Open Access Article
Open Access ArticleSimultaneous reductive and sorptive removal of Cr(VI) by activated carbon supported β-FeOOH†
Tingting Yanga,
Lirong Menga,
Shuwen Hana,
Jianhua Hou ab,
Shengsen Wangab and
Xiaozhi Wang
ab,
Shengsen Wangab and
Xiaozhi Wang *ab
*ab
aCollege of Environmental Science and Engineering, Yangzhou University, Jiangsu 225127, China. E-mail: xzwang@yzu.edu.cn
bJiangsu Collaborative Innovation Center for Solid Organic Waste Resource Utilization, Nanjing 210095, China
First published on 11th July 2017
Abstract
An activated carbon (AC)-supported nanocomposite was prepared by precipitating β-FeOOH onto KOH activated soybean meal-derived biochar (SYBK). The as-prepared β-FeOOH/SYBK composites were characterized by N2-Brunauer–Emmett–Teller (BET), scanning electron microscopy (SEM), X-ray diffractions (XRD) and X-ray photoelectron spectroscopy (XPS). XRD results confirmed that β-FeOOH was impregnated by AC. The chromate (Cr(VI)) removal capacity was investigated in a batch experiment with different conditions. The ratios of β-FeOOH and AC were compared for Cr(VI) removal and a loading quantity of 20 wt% β-FeOOH was considered as the most efficient amount. This was possibly ascribed to it having the highest surface area (670.65 m2 g−1) of the β-FeOOH/SYBK nanocomposites. It was found that 20β-FeOOH/SYBK could remove as much as 96% Cr(VI) at pH 1–2 with 2 mmol L−1 EDTA and 2.0 g L−1 nanocomposites. The maximal Cr(VI) removal by 20β-FeOOH/SYBK was 37.04 g kg−1, as estimated by a Langmuir isotherm model. The removal mechanisms were examined by studying the speciation of Cr on sorbents as well as in aqueous solution. The XPS analysis of spent sorbents and chemical speciation of Cr in aqueous solutions revealed that partial Cr(VI) was reduced to Cr(III) on sorbents and in aqueous solution. This suggests that Cr(VI) can be removed by simultaneous sorption and reduction by the as-prepared nanocomposites.
1. Introduction
Chromium (Cr) is a typical toxic heavy metal, which has been extensively used in electroplating, leather tanning and other industrial applications.1,2 Excessive accumulation in the human body may cause serious health problem.3 The U.S. Environmental Protection Agency has prescribed a maximal allowance concentration of 0.1 mg L−1 in drinking water.4 Cr exists mainly in two stable valence states, namely hexavalent Cr (Cr(VI)) and trivalent Cr (Cr(III)), with different toxicity, mobility and biological availability. In contrast to Cr(III), Cr(VI) exists as CrO42− and Cr2O72− in the solution, which is more toxic and soluble. The toxicity of Cr(VI) is 100 times higher than Cr(III).5 To reduce Cr(VI) toxicity in contaminated soil and water, Cr(VI) can be removed by sorption or reduced to its less toxic form.6,7Activated carbon (AC) is a carbon-enriched material, which is characterized with high surface area and abundant functional groups.8 The favorable properties enable it to remove many kinds of organic and inorganic contaminants from aqueous solutions. Recently, the reduction of Cr(VI) in water treatment has been achieved by various biochar,9 e.g., derived from agricultural and forestry waste. In this study, soybean meal was used to produce biochar (BC) with abundant elements such as Si, Mg, Al, and so on. Chemical activation may increase the surface area and functional groups of biochars. KOH activation was an effective method to prepare AC and to increase its surface area,10,11 and activated carbon can be widely used as an sorbent in waste water treatment.12,13 However, most carbonaceous materials such as activated carbon are not efficient for Cr(VI) removal,14,15 which is possibly ascribed to the low point zero charges and negative zeta potential at neutral pH. The negative charges at normal environmental conditions of the sorbents is not favorable for sorption of negatively charged Cr(VI).16 As a result, various materials are incorporated in the carbon matrix to improve its performance for Cr(VI) removal.
Iron oxide is an ideal candidate which can not only adsorb Cr(VI) but also reduce it to a less toxic species.17 Akaganéite (β-FeOOH) is a typical iron oxyhydroxide mineral and characterized with a channel structure parallel to the c-axis.18 β-FeOOH is widely used as sorbents as well as a Fenton-like catalyst to remove contaminants from aqueous solutions.19 Both sorptive and reductive removal of Cr(VI) by β-FeOOH has been reported elsewhere.20 The Fe(III) can then be transformed to Fe(II),21 largely dependent onto conditions of the reactions. β-FeOOH is usually synthesized by wet-chemistry approach, and the prepared nanoparticles may aggregate which is difficult to separate from aqueous solutions. Activated carbon is a commonly-used support matrix to stabilize nanoparticles. Thus, we propose to immobilize β-FeOOH with activated carbon to improve its surface area and thus Cr(VI) removal capacity.
In this work, the KOH-activated carbons were impregnated with β-FeOOH. The purposes of this study were to (1) prepare and characterize the KOH activated soybean meal derived biochar, (2) select the most efficient β-FeOOH loadings onto activated carbon for Cr(VI) removal, (3) investigate the sorptive and reductive removal capacity of Cr(VI), and (4) find out the possible mechanisms associated with Cr(VI) removal.
2. Materials and methods
2.1 Chemical reagents
Cr(VI) aqueous solution was prepared by using potassium dichromate (K2Cr2O7) of analytical grade. Potassium hydroxide (KOH), ethylene diamine tetra acetic acid (EDTA), ferric chloride hexahydrate (FeCl3·6H2O), urea (H2NCONH2), potassium permanganate (KMnO4) used were of analytical grade. 1 M HCl and NaOH were used to adjust the pH of Cr(VI) solution. Deionized (DI) water was used to dissolve the chemicals.2.2 Modified biocahr preparation
To prepare soybean meal derived biochars (SYB), a commercial soybean meal was oven dried at 80 °C, and then pyrolyzed in a tube furnace at 700 °C for two hours purged with nitrogen gas. The BC was washed with DI water and oven dried overnight at 80 °C. The obtained SYB was mixed with KOH solution, with KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BC mass ratio of 1
BC mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The suspension was stirred for two hours and then heated at 80 °C until the mixture turned into a paste. The resulting product was heated (10 °C min−1) in a tube furnace at a highest temperature of 800 °C for 1 h in N2 flow. After cooling, the sample was washed with a 0.1 N HCl acid and DI water, oven dried overnight at 80 °C. The resulting activated carbon was denoted as SYBK.
1. The suspension was stirred for two hours and then heated at 80 °C until the mixture turned into a paste. The resulting product was heated (10 °C min−1) in a tube furnace at a highest temperature of 800 °C for 1 h in N2 flow. After cooling, the sample was washed with a 0.1 N HCl acid and DI water, oven dried overnight at 80 °C. The resulting activated carbon was denoted as SYBK.
Four β-FeOOH/SYBK composites, with the different loading percentage of β-FeOOH, were obtained by following procedures according to the previous method.3 0.5 g SYBK was added to 60 mL deionized water and magnetically stirred for 24 h. Then, the required amount of FeCl3·6H2O and urea (with β-FeOOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SYBK mass ratio of 5
SYBK mass ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95, 10
95, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, 20
90, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, 40
80, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, and Fe3+/urea mole ratio of 1
60, and Fe3+/urea mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) were added to the above suspension, respectively. After stirring for 60 min, the obtained slurry was transferred into a Teflon-lined stainless steel autoclave (100 mL) and was maintained at 90 °C for 8 h. The resulting composites were cooled to room temperature and filtered by suction filtration. The composites were then washed several times with ethanol and DI water, and oven dried overnight at 80 °C. The obtained samples were denoted as 5β-FeOOH/SYBK, 10β-FeOOH/SYBK, 20β-FeOOH/SYBK, 40β-FeOOH/SYBK, respectively. For comparison, β-FeOOH/SYB was prepared by SYB (without activated by KOH) following same procedure but with no KOH activation (with β-FeOOH
4) were added to the above suspension, respectively. After stirring for 60 min, the obtained slurry was transferred into a Teflon-lined stainless steel autoclave (100 mL) and was maintained at 90 °C for 8 h. The resulting composites were cooled to room temperature and filtered by suction filtration. The composites were then washed several times with ethanol and DI water, and oven dried overnight at 80 °C. The obtained samples were denoted as 5β-FeOOH/SYBK, 10β-FeOOH/SYBK, 20β-FeOOH/SYBK, 40β-FeOOH/SYBK, respectively. For comparison, β-FeOOH/SYB was prepared by SYB (without activated by KOH) following same procedure but with no KOH activation (with β-FeOOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SYB mass ratio of 20
SYB mass ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, and the Fe3+/urea mole ratio 1
80, and the Fe3+/urea mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4).
4).
2.3 Characterization and analysis β-FeOOH/SYBK
The structure and phase of as-prepared β-FeOOH/SYBK samples were determined using an X-ray diffractometer (XRD, D8-ADVANCE) and X-ray electron spectroscopy (XPS, ESCALAB 250Xi). The surface morphology of β-FeOOH/SYBK was examined by scanning electron microscope (SEM, S-4800II). Nitrogen adsorption–desorption isotherms and specific surface areas were measured on a Micromeritics ASAP 2460, and surface area and pore volume was calculated with Brunauer–Emmett–Teller (BET) theory.2.4 Cr(VI) removal experiments
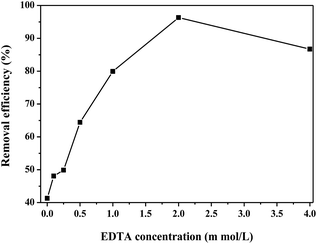
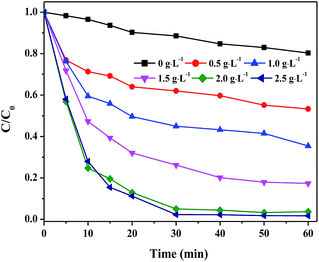
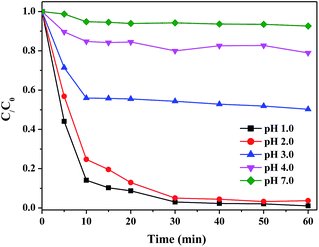
The sorption of Cr(VI) by nanocomposites was carried out in a batch experiment. First, the optimal β-FeOOH loading was determined by adding different sorbents to 50 mg L−1 Cr(VI) solution, with pH adjusted at 2 and in presence of 2 mM EDTA. The Cr(VI) removal efficiency was examined as a function of time. A reaction time of 60 min was selected for subsequent experiments. Second, the most appropriate sorbent dosage was compared by adding different amounts of sorbents (0, 0.5, 1.0, 1.5, 2.0, and 2.5 g L−1) to 50 mg L−1 Cr(VI) solution, with pH adjusted at 2 and in presence of 2 mM EDTA. Third, the sorption of Cr(VI) (50 mg L−1) by 20β-FeOOH/SYBK at different pH was determined at pH of 1, 2, 3, 4 and 7, with EDTA of 2 mM. Then, the sorption isotherm by 20β-FeOOH/SYBK was conducted at pH of 2, with different Cr(VI) concentrations (10, 15, 25, 40, 50, 60 and 80 mg L−1). The reactions were initiated in 50 mL vessels, and the suspension was shaken on a rotary shaker at 25 °C.After reaction, the solutions were sampled at different time and then filtered through 0.45 μm membranes. Total Cr(VI) was analyzed with the 1,5-diphenylcarbazide colorimetric method (with potassium permanganate).22 The concentrations of Cr(VI) was measured using the 1,5-diphenylcarbazide colorimetric method23,24 with an UV/Vis spectrophotometer at 540 nm. The Cr(III) in solutions was analyzed by the difference of total Cr and Cr(VI).25 The spent sorbents were analyzed with XPS to obtain the Cr speciation after sorption. The Fe(III) and Fe(II) in solutions was analyzed with 1,10-phenanthroline.26
3. Results and discussion
3.1 Characteristics of as-synthesized β-FeOOH/SYBK
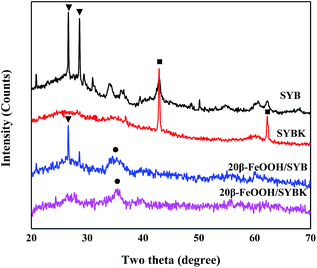
Fig. 1 showed the XRD patterns of different BC, AC and β-FeOOH nanocomposites. The diffraction peaks of SYB at 2θ° = 26.62, 28.62, 42.92 could be indexed to (011) and (211) planes of quartz and (200) plane of periclase. After KOH activation, (200) and (220) planes of SYBK were observed, corresponding to periclase. After β-FeOOH loading, the characteristic peaks at 2θ° = 35.25 was observed for 20β-FeOOH/SYB and 20β-FeOOH/SYBK, indicating formation of β-FeOOH.20Nitrogen adsorption/desorption presents a type-IV isotherm (Fig. S1†) demonstrating typical mesoporous characteristics of nanoparticles. The average pore sizes of BC, AC and β-FeOOH nanocomposites ranged between 2.67 and 6.96 nm (Table 1). 20β-FeOOH/SYBK has a specific surface of 670.65 m2 g−1. Compared with SYB, the specific surface area of SYBK increased nearly by 32 times from 14.76 m2 g−1 to 477.71 m2 g−1, and the pore volume also increased about 12 times from 0.026 cm3 g−1 to 0.324 cm3 g−1. This is possibly attributed to the surface reaction occurred on the interface of BC and KOH during the activation, which promote pore formation.27 β-FeOOH impregnation also increased the specific area of SYB and SYBK, possibly because the blocked pores are liberated or new pores were crated during β-FeOOH loading.28
| Sample | SBETa (m2 g−1) | Average pore sizeb (nm) | Pore volumec (cm3 g−1) |
|---|---|---|---|
| a Note: surface area was calculated with Brunauer–Emmett–Teller (BET) method.b Estimated from the Barrett–Joyner–Halenda (BJH) formula.c Single point adsorption total pore volume of pores. | |||
| SYB | 14.76 | 6.955 | 0.026 |
| SYBK | 477.71 | 2.713 | 0.324 |
| 20β-FeOOH/SYB | 171.24 | 3.835 | 0.164 |
| 20β-FeOOH/SYBK | 670.65 | 2.666 | 0.447 |
SEM images showed that SYB exhibited carbon skeletons (Fig. S2a†). After KOH activation, the surface of SYBK (Fig. S2b†) became more porous, and the most pores were maintained even after β-FeOOH loading (Fig. S2d†).
The XPS spectra of 20β-FeOOH/SYBK showed a considerable amount of Fe appeared in composites (Fig. 2). The C 1s spectra showed peaks at binding energies (BEs) of 287.3 eV, 285.98 eV and 289.48 eV, corresponding to C–O, hydroxyl (C–OH) bond,29 carbonyl (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) bond,30 respectively. The O 1s spectra (Fig. 2c) show the positions of Fe–O–H bonds with BE of 531.06 eV, Fe–O–C bonds at 532.2 eV,31,32 organic C
O) bond,30 respectively. The O 1s spectra (Fig. 2c) show the positions of Fe–O–H bonds with BE of 531.06 eV, Fe–O–C bonds at 532.2 eV,31,32 organic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds at 533.38 eV. The peak with BE of 534.38 eV indicated the formation of C–O. The presence of Fe–O and Fe–O–H bonds suggest the formation of β-FeOOH.33 BE peaks of Fe 2p spectrum with 712.2, 718.9, 726.3 and 733.6 eV corresponded to Fe(III).34
O bonds at 533.38 eV. The peak with BE of 534.38 eV indicated the formation of C–O. The presence of Fe–O and Fe–O–H bonds suggest the formation of β-FeOOH.33 BE peaks of Fe 2p spectrum with 712.2, 718.9, 726.3 and 733.6 eV corresponded to Fe(III).34
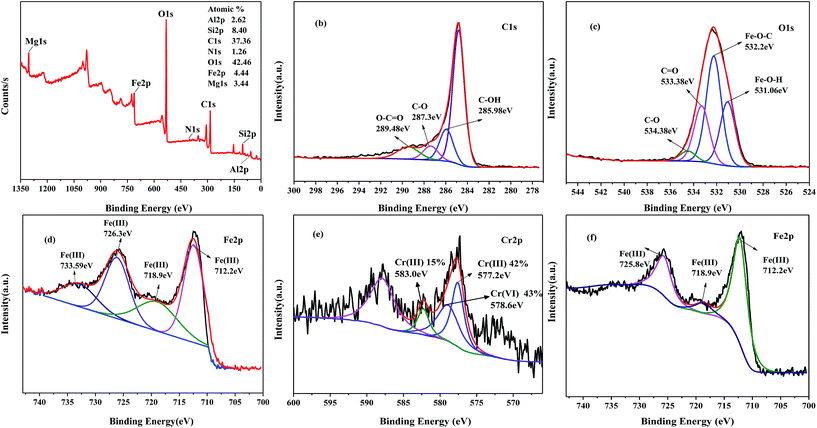 | ||
| Fig. 2 (a) XPS spectra of 20β-FeOOH/SYBK before, high-resolution XPS spectra of (b) C 1s, (c) O 1s, (d) Fe 2p and after Cr(VI) reduction Cr 2p (e), Fe 2p (f). | ||
3.2 Cr(VI) removal from aqueous solutions
| Synthesis | Kinetic constant, k (10−2 min−1) | Coefficient of determination, R2 |
|---|---|---|
| SYB | 0.801 | 0.762 |
| SYBK | 0.802 | 0.569 |
| 20β-FeOOH/SYB | 1.255 | 0.706 |
| 5β-FeOOH/SYBK | 3.536 | 0.916 |
| 10β-FeOOH/SYBK | 5.602 | 0.901 |
| 20β-FeOOH/SYBK | 9.710 | 0.975 |
| 40β-FeOOH/SYBK | 6.728 | 0.905 |
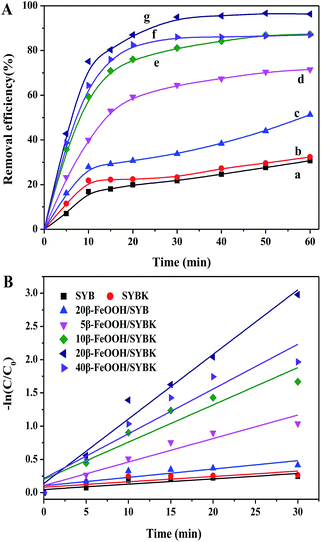
Cr(VI) removal efficiency was 30.7% and 32.4% for SYB and SYBK within 60 min. β-FeOOH greatly increased Cr(VI) removal efficiency. The Cr(VI) removal decreased in the following order: 20β-FeOOH/SYBK > 10β-FeOOH/SYBK > 40β-FeOOH/SYBK > 5β-FeOOH/SYBK. β-FeOOH/SYBK was more capable of Cr(VI) removal than β-FeOOH/SYB (Fig. 3A).
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kf + 1/n Kf + 1/n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (1) |
| Ce/qe = 1/(bqm) + Ce/qm | (2) |
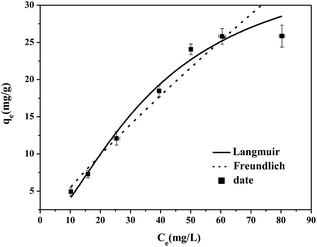 | ||
| Fig. 7 Cr(VI) sorption isotherm data and fitted models for modified activated carbon (20β-FeOOH/SYBK). | ||
Both models fitted the isotherm data well with R2 above 0.91 (Table 3). The better fit was observed for Langmuir model with greater R2. The maximal Cr(VI) removal by 20β-FeOOH/SYBK composite was estimated as 37.04 g kg−1 (Table 3). Thus, the adsorption of Cr(VI) to the 20β-FeOOH/SYBK mainly followed the Langmuir surface adsorption mechanisms. Comparison of Cr(VI) removal capacity with other work (Table 4) showed that the as-prepared β-FeOOH nanoparticles showed excellent Cr(VI) removal capacity than many other sorbents and was suggested as the best sorbent among carbon-based materials.
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (g kg−1) | b (L mg−1) | R2 | Kf/(g kg−1) | 1/n | R2 | |
| 20β-FeOOH/SYBK | 37.036 | 0.0054 | 0.9799 | 0.764 | 0.8541 | 0.9483 |
| Adsorbents | Sorbent dosage (g L−1) | pH | Time | Cr sorption capacity (g kg−1) | Reference |
|---|---|---|---|---|---|
| Biochar | 4.0 | 2 | 24 h | 24.6 | 40 |
| Carbon slurry | 4.0 | 2 | 70 min | 15.24 | 41 |
| Activated alumina | 10 | 3 | 240 min | 25.57 | 42 |
| Activated carbon | 4.0 | 3 | 180 min | 3.46 | 14 |
| Pomegranate husk carbon | 3.0 | 1 | 180 min | 35.2 | 43 |
| Carbon nanotube | 0.4 | 2 | 240 min | 9.0 | 44 |
| Fe3O4 nanoparticles | 20 | 2 | 30 min | 12.43 | 45 |
| nZVI | 1.0 | 2 | 250 min | 148 | 46 |
| α-Fe2O3 | 2.0 | 2 | 120 min | 17.65 | 47 |
| Crosslinked chitosan resins | 2.0 | 3 | 120 min | 84.19 | 48 |
| Activated carbon supported β-FeOOH | 2.0 | 2 | 60 min | 37.04 | Present work |
In order to examine Cr(VI) removal mechanism by β-FeOOH, XPS was used to investigate the valence states of Cr and Fe on surface of 20β-FeOOH/SYBK after the reaction. The BE of Fe 2p at 725.8 eV, 718.9 eV and 712.4 eV can be interpreted as Fe(III) (Fig. 2f). The spectra of the Cr 2p have three BE peaks, namely Cr 2p3/2 (578.2 eV), Cr 2p1/2 (583.0 eV) and Cr 2p1/2 (587.7 eV) (Fig. 2e). The XPS-peak-differentiating analysis revealed Cr 2p3/2 could be divided into two peaks at BEs of 578.6 and 577.2 eV, corresponding to Cr(VI) and Cr(III), respectively. The peak at 783.0 eV is a characteristic peak of Cr(III). This suggests that both Cr(VI) and Cr(III) coexist on the surface of 20β-FeOOH/SYBK after reacting with 50 mg L−1 Cr(VI). The ratio of Cr(III)/Cr(VI) was found to be 1.4, indicating the sorption along with surface reduction was the dominant mechanisms. Besides, the concentration of total Cr decreased by 43% compared with control treatment. Cr speciation was analyzed in the solution, which suggested that about 95% of Cr(VI) was reduced to Cr(III) after 60 min (Table 5). Thus, the Cr(VI) can be removed by sorption onto and reduction by the β-FeOOH.
| Total Cr (mg L−1) | Cr(VI) (mg L−1) | Cr(III) (mg L−1) | |
|---|---|---|---|
| EDTA | 49.73 | 40.15 | 9.58 |
| 20β-FeOOH/SYBK + EDTA | 28.75 | 1.59 | 27.16 |
According to high-resolution XPS spectra, Fe(III) was the ultimate species, but conversion of Fe(III) to Fe(II) was possibly happened in acidic condition during the process.49 To further investigate the mechanisms associated with Cr(VI) removal by β-FeOOH nanocomposites, we measured content of total Fe, Fe(III) and Fe(II) concentrations in acidic solution (pH = 2) after 60 min reaction. The results showed Fe(II) percent was 20% (out of total dissolved Fe 6.88 mg L−1) and 4% (out of total dissolved Fe 21.73 mg L−1) in system with no and with Cr(VI). Lower Fe(II) with presence of Cr(VI) suggests partial Fe(II) can be oxidized by Cr(VI), which was evidenced by appearance of Cr(III) in XPS spectra. Consequently, the following reaction mechanisms were proposed in the low pH conditions. On one hand, Cr(VI) was sorbed by β-FeOOH nanocomposites. One other hand, the Fe(III) was partially converted to Fe(II) in very acidic condition (eqn (3)) on both surfaces of the sorbents and in bulk solutions. The Cr(VI) was then reduced by Fe(II) (eqn (4)), which was evidenced by identification of Cr(III) in both solutions and on surfaces of sorbents.
| β-FeOOH/activated carbon → Fe2+ + Fe3+ | (3) |
| 3Fe2+ + CrO42− + 4H2O → 3Fe3+ + Cr3+ + 8OH− | (4) |
4. Conclusions
The SYBK supported β-FeOOH nanocomposites were synthesized in a hydrothermal reaction with different β-FeOOH loading. The XRD analysis confirmed the successful synthesis of β-FeOOH on the activated carbon surfaces. The batch sorption experiment revealed that the efficient and economic β-FeOOH loading, pH and sorbent dosage were 20 wt%, 2, and 2 g L−1, respectively. The predicted maximum Cr(VI) removal capacity was 37.04 g kg−1 for 20β-FeOOH/SYBK composite. The enhanced removal efficiency of β-FeOOH/SYBK can be related to high surface area of sorbents. Both Cr(VI) and Cr(III) were identified in spent 20β-FeOOH/SYBK and solutions with XPS analysis. Thus, sorption and reduction were dominant Cr(VI) removal mechanisms by β-FeOOH/SYBK. The facile synthesis of sorbents and excellent Cr(VI) removal capacity indicated that as-prepared sorbents are good candidate for Cr(VI) remediation in aqueous solutions.Acknowledgements
This work was supported in part by State Key Laboratory of Pollution Control and Resource Reuse (PCRRF1102), Social development project of Jiangsu Province (BE2015661), Six talent peaks project in Jiangsu Province (2013-NY-017). We thank the Testing Center of Yangzhou University for Sample Characterization.References
- A. Broadway, M. R. Cave, J. Wragg, F. M. Fordyce, R. J. F. Bewley, M. C. Graham, B. T. Ngwenya and J. G. Farmer, Sci. Total Environ., 2010, 409, 267–277 CrossRef CAS PubMed.
- C. Namasivayam and M. V. Sureshkumar, Process Saf. Environ. Prot., 2007, 85, 521–525 CrossRef CAS.
- Z. Xu, H. Jiang, Y. Yu, J. Xu, J. Liang, L. Zhou and F. Hu, Appl. Clay Sci., 2017, 135, 547–553 CrossRef CAS.
- Y. Xu and D. Zhao, Water Res., 2007, 41, 2101–2108 CrossRef CAS PubMed.
- P. L. Di, M. T. Gueye and E. Petrucci, J. Hazard. Mater., 2015, 281, 70–76 CrossRef PubMed.
- T. Ölmez, J. Hazard. Mater., 2009, 162, 1371–1378 CrossRef PubMed.
- K. P. Singh, A. K. Singh, S. Gupta and S. Sinha, Desalination, 2011, 270, 275–284 CrossRef CAS.
- M. B. Ahmed, J. L. Zhou, H. H. Ngo and W. Guo, Biomass Bioenergy, 2016, 84, 76–86 CrossRef CAS.
- Y. Han, X. Cao, X. Ouyang, S. P. Sohi and J. Chen, Chemosphere, 2016, 145, 336–341 CrossRef CAS PubMed.
- Z. Hu and E. F. Vansant, Carbon, 1995, 33, 1293–1300 CrossRef CAS.
- A. Ahmadpour and D. D. Do, Carbon, 1996, 34, 471–479 CrossRef CAS.
- D. Mohan, K. P. Singh and V. K. Singh, J. Hazard. Mater., 2008, 152, 1045–1053 CrossRef CAS PubMed.
- X. Wang, N. Zhu and B. Yin, J. Hazard. Mater., 2008, 153, 22–27 CrossRef CAS PubMed.
- K. Selvi, S. Pattabhi and K. Kadirvelu, Bioresour. Technol., 2001, 80, 87 CrossRef CAS PubMed.
- I. Han, M. A. Schlautman and B. Batchelor, Water Environ. Res., 2000, 72, 29–39 CrossRef CAS.
- J. Wang, K. Zhang and L. Zhao, Chem. Eng. J., 2014, 239, 123–131 CrossRef CAS.
- I. B. Singh and D. R. Singh, Environ. Technol., 2003, 24, 1041 CrossRef CAS PubMed.
- Z. Y. Yuan, T. Z. Ren and B. L. Su, Catal. Today, 2004, 93, 743–750 CrossRef.
- Y. Zhao, H. Jiangyong and H. Chen, J. Photochem. Photobiol., A, 2010, 212, 94–100 CrossRef CAS.
- M. Zhang, Z. Xu, J. Liang, L. Zhou and C. Zhang, Int. J. Environ. Sci. Technol., 2015, 12, 1669–1676 CrossRef CAS.
- Z. Xu, S. Bai, J. Liang, L. Zhou and Y. Lan, Mater. Sci. Eng., C, 2013, 33, 2192 CrossRef CAS PubMed.
- S. C. Ponce, C. Prado, E. Pagano, F. E. Prado and M. Rosa, Ecol. Eng., 2015, 74, 33–41 CrossRef.
- Q. Wang, X. Chen, K. Yu, Y. Zhang and Y. Cong, J. Hazard. Mater., 2013, 246–247, 135–144 CrossRef CAS PubMed.
- A. Idris, N. Hassan, N. S. Mohd Ismail, E. Misran, N. M. Yusof, A. F. Ngomsik and A. Bee, Water Res., 2010, 44, 1683–1688 CrossRef CAS PubMed.
- R. M. Cespón-Romero, M. C. Yebra-Biurrun and M. P. Bermejo-Barrera, Anal. Chim. Acta, 1996, 327, 37–45 CrossRef.
- A. E. Harvey Jr, J. A. Smart and E. S. Amis, Anal. Chem., 1955, 27, 26–29 CrossRef.
- X. Yan, Q. Hu, X. Liu and Z. Yan, Asia-Pac. J. Chem. Eng., 2012, 7, 598–603 CrossRef CAS.
- A. Miura, K. Nakazawa, T. Takei, N. Kumada, N. Kinomura, R. Ohki and H. Koshiyama, Ceram. Int., 2012, 38, 4677–4684 CrossRef CAS.
- D. Yang, A. Velamakanni, G. Bozoklu, S. Park, M. Stoller, R. D. Piner, S. Stankovich, I. Jung, D. A. Field and C. A. Ventrice Jr, Carbon, 2009, 47, 145–152 CrossRef CAS.
- A. Ganguly, S. Sharma, P. Papakonstantinou and J. Hamilton, J. Phys. Chem. C, 2011, 115, 17009–17019 CAS.
- A. N. Mansour and R. A. Brizzolara, Surf. Sci. Spectra, 1996, 4, 357–362 CrossRef CAS.
- H. Abdel-Samad and P. R. Watson, Appl. Surf. Sci., 1998, 136, 46–54 CrossRef CAS.
- Y. Zheng, Z. Zhang and C. Li, J. Mol. Catal. A: Chem., 2016, 423, 463–471 CrossRef CAS.
- M. Ding, B. H. W. S. D. Jong, S. J. Roosendaal and A. Vredenberg, Geochim. Cosmochim. Acta, 2000, 64, 1209–1219 CrossRef CAS.
- W. Qi, X. Shi, J. Xu, J. C. Crittenden, E. Liu, Z. Yi and Y. Cong, J. Hazard. Mater., 2016, 307, 213–220 CrossRef PubMed.
- K. Kim and W. Choi, Environ. Sci. Technol., 2011, 45, 2202 CrossRef CAS PubMed.
- Y. Ku and I. L. Jung, Water Res., 2001, 35, 135–142 CrossRef CAS PubMed.
- T. Papadam, N. P. Xekoukoulotakis, I. Poulios and D. Mantzavinos, J. Photochem. Photobiol., A, 2007, 186, 308–315 CrossRef CAS.
- W. D. Zhang, L. C. Jiang and J. S. Ye, J. Phys. Chem. C, 2009, 113, 16247–16253 CAS.
- A. Tytłak, P. Oleszczuk and R. Dobrowolski, Environ. Sci. Pollut. Res., 2015, 22, 5985–5994 CrossRef PubMed.
- V. K. Gupta, A. Rastogi and A. Nayak, J. Colloid Interface Sci., 2010, 342, 135–141 CrossRef CAS PubMed.
- A. K. Bhattacharya, T. K. Naiya, S. N. Mandal and S. K. Das, Chem. Eng. J., 2008, 137, 529–541 CAS.
- A. E. Nemr, J. Hazard. Mater., 2009, 161, 132–141 CrossRef PubMed.
- M. A. Atieh, Procedia Environ. Sci., 2011, 4, 281–293 CrossRef CAS.
- S. H. Huang and D. H. Chen, J. Hazard. Mater., 2009, 163, 174–179 CrossRef CAS PubMed.
- H. Jabeen, V. Chandra, S. Jung, J. W. Lee, K. S. Kim and S. B. Kim, Nanoscale, 2011, 3, 3583–3585 RSC.
- Z. Jia, Q. Wang, D. Ren and R. Zhu, Appl. Surf. Sci., 2013, 264, 255–260 CrossRef CAS.
- Z. Wu, S. Li, J. Wan and Y. Wang, J. Mol. Liq., 2012, 170, 25–29 CrossRef CAS.
- S. S. Chen, C. Y. Cheng, C. W. Li, P. H. Chai and Y. M. Chang, J. Hazard. Mater., 2007, 142, 362 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra06440c |
| This journal is © The Royal Society of Chemistry 2017 |