procedure was repeated on the catalytically used sample. The corresponding curve was obtained and denoted as 2nd; interestingly, the RTW moved towards low temperature direction for about 60 °C in general, indicating a dramatic enhancement in the activity on the used sample for CO oxidation. To further understand the phenomenon, the examination procedures were repeated on the sample; the curves were obtained and denoted as 3rd to 9th. Among the 2nd to 9th curves, only small fluctuations of the RTW were observed, suggesting that the enhancement in CO conversion within this low RTW range was steady on the sample after just one catalytic run. The phenomenon was further disclosed by comparing the CO conversion at 80 °C and 140 °C (from the data of Fig. 1A) of the fresh sample and used samples obtained from different catalytic runs, as shown in Fig. 1B. It clearly exhibited a dramatic and sustainable enhancement in the activity on the used sample for CO oxidation after just one catalytic run as compared to that on its fresh counterpart. In addition, a reference CuO sample (obtained from the calcination procedure on fresh Cu2O) was also examined (seen in Fig. 1A); its performance was between that of the fresh Cu2O and used Cu2O samples. These results implied that the used Cu2O samples neither had a pure Cu2O nor a pure CuO structure.To clarify the reason for the abovementioned observations, a series of characterizations was performed on fresh Cu2O, reference CuO, and typically used Cu2O samples, such as the sample obtained after the first catalytic run, i.e., the sample directly derived from imposing one CO oxidation catalytic run on fresh Cu2O (denoted as Cu2O-1st CR), and the sample after the sixth catalytic run (denoted as Cu2O-6th CR). Fig. 2 shows the XRD patterns (A) and H2-TPR profiles (B) of the samples. In Fig. 2A, for fresh Cu2O (a) and reference CuO (d), the 2θ values of the diffraction peaks are in agreement with the standard diffraction angles of Cu2O (JCPDS cards no. 65-3288#) and those of CuO (JCPDS cards no. 65-2309#), indicating the well-developed and highly-ordered phase structure of both samples. For the Cu2O-1st CR and Cu2O-6th CR samples (b and c), although two small and weak diffractions located at around 2θ of 38.8° and 66.2° suggested the existence of CuO structure, the main diffractions were closer to those of fresh Cu2O, indicating that the main phase or body of the two samples undergoing the catalytic runs was retained as Cu2O crystalline. The abovementioned phase differences among samples also led to their different redox properties; as shown in Fig. 2B, the H2-consumption peaks of the two used Cu2O samples nearly overlapped, and the area was higher than that of fresh Cu2O, but lower than that of CuO, suggesting that there were additional species to exhaust H2 on used samples as compared to those on fresh Cu2O. In addition, the lower temperature for the H2-consumption peak for Cu2O-1st CR and Cu2O-6th CR samples (205 °C) as compared to that for the fresh substrate (250 °C) was observed, suggesting that the redox ability of Cu2O was obviously improved after just one catalytic run, which could have caused the enhanced activity observed on the used samples.
Fig. 3 shows the images of four samples obtained from the TEM measurements. The fresh Cu2O sample (Fig. 3a) is shown as the nanoporous sphere within the size range of 250–400 nm. Cu2O-1st CR (Fig. 3b), Cu2O-6th CR (Fig. 3c), and CuO (Fig. 3d) appeared similar to Cu2O substrate; this suggested the insignificant changes in shape among the samples. However, from the detailed local features shown in the HRTEM images of the samples, d-spacing values measured at 0.302 nm and 0.246 nm for the fresh Cu2O sample were close to the standard values of Cu2O-[110] and Cu2O-[111] planes (JCPDS cards no. 65-3288). Only the d-spacing value around 0.252 nm pertinent to the CuO-[002] plane (JCPDS cards no. 65-2309) was observed in the CuO sample, confirming the pure CuO crystalline feature of the sample, consistent with the XRD observation. In contrast, both Cu2O-[110], -[200] (d-spacing value at 0.211 nm) and CuO-[002], -[111] (d-spacing value at 0.231 nm) planes could be resolved on Cu2O-1st CR and Cu2O-6th CR samples; this indicated the co-existence of the Cu2O and CuO structures, i.e., the Cu(I)–Cu(II) multivalent oxide composite interface derived from Cu2O during the CO oxidation reaction, in both the used Cu2O samples.
 | ||
| Fig. 3 General appearance and local details of morphology for the representative samples. (a) Fresh Cu2O; (b) Cu2O-1st CR; (c) Cu2O-6th CR; and (d) CuO (up: TEM images; down: HRTEM images). | ||
XPS measurements were employed to quantify the surface composition of the samples, and the spectra are shown in Fig. 4. For our Cu2O sample (Fig. 4A(a)), two peaks located at 932.2 eV and 951.9 eV were close to the reported Cu 2p3/2 and Cu 2p1/2 spectra of the common Cu2O particles.24,25 In contrast, other samples produced two main peaks at the higher binding energy range around 934.0 eV and 954.1 eV, which were close to the standard Cu 2p3/2 and Cu 2p1/2 spectra of CuO, respectively. Furthermore, the shakeup satellite peaks within 939.6 and 945.8 eV and around 962.7 eV were also the features of CuO, being different from those of Cu2O;26,27 these features indicated that CuO was the main component on the surfaces of Cu2O-1st CR, Cu2O-6th CR, and CuO samples (Fig. 4A(b–d), respectively). In addition, it should be noted that the Cu 2p spectra for Cu2O-1st CR and Cu2O-6th CR samples displayed broader shoulder peaks in comparison with the relatively symmetrical peaks for the CuO sample. Moreover, the co-existence of Cu2O and CuO in Cu2O-1st CR and Cu2O-6th CR samples was confirmed via the HRTEM measurements. Hence, careful peak-fitting measurements focused on Cu 2p peaks of Cu2O and CuO for the two samples were performed, as shown in Fig. 4A(b and c). Interestingly, these two samples showed close ratios of Cu(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(I), which were all around 4.0, suggesting that a stable Cu(I)–Cu(II) multivalent oxide composite surface with almost unchanged Cu(II)
Cu(I), which were all around 4.0, suggesting that a stable Cu(I)–Cu(II) multivalent oxide composite surface with almost unchanged Cu(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(I) ratio was derived from the fresh Cu2O surface through just one catalytic run of CO oxidation. Besides Cu 2p spectra, O 1s spectra were also resolved for the samples, as shown in Fig. 4B. In general, two distinguished peaks within the 526.0–538 eV range were observed for all the samples, which could be assigned to lattice oxygen OL and chemisorbed oxygen OCA (at higher binding energy).28,29 The ratios of OCA
Cu(I) ratio was derived from the fresh Cu2O surface through just one catalytic run of CO oxidation. Besides Cu 2p spectra, O 1s spectra were also resolved for the samples, as shown in Fig. 4B. In general, two distinguished peaks within the 526.0–538 eV range were observed for all the samples, which could be assigned to lattice oxygen OL and chemisorbed oxygen OCA (at higher binding energy).28,29 The ratios of OCA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OL in Cu2O, Cu2O-1st CR, Cu2O-6th CR, and CuO were estimated to be 0.46, 5.3, 5.7, and 1.9, respectively; moreover, Cu2O-1st CR and Cu2O-6th CR showed similar OCA
OL in Cu2O, Cu2O-1st CR, Cu2O-6th CR, and CuO were estimated to be 0.46, 5.3, 5.7, and 1.9, respectively; moreover, Cu2O-1st CR and Cu2O-6th CR showed similar OCA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OL ratio (averaged at 5.5) on their surfaces, further confirming the stable surface composition of this reaction-induced or in situ restructured sample from the Cu2O substrate. Notably, OCA was generally believed to be the key active oxygen species for the oxidation reactions;28,29 however, the highest ratios between the surface oxygen and lattice oxygen were always found to be not more than or just around 2.0 among most of the reported CuxO-based catalysts such as Cu2O-, CuO-based, or Cu(I)–Cu(II) composite oxide catalysts.28–30 In total contrast, a particularly high OCA surface composition with the OCA
OL ratio (averaged at 5.5) on their surfaces, further confirming the stable surface composition of this reaction-induced or in situ restructured sample from the Cu2O substrate. Notably, OCA was generally believed to be the key active oxygen species for the oxidation reactions;28,29 however, the highest ratios between the surface oxygen and lattice oxygen were always found to be not more than or just around 2.0 among most of the reported CuxO-based catalysts such as Cu2O-, CuO-based, or Cu(I)–Cu(II) composite oxide catalysts.28–30 In total contrast, a particularly high OCA surface composition with the OCA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OL ratio around 5.5 was observed in our Cu2O-1st CR and Cu2O-6th CR samples, which could be an important factor to facilitate CO oxidation. Considering that oxygen content was excessive in our catalytic measurements, based on a referee's advice, a reference sample obtained by heating fresh Cu2O to 200 °C in air for 2 h was also measured by XPS to investigate the influence of this atmosphere; the spectra of the sample are labeled as (e) Cu2O-A200 in Fig. 4. The general feature of the sample was similar to that of the CuO sample, except for the distinguished Cu(II)
OL ratio around 5.5 was observed in our Cu2O-1st CR and Cu2O-6th CR samples, which could be an important factor to facilitate CO oxidation. Considering that oxygen content was excessive in our catalytic measurements, based on a referee's advice, a reference sample obtained by heating fresh Cu2O to 200 °C in air for 2 h was also measured by XPS to investigate the influence of this atmosphere; the spectra of the sample are labeled as (e) Cu2O-A200 in Fig. 4. The general feature of the sample was similar to that of the CuO sample, except for the distinguished Cu(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(I) ratio at 6.0 and OCA
Cu(I) ratio at 6.0 and OCA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OL ratio of 2.2. Although better CO oxidation activity with a lowered reaction temperature range of 80–180 °C as compared to that on the Cu2O sample was observed in the sample (not shown), due to different surface compositions, the sample was still less active in comparison with Cu2O-1st CR and Cu2O-6th CR samples, which were restructured during CO oxidation. The result suggested that the atmosphere played an important role in restructuring the surface composition of the Cu2O substrate. However, the deliberately imposed conditions to process the sample need more careful investigation for further improving the performance. Based on the abovementioned characterizations, it can be recognized that due to the surface restructuring effect of fresh Cu2O present in the first catalytic run of CO oxidation, the pure Cu2O surface would in situ and spontaneously transform into distinguished Cu-oxides composite interface with certain and steady surface distributions of oxidative Cu and active oxygen species.
OL ratio of 2.2. Although better CO oxidation activity with a lowered reaction temperature range of 80–180 °C as compared to that on the Cu2O sample was observed in the sample (not shown), due to different surface compositions, the sample was still less active in comparison with Cu2O-1st CR and Cu2O-6th CR samples, which were restructured during CO oxidation. The result suggested that the atmosphere played an important role in restructuring the surface composition of the Cu2O substrate. However, the deliberately imposed conditions to process the sample need more careful investigation for further improving the performance. Based on the abovementioned characterizations, it can be recognized that due to the surface restructuring effect of fresh Cu2O present in the first catalytic run of CO oxidation, the pure Cu2O surface would in situ and spontaneously transform into distinguished Cu-oxides composite interface with certain and steady surface distributions of oxidative Cu and active oxygen species.
 | ||
| Fig. 4 Cu 2p (A) and O 1s (B) XPS spectrum of representative samples. (a) Fresh Cu2O; (b) Cu2O-1st CR; (c) Cu2O-6th CR; (d) CuO; and (e) Cu2O-A200 (obtained by heating Cu2O in air to 200 °C for 2 h). | ||
For CO catalytic oxidation, the interaction between CO and catalyst surface was believed to be a key factor influencing the reaction behavior and activity. As an attempt to illustrate this interaction in our samples, herein, CO-TPD measurements were employed, as shown in Fig. 5. In comparison with those of the Cu2O and CuO samples, the desorption peaks of the two used Cu2O moved towards lower temperature range, indicating superior low-temperature activation effect on the multivalent composite surface of the two used Cu2O samples as compared to that of the unitary Cu2O or CuO surface. Notably, the desorption peaks for the two used Cu2O samples almost overlapped, and the observation was similar to previous characterizations, which further confirmed that even through just one catalytic run, not only a stable composite surface but also steady chemical properties could be achieved on the Cu2O substrate. Djurišić et al.29 also carefully inspected the surface change between the as-prepared and used states on a hydrothermally synthesized CuxO sample for CO oxidation. They found that the surface of their sample was not stable since a continuous decrease in the ratio of lattice oxygen to surface oxygen with the increasing catalytic cycling was observed. This observation suggested that although multivalent CuxO interface had been demonstrated to be catalytically more competitive than pure Cu2O and CuO surfaces, obtaining a stable surface with desirable properties could still require extensive study and attention. Relatively, present findings suggested that employment of a reactive redox atmosphere and certain thermal conditions to process Cu2O, such as CO oxidation on our sample, could be a convenient way to acquire Cu2O-based materials with finely modulated and stable surfaces. In addition, the outlet gas from CO-TPD was analyzed via mass spectrometry; in contrast to the mixture of CO and CO2 desorbed from the pure Cu2O surface, the desorption from Cu2O-1st CR and Cu2O-6th CR samples was almost fully composed by CO2; this indicated that the introduced CO could be more easily and fully converted to CO2 on our surface-restructured Cu2O samples. The phenomenon was directly connected to the particularly higher surface compositions of active chemisorbed oxygen as compared to that of the pure Cu2O sample (disclosed by previous XPS measurements) since there was no other O-source in the environment of the CO-TPD measurements. Therefore, it was reasonable to believe that the most attractive advantage of our surface-restructured Cu2O sample could be its high efficiency in producing large amounts of the active chemisorbed oxygen during CO oxidation, which was the main impetus to speed up CO conversion to CO2 in low temperature range.
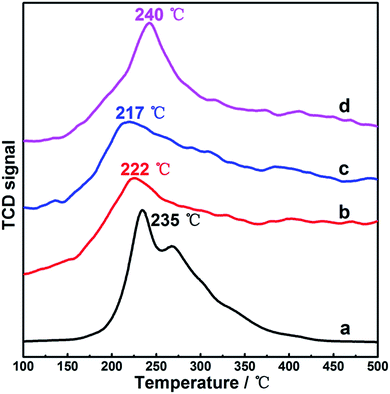 | ||
| Fig. 5 CO-TPD profiles on representative samples. (a) Fresh Cu2O; (b) Cu2O-1st CR; (c) Cu2O-6th CR; and (d) CuO. | ||
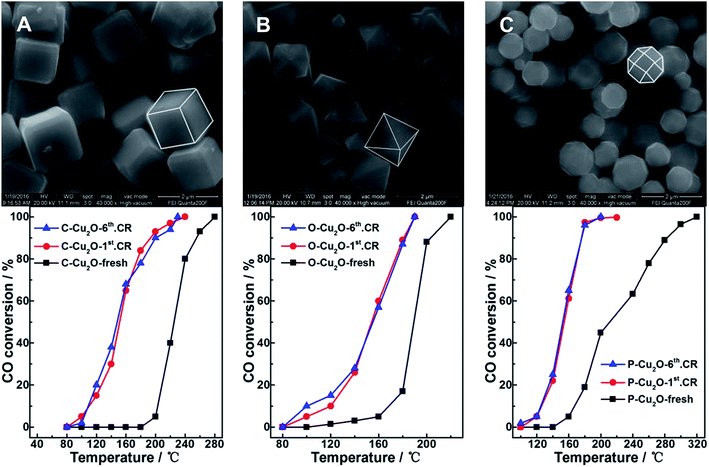
As is known, many studies on Cu2O-based materials for catalysis applications have been focused on the influence of different physical features of samples;13,14,19 for example, Huang et al.14 showed the detailed impact of crystalline surface on the CO oxidation behavior by employing Cu2O nanocrystals in different shapes. We also extended similar catalytic measurements on other Cu2O nanosubstrates with different morphologies such as cubic (C-Cu2O), octahedral (O-Cu2O), and 18-facet polyhedral (P-Cu2O) nanoparticles. Fig. 6 shows the morphology and corresponding CO conversion curves of these samples. The SEM images show the well-constructed shape of the samples, and the corresponding CO oxidation measurements on the fresh sample, the used sample after one catalytic run, and the used sample after six catalytic runs were conducted. Irrespective of the initial state and shape of the Cu2O nanoparticles, the dramatic and sustainable enhancement in the activity on the used samples as compared to that on their fresh counterparts were repeatedly observed, similar to the observations on nanoporous sphere sample. Hence, these observations suggested that the reaction-induced or in situ restructuring effect under the presence of redox atmosphere discovered in this study might be a common feature of the Cu2O substrates, which could lead to notable changes in both the surface structure and corresponding performance of the Cu2O-containing samples.
4. Conclusions
Irrespective of the initial states and shapes, the pure Cu(I) surface of the fresh Cu2O nanomaterials would spontaneously transform in situ into a multivalent and stable Cu(I)–Cu(II) composite surface (with almost unchanged Cu(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(I) and OCA
Cu(I) and OCA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OL ratios) through just one catalytic run of CO oxidation. The higher surface composition of active chemisorbed oxygen and the corresponding more effective interaction with CO contributed to the dramatic activity enhancement on the used samples as compared to that on the fresh Cu2O substrates. These observations suggest that this in situ restructuring effect of the Cu2O component should be noted as a common feature of the Cu2O-based materials, especially when serious application conditions such as redox atmosphere and certain thermal conditions are employed; these findings can be referenced as clues not only for understanding the real state of the Cu2O-containing samples in applications, but also for manipulating the surface structure of Cu2O-based materials for advanced applications through convenient atmosphere-controlled measurements.
OL ratios) through just one catalytic run of CO oxidation. The higher surface composition of active chemisorbed oxygen and the corresponding more effective interaction with CO contributed to the dramatic activity enhancement on the used samples as compared to that on the fresh Cu2O substrates. These observations suggest that this in situ restructuring effect of the Cu2O component should be noted as a common feature of the Cu2O-based materials, especially when serious application conditions such as redox atmosphere and certain thermal conditions are employed; these findings can be referenced as clues not only for understanding the real state of the Cu2O-containing samples in applications, but also for manipulating the surface structure of Cu2O-based materials for advanced applications through convenient atmosphere-controlled measurements.
Acknowledgements
Authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (NSFC, No. 21003071 and No. 21563018) and Doctoral Fund of Ministry of Education of China (No. 20093601120007).References
- C. H. Kuo and M. H. Huang, Nano Today, 2010, 5, 106–116 CrossRef CAS.
- P. Lignier, R. Bellabarba and R. P. Tooze, Chem. Soc. Rev., 2012, 41, 1708–1720 RSC.
- J. Zhang, J. Liu, Q. Peng, X. Wang and Y. Li, Chem. Mater., 2006, 18, 867–871 CrossRef CAS.
- L. Zhang, Z. Cui, Q. Wu, D. Guo, Y. Xu and L. Guo, CrystEngComm, 2013, 15, 7462–7467 RSC.
- H. Bao, Z. Zhang, Q. Hua and X. W. Huang, Langmuir, 2014, 30, 6427–6436 CrossRef CAS PubMed.
- Z. Wang, R. Li and Q. Chen, ChemPhysChem, 2015, 16, 2415–2423 CrossRef CAS PubMed.
- J. Chen, S. Shen, P. Guo, M. Wang, P. Wu, X. Wang and L. J. Guo, Appl. Catal., B, 2014, 152–153, 335–341 CrossRef CAS.
- R. M. Mohamed and E. S. Aazam, Appl. Catal., A, 2014, 480, 100–107 CrossRef CAS.
- L. Wu, L. K. Tsui, N. Swami and G. Zangari, J. Phys. Chem. C, 2010, 114, 11551–11556 CAS.
- R. Ji, W. Sun and Y. Chu, ChemPhysChem, 2013, 14, 3971–3976 CrossRef CAS PubMed.
- Y. P. Zhang, Y. C. Jiao, Y. S. Yang and C. L. Li, Tetrahedron Lett., 2013, 54, 6494–6497 CrossRef CAS.
- N. Chatterjee, R. Pal, S. Sarkar and A. K. Sen, Tetrahedron Lett., 2015, 56, 3886–3889 CrossRef CAS.
- M. Leng, M. Liu, Y. Zhang, Z. Wang, C. Yu, X. Yang, H. Zhang and C. Wang, J. Am. Chem. Soc., 2010, 132, 17084–17087 CrossRef CAS PubMed.
- Q. Hua, T. Cao, H. Bao, Z. Jiang and X. W. Huang, ChemSusChem, 2013, 6, 1966–1972 CrossRef CAS PubMed.
- J. Shi, J. Li, X. Huang and Y. Tan, Nano Res., 2011, 4, 448–459 CrossRef CAS.
- W. C. Huang, L. M. Lu, Y. C. Yang and M. H. Huang, J. Am. Chem. Soc., 2012, 134, 1261–1267 CrossRef CAS PubMed.
- B. D. Yu and P. Yang, J. Am. Chem. Soc., 2009, 131, 3756–3761 CrossRef PubMed.
- J. Wang, G. Ji, Y. Liu, M. A. Gondal and X. Chang, Catal. Commun., 2014, 46, 17–21 CrossRef CAS.
- S. Sun, Nanoscale, 2015, 7, 10850–10882 RSC.
- S. Y. Zhang, H. Liu, C. C. Sun, P. F. Liu, L. C. Li, Z. H. Yang, X. Feng, F. W. Huo and X. H. Lu, J. Mater. Chem. A, 2015, 3, 5294–5298 CAS.
- D. R. Lide, Standard Thermodynamic Properties of Chemical Substances, in CRC Handbook of Chemistry and Physics, P788-P804, CRC, Boca Raton, Florida, 90th edn, 2009 Search PubMed.
- L. Gou and C. J. Murphy, Nano Lett., 2003, 3, 231–234 CrossRef CAS.
- C. Lu, L. Qi, J. Yang, X. Wang, D. Zhang, J. Xie and J. Ma, Adv. Mater., 2005, 17, 2562–2567 CrossRef CAS.
- C. K. Wu, M. Yin, S. O. Brien and J. T. Koberstein, Chem. Mater., 2006, 18, 6054–6058 CrossRef CAS.
- C. C. Chusuei, M. A. Brookshier and D. W. Goodman, Langmuir, 1999, 15, 2806–2808 CrossRef CAS.
- K. Zhong, J. Xue, Y. Mao, C. Wang, T. Zhai, P. Liu, X. Xia, H. Li and Y. Tong, RSC Adv., 2012, 2, 11520–11528 RSC.
- M. S. P. Francisco and V. R. Mastelaro, J. Phys. Chem. B, 2001, 105, 10515–10522 CrossRef CAS.
- D. A. Svintsitskiy, A. P. Chupakhin, E. M. Slavinskaya, O. A. Stonkus, A. I. Stadnichenko, S. V. Koscheev and A. I. Boronin, J. Mol. Catal. A: Chem., 2013, 368–369, 95–106 CrossRef CAS.
- M. Y. Guo, F. Liu, J. Tsui, A. A. Voskanyan, A. M. C. Ng, A. B. Djurišić, W. K. Chan, K. Y. Chan, C. Liao, K. Shih and C. Surya, J. Mater. Chem. A, 2015, 3, 3627–3632 CAS.
- Y. L. Zheng, D. S. Mao, S. S. Sun and G. Y. Fu, J. Nanopart. Res., 2015, 17, 471 CrossRef.
| This journal is © The Royal Society of Chemistry 2017 |