Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly promoted removal of Hg(II) with magnetic CoFe2O4@SiO2 core–shell nanoparticles modified by thiol groups
He Zhu,
Yi Shen,
Qin Wang,
Kuan Chen,
Xi Wang,
Ganwei Zhang,
Jingjing Yang,
Yongfu Guo * and
Renbi Bai*
* and
Renbi Bai*
Center for Separation and Purification Materials &Technologies, Suzhou University of Science and Technology, Suzhou 215009, P. R. China. E-mail: yongfuguo@163.com; Tel: +86-512-68092987
First published on 10th August 2017
Abstract
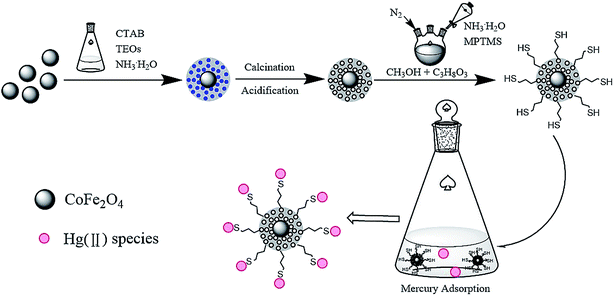
A simple and environmentally friendly material, CoFe2O4@SiO2–SH, was prepared successfully with CoFe2O4 nanoparticles coated by SiO2 which was further functionalized with thiol groups (–SH). CoFe2O4@SiO2–SH nanoparticles were structurally and thermally characterized. The results show that the additive of 3-mercaptopropyltriethoxysilane was successfully grafted onto CoFe2O4@SiO2. The affecting factors, including pH, adsorbent dosage, and the initial concentration of Hg Hg(II) and coexisting ions were fully investigated and well elucidated. The fitting of adsorption kinetics and isotherm model indicated a good match with pseudo-second-order kinetics and Freundlich models. The Langmuir adsorption capability realized 641.0 mg g−1. The data of thermodynamics illustrated that the removal for Hg(II) was exothermic and spontaneous. Last, an application evaluation of the material was also carried out. The adsorbent can achieve a quick and effective separation via a magnetic field after adsorption. Hence, the CoFe2O4@SiO2–SH material will be a favorable and promising adsorbent to remove Hg(II) from water.
1. Introduction
With the economic globalization and the large-scale development of industrialization, water pollution of heavy metals caused by a large amount of sewage from chemical plants and mining fields, has attracted a lot of research attention. Mercury, one of the most toxic pollutants exhibiting properties of persistence, bioaccumulation and migration,1 can enter the human body through the food chain, causing irreversible serious damage to human body function and the nervous system.2In many countries, mercury pollution incidents occur frequently.3 In the 1980s in China, several serious mercury pollution incidents, occurring along the Songhua River, in the lower reaches of Tianjin Jiyun River and Jinzhou Bay in Liaoning Province, etc. greatly attracted people's attention. So, it is imperative to solve the problem of mercury pollution in water.
To solve this issue, there are many methods developed for the recovery of mercury, such as precipitation, ion exchange, physical separation and microbiological method and so on.4–7 Nevertheless, these technologies always have shortcomings like poor removal capacity, low metal removal efficiency and the risk of secondary pollution.8 Hence, it is necessary to explore economic and effective technologies to treat the pollution. Adsorption has been recognized as an economic and highly effective method used in water treatment due to its cost-effective and efficient.9 However, most of adsorbent materials have some issues like poor regeneration, easy to produce secondary pollution and poor selectivity. Especially after the completion of the adsorption, the material is difficult to be separated from water, which is the most difficult problem to apply on the real projects.
MFe2O4 (M represents metal elemental), a kind of magnetic substance with a cubic spinel structure, has well received by the people's favorites and been widely used in many fields.10 Among them, CoFe2O4 gets more attention due to its advantages, such as large saturation magnetization and good thermal stability. Fe3O4 was also one of magnetic materials and got more modification and researches in recent years.11–13
Compared with the production process of Fe3O4, the synthesis method of CoFe2O4 is simpler and the process of reaction can underway without the need of nitrogen environment. According to the study of Zhang,14 the number of hydroxyl group (M–OH) on the surface of CoFe2O4 (38.1%) was higher than that of Fe3O4 (25.4%). The amount of M–OH has a significant effect on the performance of the adsorbents, and the more M–OH, the more easily material can be modified.
In addition to the above advantages, CoFe2O4 also has some shortcomings that cannot be overcome by itself, such as easy agglomeration, corrosion in acidic environments and so on, which is not conducive to the material adsorption and adsorption selectivity. In general, the methods of adding surfactant or forming silica layer outside CoFe2O4 through hydrolyzing tetraethyl silicate (TEOs) can greatly improve corrosion resistance and dispersion of CoFe2O4 in water.15,16
According to the Lewis acid–base interactions,17 thiol group and mercury should have a high affinity to each other, for thiol group is characteristic of soft base and mercury is a soft acid. Thiol group is also an excellent ligand. CoFe2O4 can be modified with thiol group to improve the adsorption properties of materials. 3-Mercaptopropyltrimethoxysilane (MPTMS) is a silane coupling agent containing mercapto functional groups. After the inorganic layer SiO2 was coated on the surface of CoFe2O4 and MPTMS was added, the mercapto group was successfully grafted.
During the process of modification, the contents of thiol in adsorbents are related to amount of catalysts used in the reaction, the choice of solvents, reaction temperature and reaction time.18
Currently, most of magnetic adsorption materials were modified with Fe3O4. However, the research with CoFe2O4 modified materials to remove heavy metal was little. One aim of this paper is to explore the adsorption performance of the prepared adsorbent modified with other magnetic base material, i.e., CoFe2O4; another aim is to prepare CoFe2O4 base adsorbent with core–shell construction and investigate its adsorption performance for Hg(II) ions when the prepared adsorbent was modified by thiol group.
So, in this paper, in order to overcome the defect of easy agglomeration and corrosion of CoFe2O4, improve its dispersion in water and simultaneously develop a novel adsorbent with high adsorption capability, a thiol-functionalized and silica-coated magnetic nanoparticle (CoFe2O4@SiO2–SH) was synthesized by a simple method and employed to remove Hg(II) ions from water. The characteristics and properties of materials before and after modification were discussed by various means of characterization. To deeply comprehend the adsorption process of materials, the adsorption kinetics, isotherm and thermodynamics were employed. The effects of foreign ions on the adsorption process and the adsorption mechanism were discussed.
2. Materials and experimental methods
2.1 Experimental materials
Cobaltous nitrate hexahydrate (Co(NO3)2·6H2O), iron(III) acetylacetonate (Fe(acac)3), ethylene glycol (EG), sodium acetate anhydrous (CH3COONa), polyethylene glycol, cetyltrimethylammonium bromide (CTAB, 99 wt%), methanol (CH3OH), glycerol (C3H8O3) and ammonia water (NH3·H2O, 25–28 wt%) were provided by Sinopharm Chemical Reagent Co., Ltd. (China). (3-Mercaptopropyl)trimethoxysilane (MPTMS, 98 wt%) and TEOs were obtained from Macklin Reagent (China). All chemical materials and solvents were analytical grade.2.2 Preparation of CoFe2O4@SiO2–SH
2.3 Sample characterizations
The obtained samples were characterized via Scanning Electron Microscope (SEM), Transmission Electron Microscopy (TEM), X-ray Diffraction analysis (XRD), Fourier Transform Infrared (FT-IR), Vibrating Sample Magnetometer (VSM), N2 adsorption–desorption isotherms, X-ray Photoelectron Spectroscopy (XPS) and Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES). SEM (FEI Quanta FEG250, USA) and TEM (JEM-2100F, Japan) were used to observe the morphology. XRD (D/MAX-2550-18KW, Japan) was used to analyze the phase and crystallinity of the adsorbents.The functional groups in materials were analyzed by FT-IR (Thermo, Nicolet-6700, USA). The magnetic property of the nanocomposites was confirmed by VSM (Quantum design, MPMS3, USA). The specific surface values and the pore distributions were got on the basis of nitrogen adsorption (Quanta, Autosorb-IQZ-MP-XR-VP, USA) at 77 K through using Brunauer–Emmett–Teller (BET) equation. Chemical elements compositions were measured by XPS (Thermo Scientific, Escalab 250Xi, USA). Quantitative determination of the contents for Hg(II) ions were performed by ICP-OES.
2.4 Adsorption experiments
To prevent the hydrolysis of mercury ions, the protective solution was configured as following: concentrated HNO3 (10 mL) and HCl (0.1 mL) were added into ultrapure water (1000 mL). A reserve solution of Hg(II) (1 g L−1) was made through dissolving HgCl2 (0.6767 g) in a 500 mL protective solution. The protective solution was diluted with according to the desired concentration before each experiment.The adsorption capacities of CoFe2O4@SiO2–SH MNPs were evaluated at various conditions of pH, adsorbent dosages, contact time (t), initial concentration of Hg(II) and co-existing ions. Effect of pH values of 2–10 was assessed through adding 0.1 mol L−1 HCl and 0.1 mol L−1 NaOH solutions. Adsorption studies were carried out in 250 mL stoppered flaskets with Hg(II) solution (100 mL, 40 mg L−1) and 0.005 g of adsorbents. The stoppered flaskets with the mixture were put in a shaker at 298 K for 12 h. The contents of Hg(II) were filtered via a 0.45 μm filter membrane and analyzed by ICP-OES. All of the tests were made in triplicate and the mean value was chosen for further analysis.
The dosage effects of adsorbents (defined as ratios of solid to liquid) were investigated at the intervals of 0.03, 0.05, 0.08, 0.1 and 0.15 g L−1 with 40 mg L−1 of Hg(II) at pH = 8 and 298 K. The contact time was examined at 1, 3, 5, 10, 30, 60, 180, 240, 300, 360, 420, 480, 540, 600, 660 and 720 min with 40 mg L−1 of Hg(II) and 0.005 g adsorbents at pH of 8 and 298 K.
The isotherms were studied at 298, 308 and 318 K at pH = 8 and t = 12 h. The initial concentration was charged between 20 and 200 mg L−1. In the research of co-existing ions, five kinds of salts with a concentration of 10 mM and 100 mM were separately added into the Hg(II) solutions, respectively. The adsorption capacities can be determined as following:
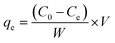 | (1) |
3. Results and discussion
3.1 Characterization
SEM images of CoFe2O4, CoFe2O4@SiO2 and CoFe2O4@SiO2–SH MNPs are compared and shown in Fig. 2. The diameters of these adsorbents were about 50–90, 70–120, and 80–130 nm, respectively. As displayed in Fig. 2(a), the size of CoFe2O4 nanoparticles was small, but not uniform. Its surface was not smooth and crowded together. The observed aggregation may be due to the small size and the priority requirement for reducing the interfacial energy of nanoparticles.19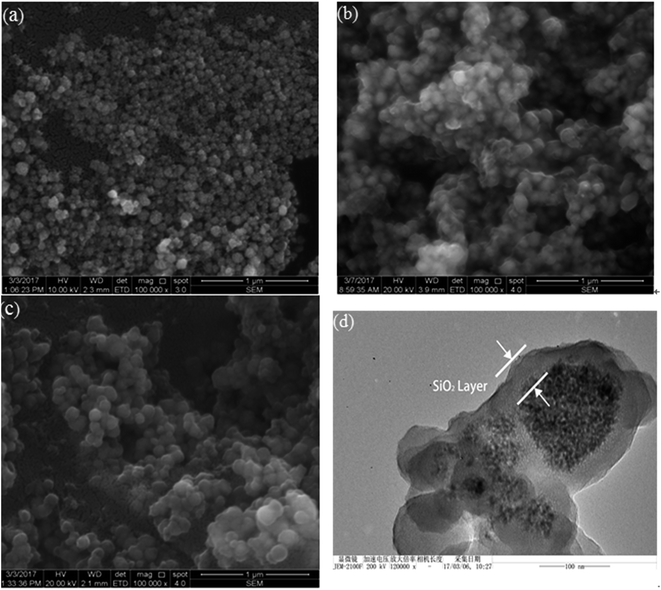 | ||
| Fig. 2 SEM pictures of (a) CoFe2O4, (b) CoFe2O4@SiO2, (c) CoFe2O4@SiO2–SH and TEM image of (d) CoFe2O4@SiO2–SH. | ||
As displayed in Fig. 2(b), the size of CoFe2O4@SiO2 became larger and the surface got smooth, indicating that CoFe2O4 successfully covered the silicon shell. Fig. 2(c) shows that the dispersity of the material had been greatly improved after modified by MPTMS. Based on the data in Fig. 2(d), the black magnetic core was wrapped around the translucent polymer coating, which was the silicon shell.
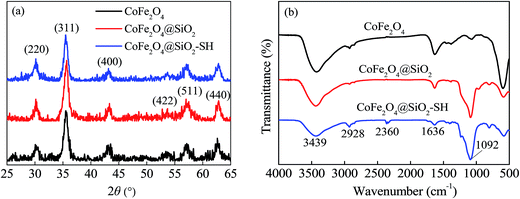
Fig. 3(a) shows the XRD patterns of CoFe2O4, CoFe2O4@SiO2 and CoFe2O4@SiO2–SH. It can observe that XRD pattern of CoFe2O4 had the diffraction peaks that appeared at 2θ = 30.1°, 35.5°, 43.3°, 53.8°, 56.8° and 62.5°, corresponding to the JCPDS file of CoFe2O4 (no. 22-1086).20 All of the diffraction peaks of CoFe2O4@SiO2 and CoFe2O4@SiO2–SH were similar to those of CoFe2O4, and no peaks of any other phases were observed, indicating that the structure and the crystal formation of CoFe2O4@SiO2 and CoFe2O4@SiO2–SH MNPs remained unchanged after silicon-covering and thiol-functionalized process.21
The average sizes of CoFe2O4, CoFe2O4@SiO2 and CoFe2O4@SiO2–SH were calculated using the Debye–Scherrer formula to be 18.1, 20.0 and 21.6 nm, respectively.
The functional groups and chemical bonds in the materials were analyzed by means of FT-IR. Fig. 3(b) indicates FT-IR spectra of CoFe2O4, CoFe2O4@SiO2 and CoFe2O4@SiO2–SH (CoFe2O4@SiO2–SH-1 was the fresh original sample and CoFe2O4@SiO2–SH-2 was prepared one month ago). Broad FT-IR peaks around at 3439 and 1636 cm−1 observed should be attributed to stretching vibration of –OH in surface adsorbed water.22
A broad adsorption band at 1092 cm−1 can be attributed to Si–O–Si.23 As reported, typical adsorption bands of thiol group should be at about 2550 cm−1. However, the two very weak peaks of –SH can be found at round 2360 cm−1. It may be owing to the aggregation of –SH group and the effect of –H bonds. The phenomenon has also been reported by other scholars.24,25 A bend at 2928 cm−1 was the C–H stretching of methylene from alkyl chain,26 which can indirectly indicate that MPTMS had been successfully grafted onto CoFe2O4@SiO2.
Moreover, the material of CoFe2O4@SiO2–SH-2 had a similar FT-IR picture compared to the CoFe2O4@SiO2–SH-1, shown in Fig. 3(b), which indicated that the prepared nanocomposite of CoFe2O4@SiO2–SH had a good stability.
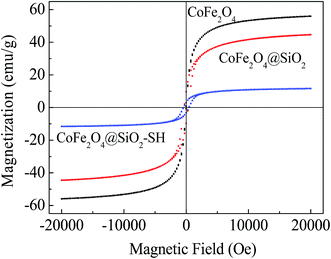
Fig. 4 shows the magnetic hysteresis loops of the three synthesized nanoparticles. The results show that saturation magnetization values for the three synthesized nanoparticles were 56.03, 44.68 and 11.61 emu g−1 at 20 kOe, respectively. It can be clearly seen that the saturation magnetization values were decreased after modified by MPTMS, which indirectly proved that the surface of CoFe2O4@SiO2 formed a non-magnetic thiol functional layer. After modified with MPTMS, the magnetic properties of the materials reduced obviously, but still can be easily separated from water via a magnetic field. It is conducive to the solid–liquid separation, making the whole adsorption process more environmentally friendly.
Based on the data in Fig. 5, the BET values, pore volumes and pore diameters of the three synthesized materials can be determined and exhibited in Table 1. From Table 1, the pore diameters and total pore volumes of CoFe2O4@SiO2 changed little under the addition of surfactant CTAB and introduction of silicone shell TEOs. However, the BET value of CoFe2O4@SiO2 is four times more than that of CoFe2O4, which may be the structure change of porous fluffy state of CoFe2O4@SiO2 and the removal of CTAB after calcination at high temperature.
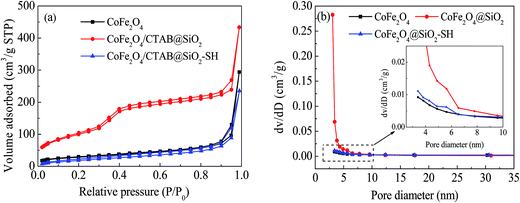 | ||
| Fig. 5 N2 adsorption desorption isotherms (a) and size distribution (b) of CoFe2O4, CoFe2O4@SiO2 and CoFe2O4@SiO2–SH. | ||
| Samples | BET values (m2 g−1) | Total pore volumes (cm3 g−1) | Pore diameters (nm) |
|---|---|---|---|
| CoFe2O4 | 48.49 | 0.424 | 3.413 |
| CoFe2O4@SiO2 | 225.36 | 0.552 | 3.062 |
| CoFe2O4@SiO2–SH | 48.90 | 0.348 | 3.409 |
After the introduction of mercapto groups by MPTMS, the BET value returned to the original value, which may be due to the existence of large amount of organic mercapto groups grafted into CoFe2O4@SiO2 mesopores.27 On one hand, it shows the successful grafting of –SH; on the other hand, it indicates that the BET value was not the main factor affecting the adsorption performance of CoFe2O4@SiO2–SH.
The wide-scan XPS spectra of the three synthesized materials are shown in Fig. 6. The elements of Fe, Co, O and C on the surface of CoFe2O4 were detected. For CoFe2O4@SiO2, the peaks of Fe and Co elements almost disappeared, and the peaks of Si and S appeared, indicating that the silicon shell has completely wrapped the magnetic core CoFe2O4.
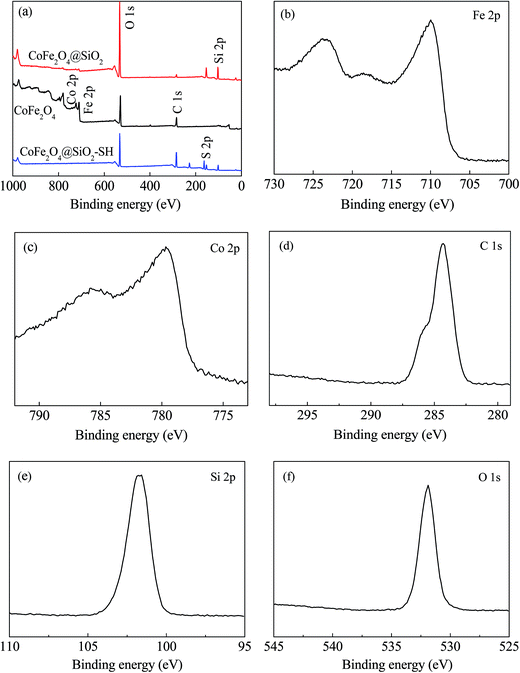 | ||
| Fig. 6 XPS scan of (a) survey scan of CoFe2O4, CoFe2O4@SiO2 and CoFe2O4@SiO2–SH; high resolution scan of CoFe2O4@SiO2–SH of (b) Fe 2p, (c) Co 2p, (d) C 1s, (e) Si 2p and (f) O 1s. | ||
After modification with MPTMS, the peaks of Si became weak and a new peak of S appeared. Through high resolution scans of CoFe2O4@SiO2–SH shown in Fig. 6(b)–(f), it can be known that the peaks of Fe 2p, Co 2p, C 1s, Si 2p and O 1s were at 724.59 eV, 781.86 eV, 284.33 eV, 101.73 eV and 531.92 eV, respectively, which directly indicated that the –SH was introduced onto the material successfully by MPTMS modification. The results agreed well with the FT-IR analysis of the sorbents.
3.2 Adsorption experiments
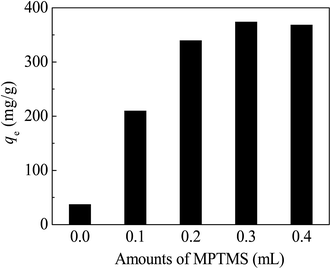 | ||
| Fig. 7 Effect of MPTMS amount in CoFe2O4@SiO2–SH. Conditions: pH = 8, dosage of 0.05 g L−1, C0 = 40 mg L−1, t = 12 h and T = 298 K. | ||
The adsorption capacity of CoFe2O4@SiO2–SH increased with the increasing amount of the MPTMS under the condition of low addition of MPTMS. The optimum amount of MPTMS was found to be 0.3 mL. If the amount of MPTMS was less than 0.3 mL, the surface of the material would not be completely grafted by –SH group, therefore, the adsorption capacity cannot be maximized. When the addition amount of MPTMS was 0.3 mL, the qe of adsorbents reached 374.4 mg g−1.
Continued to increase the amount of MPTMS, the adsorption capacity of the adsorbents had not been further improved, but slightly decreased. So, the optimal amount of MPTMS should be 0.3 mL. The reason may be owing to no excess active sites grafting with MPTMS on the surface of CoFe2O4@SiO2. It also may be owing to the aggregation of magnetic core because of the uncontrolled self-condensation of trimethoxysilane groups, as Sun et al. reported.28
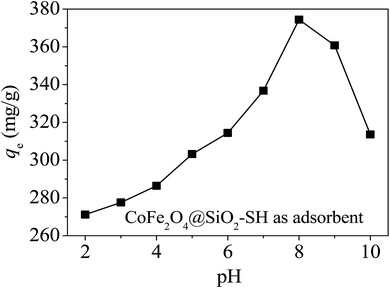
Based on the data from Fig. 8, qe of CoFe2O4@SiO2–SH for Hg(II) reached a maximum of 374.4 mg g−1 at pH of 8. Furthermore, the CoFe2O4@SiO2–SH exhibited better adsorption performance at pH of 2–10. The adsorption capacity had also a high value of 271.2 mg g−1 even at pH = 2.
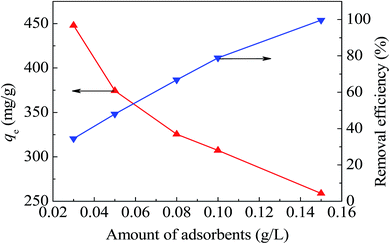
As shown in Fig. 9, along with the increasing addition of CoFe2O4@SiO2–SH, the adsorption capacity for Hg(II) decreased quickly and the adsorption efficiency increased remarkably. Fig. 9 shows that CoFe2O4@SiO2–SH had great high adsorption capacities for Hg(II) even with pretty low dosage, for instance, 0.05 g L−1.
3.3 Adsorption kinetics
The adsorption kinetic data of heavy metal ions were analyzed by testing pseudo-first-order (eqn (2)),29 pseudo-second-order (eqn (3))30 and the intra-particle diffusion models (eqn (4)).31
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (2) |
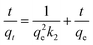 | (3) |
| qt = kdit0.5 + Ci | (4) |
Thereinto, k1 (min−1), k2 (g mg−1 min) and kdi (mg g−1 min0.5) are rate constants, respectively. qt (mg g−1) is instantaneous adsorption capacity at time of t, and Ci (mg g−1) represents the thickness of boundary layer.
The diffusion and chemisorption are normally regarded as the rate limiting step by pseudo-first-order and pseudo-second-order models, separately. The Weber and Morris diffusion is used to analyze the suitability and effectiveness of the adsorption process. Fig. 10(a) shows that when t ≤ 60 min, the adsorption rate was very fast, which may be due to the surface of the material had a rich activity of adsorption sites at the beginning of the reaction. With the passage of time, more and more Hg(II) was adsorbed onto the materials, and meanwhile, the concentration gradient between the surface of the material and the interior of solution decreased, resulting in a decreasing adsorption rate, and ultimately tending to a balance.
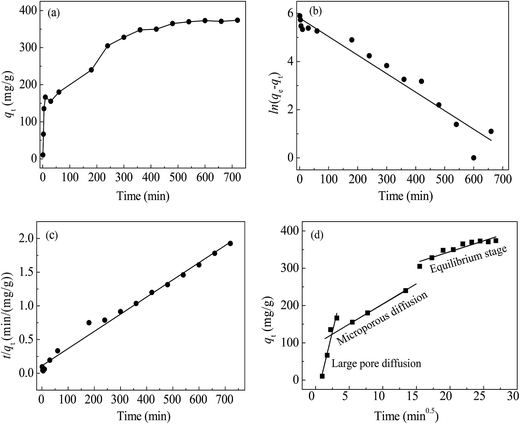 | ||
| Fig. 10 Adsorption curves of Hg(II) by qt vs. t (a), kinetic fitting of pseudo-first-order (b), pseudo-second-order (c) and intra-particle diffusion (d). | ||
The goodness of fitting was estimated from the measurement coefficient R2. The calculated results were listed in Table 2. The data from pseudo-first-order model in Fig. 10(b) did not show good agreement (R2 = 0.941) with the experimental data, and the difference between the theoretical qe,cal (332.8 mg g−1) and the experimental qe,exp (374.4 mg g−1) was a little large. The data from pseudo-second-order model in Fig. 10(c) fitted well (R2 = 0.989), and the theoretical qe,cal (392.2 mg g−1) was close to the actual qe,exp. Therefore, the experimental results can be well represented by pseudo-second-order model and indicated that the removal of Hg(II) with CoFe2O4@SiO2–SH involved chemical reaction.
| Pseudo-first-order | Pseudo-second-order | |||||
|---|---|---|---|---|---|---|
| qe,exp | qe,cal | k1 | R2 | qe,cal | k2 | R2 |
| 374.4 | 332.8 | 0.0077 | 0.941 | 392.2 | 5.81 × 10−5 | 0.989 |
| Intra-particle diffusion | ||||||||
|---|---|---|---|---|---|---|---|---|
| kd1 | C1 | R12 | kd2 | C2 | R22 | kd3 | C3 | R32 |
| 74.69 | −57.21 | 0.910 | 10.66 | 97.06 | 0.999 | 5.80 | 228.39 | 0.859 |
In Fig. 10(d), the initial linear portion represented instantaneous adsorption on the surface of the material, indicating that the mass transfer in the early stage was achieved by large pore diffusion. The second stage was the gradual adsorption, indicating that mass transfer at this stage was achieved via microporous diffusion. The second stage was the main diffusion rate limitation. The third line was tending to equilibrium stage. Straight lines did not go through the origin, indicating that intra-particle diffusion was not the only rate limitation.
3.4 Adsorption isotherms
To further investigate the capacity of Hg(II) onto CoFe2O4@SiO2–SH, Langmuir (eqn (5))32 and Freundlich models (eqn (7))33 were employed. The Langmuir model supposes the adsorption as monolayer and occurring on a homogeneous surface shown as eqn (5), whereas Freundlich model is built on the theory of multilayer adsorption shown as eqn (6).
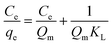 | (5) |
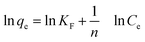 | (6) |
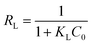 | (7) |
Fig. 11(a) and (b) show the isotherm fitting of Hg(II) on the surface of CoFe2O4@SiO2–SH. Table 3 exhibits the isotherm parameters under three temperatures. R2 (0.995) from Freundlich model was higher than that from Langmuir model (0.989), indicating a better fitting with Freundlich model compared with Langmuir model. The n from Freundlich constants was greater than 1, indicating a favourable adsorption.34 Three R2 from Langmuir model were all greater than 0.980, indicating a good fitting degree. So Langmuir model can be employed to simulate the removal process for Hg(II).
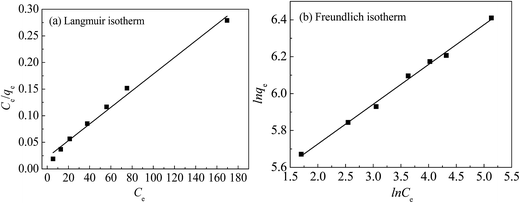 | ||
| Fig. 11 The Langmuir (a) and Freundlich (b) isotherm curves with CoFe2O4@SiO2–SH (T = 298 K, pH = 8, dosage of 0.05 g L−1 and t = 12 h). | ||
| T (K) | Langmuir model | Freundlich model | |||||
|---|---|---|---|---|---|---|---|
| Qm | KL | R2 | RL | 1/n | KF | R2 | |
| 298 | 641.0 | 0.071 | 0.989 | 0.123 | 0.216 | 199.5 | 0.995 |
| 308 | 628.9 | 0.066 | 0.991 | 0.132 | 0.232 | 180.8 | 0.997 |
| 318 | 591.7 | 0.061 | 0.987 | 0.142 | 0.226 | 171.1 | 0.994 |
Langmuir also suggested a monolayer adsorption process and the Qm from CoFe2O4@SiO2–SH was 641.0 mg g−1. This value was much larger than the maximum adsorption capacity of many similar materials (Table 4).35,36 The separation factor RL can determine if adsorbents have an ability to effectively remove contaminants. The data in Table 3 showed that RL value changed from 0 to 1, indicating a spontaneous adsorption.
| Materials | pH | Fitting models | Qm | Ref. |
|---|---|---|---|---|
| Tannic acid modified Fe3O4 core–shell nanoparticles | 5 | Langmuir isotherm | 96 | 37 |
| Starch/SnO2 | 6 | Freundlich isotherm | 192 | 38 |
| Polypyrrole/SBA-15 | 8 | Langmuir isotherm | 200 | 39 |
| Amino-functionalized CoFe2O4–chitosan–graphene | 7 | Langmuir isotherm | 361 | 40 |
| CoFe2O4–rGO | 4.6 | Langmuir isotherm | 157.9 | 41 |
| Magnetic Fe3O4 GO | 6 | Langmuir isotherm | 71.3 | 42 |
| Polystyrene coated CoFe2O4 modified with 2-(3-(2-aminoethylthio)propylthio) ethanamine | 7–8 | Langmuir isotherm | 84 | 43 |
| Fe3O4@SiO2–SH | 6 | Langmuir isotherm | 132 | 44 |
| Mercaptoamine-functionalised silica-coated MNPs | 5–6 | Freundlich isotherm | 355 | 45 |
| Three types of activated carbons | 4 | Langmuir isotherm | 40–60 | 46 |
| CoFe2O4@SiO2–SH | 8 | Freundlich isotherm | 641.0 | This work |
3.5 Adsorption thermodynamics
Three thermodynamic parameters, Gibbs free energy (ΔG°, kJ mol−1) (eqn (8)), enthalpy (ΔH°, kJ mol−1) (eqn (9)) and entropy (ΔS°, kJ mol−1 K) (eqn (9)) of adsorption are listed as follows:
ΔG0 = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd Kd
| (8) |
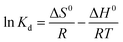 | (9) |
| C0 | ΔH0 | ΔS0 | ΔG0 | ||
|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | |||
| 40 | −0.0116 | 40.52 | −23.68 | −24.05 | −24.49 |
3.6 Effect of coexisting ions
In the real application of materials, the anions and cations in the natural water may have an effect on adsorption capacity. Hence, six common types of ions, including three cations (Na+, K+ and Ca2+) and three anions (Cl−1, NO3− and SO42−) were used to assess the removal of Hg(II) with CoFe2O4@SiO2–SH.From Fig. 12, the adsorption properties of materials are affected by the individual ions and decreased as the ionic strength increases. This may be owing to the fact that the cations in the water compete with the active adsorption sites, resulting in a descending adsorption.47
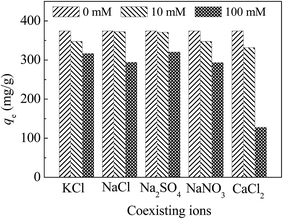 | ||
| Fig. 12 Effect of coexisting ions and ion strength with CoFe2O4@SiO2–SH as adsorbents. Conditions: pH = 8, C0 = 40 mg L−1, T = 298 K, dosage of 0.05 g L−1 and t = 12 h. | ||
Among these ions, Ca2+ has the greatest effect on the capacity of materials, probably because Ca2+ is a divalent cation with two positive charges, which will occupy two active sites.48 Therefore, the effect of Ca2+ on the performance of the material was larger than that of other monovalent cations including K+ and Na+. For anions, Cl− has the greatest effect on the adsorption. Cl− can complex with mercury to form HgCl2, HgCl3− and HgCl42−.43 However, higher concentration of Cl− can compete for the surface of the active sites with mercury, resulting in a decreased adsorption.49
3.7 Application evaluation
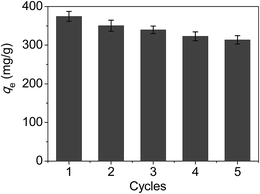
In order to further reveal the performance of the material of CoFe2O4@SiO2–SH in a real engineering, an application evaluation was carried out. Firstly, desorption and regeneration experiments were made to investigate the reusable performance of the material, and the results were shown in Fig. 13. From the data in Fig. 13, it can be seen that the adsorption capacities declined slowly with the increasing cycles, and the decrease of adsorption capabilities of CoFe2O4@SiO2–SH was about 16.2% after five cycles.Besides, as we know, electroplating wastewater is a common and typical wastewater and contains many kinds of harmful heavy metals, such as mercury, chromium, nickel, lead, and so on. To investigate the adsorption activity of the material, a real electroplating wastewater sample was employed to be treated by the material of CoFe2O4@SiO2–SH.
The concentrations of Hg(II), Cr(VI), Ni(II) and Pb(II) in the wastewater sample are 0.04–0.8 mg L−1, 0.6–1.3 mg L−1, 0.5–1.1 mg L−1 and 0.15–0.3 mg L−1, respectively. The CODcr value of wastewater is about 43.5–55.1 mg L−1. The experiments were carried out at the conditions of dosage of 0.15 g L−1 and pH of 8 ± 0.2.
The test shows that the removal efficiency of Hg(II) achieved over 99% after adsorption and the effluent basically met the Chinese National Standard “Emission Standard of Pollutants for Electroplating” (GB 21900-2008). The above data indicated that the as-prepared material of CoFe2O4@SiO2–SH could be employed as a promising and efficient adsorbent in the real water treatment engineering.
3.8 Mechanism speculation
By comparing the XPS spectra of a wide range and individual important elements of CoFe2O4@SiO2–SH before and after adsorption for mercury, it can further investigate the removal mechanism of materials.Fig. 14(a) exhibits the wide scans of CoFe2O4@SiO2–SH (before adsorption) and CoFe2O4@SiO2–SH–Hg (after adsorption). Before adsorption, the wide scan clearly shows the peaks of O 1s, C 1s, Si 2p and S 2p. After the material adsorbed Hg(II), the peak of S 2p became weak and the new peaks of Hg 4d and Hg 4p were clearly observed. The presence of Hg 4p and Hg 4f demonstrated the successful adsorption of CoFe2O4@SiO2–SH for Hg(II) ions.
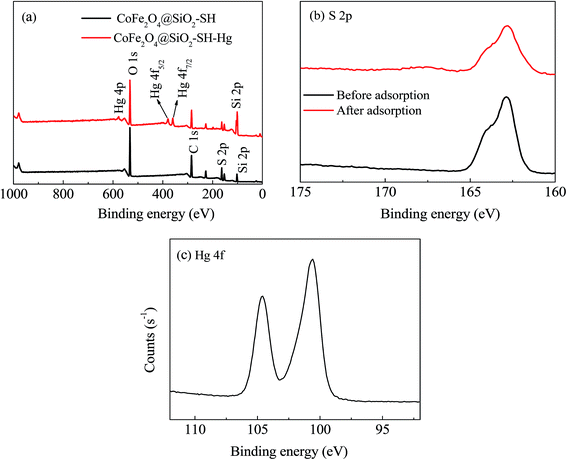 | ||
| Fig. 14 XPS spectra of wide scan (a), high resolution scan of S 2p (b) before and after adsorption, and Hg 4f (c). | ||
Fig. 14(b), through comparing the XPS spectra of S elements before and after adsorption for Hg(II), it can be known that the peak of S 2p after adsorption was weaker than that before adsorption, and was slightly shifted. Also, a peak of S 2p appeared at 162.91 eV before adsorption may belong to –SH. The peak of S 2p was slightly moved to 162.82 eV after adsorption. It may be that S atoms in mercapto group donated parts of electrons to Hg during the process of adsorption.
From Fig. 14(c), the high resolution XPS spectra of Hg, there is a double-peaks, which may be the Hg 4f7/2 (100.8 eV) and Hg 4f5/2 (104.8 eV), which further demonstrates the successful adsorption of mercury onto the material.
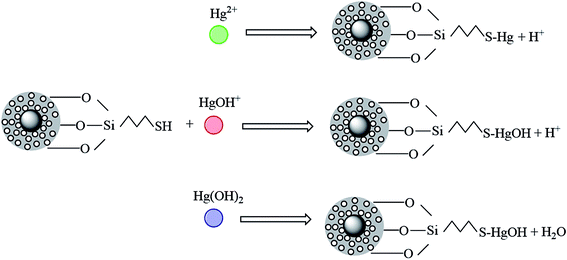
As we know, mercury in aqueous solution exists mainly in three forms: Hg2+, Hg(OH)+ and Hg(OH)2. In acidic solution (pH < 3), the three forms are all present. Hg2+ was the main form of existence, and its amount will decrease with the pH increasing, until disappearance. The amount of the other two forms of Hg(OH)+ and Hg(OH)2 will increase as pH increases.
In addition, the number of Hg(OH)+ will reach the maximum at pH = 4, and Hg(OH)2 will become the main form at pH > 6. To better understand the removal mechanism, the probable reaction processes between Hg(II) and the mercapto-modified materials are plotted in Fig. 14.
Fig. 15 shows that Hg2+ can form –SH–Hg+ with the mercapto group under electrostatic attraction, but the existence of large amount of –SH–Hg+ might be also slightly prevent the further progress of the reaction. Hg(OH)+ and Hg(OH)2 in water can form complexes directly with mercapto groups with/without electrostatic attraction. In the solutions with various pH, the above three forms of mercury exist in different amounts and are adsorbed by the mercapto group by different ways.
In the aforementioned study of pH on adsorption, the gaps between adsorption performances of CoFe2O4@SiO2–SH were not large at pH of 2–10. Coupled with results of the effect of mercapto content on the adsorption capacities, it can be concluded that the adsorption capacity of CoFe2O4@SiO2 was greatly improved after the introduction of mercapto. CoFe2O4@SiO2–SH had an excellent adsorption for mercury, mainly due to the affinity between –SH and mercury, apart from the electrostatic interactions between the material and mercury. Based on the Hard Soft Acid–Base theory, mercapto (soft base) and Hg (soft acid) can have a strong affinity, which is very conducive to the progress of the adsorption reaction.
4. Conclusions
In this study, a thiol-functionalized and silica-coated magnetic nanoparticle (CoFe2O4@SiO2–SH) was successfully synthesized via a simple and cost effective method, and employed to remove Hg(II) in water.The adsorption process showed a good performance at pH of 2–10, which implied a probable practical application. And CoFe2O4@SiO2–SH had great high adsorption capacities for Hg(II) even with pretty low dosage of 0.05 g L−1.
Kinetic fitting exhibited a good correlation to pseudo-second-order model and adsorption process was controlled by a chemical reaction with three diffusion stages. The isotherm data were in accord with Freundlich model with maximum capacities of 641.0 mg g−1 at 298 K and pH of 8, substantially higher than many similar materials. Thermodynamic data displayed an exothermic and spontaneous adsorption process.
Hg2+ can form –SH–Hg+ with the mercapto group or complexes with/without electrostatic attraction. Furthermore, after adsorption, CoFe2O4@SiO2–SH MNPs can be collected from water at a low magnetic field gradient, which helps to prevent secondary pollution and reduce the costs of water treatment. In general, CoFe2O4@SiO2–SH MNPs is an economy and efficient material with great potential to remove mercury from water.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51578354, 51678381), Natural Science Foundation of Jiangsu Province (BK20141179), Six Talent Peaks Program (2016-JNHB-067), Suzhou Science and Technology Bureau (SS201667), Qing Lan Project and Research Innovation Project for College Graduates of Jiangsu Province (SJZZ16_0248, KYCX17_2067).References
- T. W. Clarkson, Crit. Rev. Clin. Lab. Sci., 1997, 34, 369 CrossRef CAS PubMed.
- N. Langford and R. Ferner, J. Hum. Hypertens., 1999, 13, 651 CAS.
- G. B. Jiang, J. B. Shi and X. B. Feng, Environ. Sci. Technol., 2006, 40, 3672 CrossRef CAS.
- M. J. González-Muñoz, M. A. Rodríguez, S. Luque and J. R. Álvarez, Desalination, 2006, 200, 742 CrossRef.
- W. Plazinski and W. Rudzinski, Environ. Sci. Technol., 2009, 43, 7465 CrossRef CAS PubMed.
- C. Visvanathan, R. B. Aim and K. Parameshwaranc, Crit. Rev. Environ. Sci. Technol., 2000, 30, 1 CrossRef CAS.
- C. H. Mo, Q. Y. Cai and Q. T. Wu, Chin. J. Appl. Environ. Biol., 2001, 7, 511 CAS.
- H. V. Tran, L. D. Tran and T. N. Nguyen, Mater. Sci. Eng., C, 2010, 30, 304 CrossRef CAS.
- S. Hokkanen, A. Bhatnagar and M. Sillanpää, Water Res., 2016, 91, 156 CrossRef CAS PubMed.
- S. Sun, H. Zeng, D. B. Robinson, S. Raoux, P. M. Rice, S. X. Wang and G. Li, J. Am. Chem. Soc., 2004, 126, 273 CrossRef CAS PubMed.
- Y. X. Ma, D. Xing, C. P. Lu, X. Y. Du and P. Q. La, Polym. Compos., 2016, 9 Search PubMed.
- Z. Wang, J. Xu, Y. Hu, H. Zhao, J. Zhou, Y. Liu, Z. Lou and X. Xu, J. Taiwan Inst. Chem. Eng., 2016, 60, 394 CrossRef CAS.
- Y. Ma, P. La, W. Lei, C. Lu and X. Du, Desalin. Water Treat., 2016, 57, 5004 CrossRef CAS.
- S. X. Zhang, H. Y. Niu, Y. Cai, X. L. Zhao and Y. Shi, Chem. Eng. J., 2010, 158, 599 CrossRef CAS.
- C. Ren, X. Ding, H. Fu, C. Meng, W. Li and H. Yang, RSC Adv., 2016, 6, 72479 RSC.
- R. Roto, Y. Yusran and A. Kuncaka, Appl. Surf. Sci., 2016, 377, 30 CrossRef CAS.
- O. Fardmousavi and H. Faghihian, C. R. Chim., 2014, 17, 1203 CrossRef CAS.
- J. Zhu, J. Yang and B. Deng, J. Hazard. Mater., 2009, 166, 866 CrossRef CAS PubMed.
- S. Zhang, H. Niu, Y. Cai, X. Zhao and Y. Shi, Chem. Eng. J., 2010, 158, 599 CrossRef CAS.
- B. Viltužnik, A. Košak, Y. L. Zub and A. Lobnik, J. Sol-Gel Sci. Technol., 2013, 68, 365 CrossRef.
- O. Hakami, Y. Zhang and C. J. Banks, Water Res., 2012, 46, 3913 CrossRef CAS PubMed.
- S. Dong, X. Dou, D. Mohan, C. U. Pittman Jr and J. Luo, Chem. Eng. J., 2015, 270, 205 CrossRef CAS.
- Y. Zhang, Q. Xu, S. Zhang, J. Liu, J. Zhou, H. Xu, H. Xiao and J. Li, Sep. Purif. Technol., 2013, 116, 391 CrossRef CAS.
- G. Li, Z. Zhao, J. Liu and G. Jiang, J. Hazard. Mater., 2011, 192, 277 CAS.
- H. Parham, B. Zargar and R. Shiralipour, J. Hazard. Mater., 2012, 205–206, 94 CrossRef CAS PubMed.
- S. Zhang, Y. Zhang, J. Liu, Q. Xu, H. Xiao, X. Wang, H. Xu and J. Zhou, Chem. Eng. J., 2013, 226, 30 CrossRef CAS.
- L. C. Lin, M. Thirumavalavan and J. F. Lee, Clean: Soil, Air, Water, 2015, 43, 775 CrossRef CAS.
- Y. Sun, X. Ding, Z. Zheng, X. Cheng, X. Hu and Y. Peng, Eur. Polym. J., 2007, 43, 762 CrossRef CAS.
- Y. S. Ho and G. Mckay, Water Res., 1999, 33, 578 CrossRef CAS.
- Y. S. Ho and G. Mckay, Process Biochem., 1999, 34, 451 CrossRef CAS.
- M. Yari, M. Rajabi, O. Moradi, A. Yari, M. Asif, S. Agarwal and V. K. Gupta, J. Mol. Liq., 2015, 209, 50 CrossRef CAS.
- I. Langmuir, J. Chem. Phys., 1918, 40, 1361 CAS.
- M. D. Levan and T. Vermeulen, J. Phys. Chem., 1981, 85, 3247 CrossRef CAS.
- L. Zhou, L. Ji, P. C. Ma, Y. Shao, H. Zhang, W. Gao and Y. Li, J. Hazard. Mater., 2014, 265, 104 CrossRef CAS PubMed.
- Y. S. Bo, Y. Eom and G. L. Tai, Appl. Surf. Sci., 2011, 257, 4754 CrossRef.
- B. Viltužnik, A. Lobnik and A. Košak, J. Sol-Gel Sci. Technol., 2015, 74, 199 CrossRef.
- H. Luo, S. Zhang, X. Li, X. Liu, Q. Xu, J. Liu and Z. Wang, J. Taiwan Inst. Chem. Eng., 2017, 72, 163 CrossRef CAS.
- M. Naushad, T. Ahamad, G. Sharma, A. A. H. Al-Muhtaseb, A. B. Albadarin, M. M. Alam, Z. A. Alothman, S. M. Alshehri and A. A. Ghfar, Chem. Eng. J., 2016, 300, 306 CrossRef CAS.
- M. Shafiabadi, A. Dashti and H. A. Tayebi, Synth. Met., 2016, 212, 154 CrossRef CAS.
- Y. Zhang, T. Yan, L. Yan, X. Guo, L. Cui, Q. Wei and B. Du, J. Mol. Liq., 2014, 198, 381 CrossRef CAS.
- Y. Zhang, L. Yan, W. Xu, X. Guo, L. Cui, L. Gao, Q. Wei and B. Du, J. Mol. Liq., 2014, 191, 177 CrossRef CAS.
- Y. F. Guo, J. Deng, J. Y. Zhu, X. J. Zhou and R. B. Bai, RSC Adv., 2016, 6, 82523 RSC.
- K. Jainae, N. Sukpirom, S. Fuangswasdi and F. Unob, J. Ind. Eng. Chem., 2014, 23, 273 CrossRef.
- Z. Wang, J. Xu, Y. Hu, H. Zhao, J. Zhou, Y. Liu, Z. Lou and X. Xu, J. Taiwan Inst. Chem. Eng., 2016, 60, 394 CrossRef CAS.
- S. Bao, K. Li, P. Ning, J. Peng, X. Jin and L. Tang, Appl. Surf. Sci., 2016, 393, 457 CrossRef.
- Y. F. Guo, Z. Wang, X. J. Zhou and R. B. Bai, Res. Chem. Intermed., 2016, 10, 1 CAS.
- L. Cui, Y. Wang, L. Gao, L. Hu, Q. Wei and B. Du, J. Colloid Interface Sci., 2015, 456, 42 CrossRef CAS PubMed.
- L. Tran, P. Wu, Y. Zhu, Y. Lin and N. Zhu, J. Colloid Interface Sci., 2015, 445, 348 CrossRef CAS PubMed.
- M. M. Benjamin and J. O. Leckie, Environ. Sci. Technol., 1982, 16, 162 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |