Open Access Article
Open Access ArticleSeparation of rare earths and other valuable metals from deep-eutectic solvents: a new alternative for the recycling of used NdFeB magnets†
Sofía Riaño a,
Martina Petranikova
a,
Martina Petranikova b,
Bieke Onghenaa,
Tom Vander Hoogerstraete
b,
Bieke Onghenaa,
Tom Vander Hoogerstraete a,
Dipanjan Banerjee
a,
Dipanjan Banerjee c,
Mark R. StJ. Foreman
c,
Mark R. StJ. Foreman b,
Christian Ekberg
b,
Christian Ekberg b and
Koen Binnemans
b and
Koen Binnemans *a
*a
aKU Leuven, Department of Chemistry, Celestijnenlaan 200F, 3001 Heverlee, Belgium. E-mail: Koen.Binnemans@kuleuven.be
bChalmers University of Technology, Nuclear Chemistry and Industrial Materials Recycling, Department of Chemistry and Chemical Engineering, SE-412 96 Gothenburg, Sweden
cDutch-Belgian Beamline (DUBBLE), ESRF – The European Synchrotron, CS 40220, F-38043 Grenoble Cedex 9, France
First published on 23rd June 2017
Abstract
Deep-eutectic solvents (DESs) are used as a promising alternative to aqueous solutions for the recovery of valuable metals from NdFeB magnets. A deep-eutectic solvent based on choline chloride and lactic acid (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was used for the leaching of rare earths and other metals from NdFeB magnets. A process for the separation of Fe, B and Co from Nd and Dy in the deep-eutectic solvent was developed by using the ionic liquid tricaprylmethylammonium thiocyanate (Aliquat 336 SCN, [A336][SCN]) diluted in toluene (0.9 M). The extraction parameters were optimized and stripping of B was efficiently carried out by HCl, while EDTA was employed for the recovery of Fe and Co. The separation of Nd and Dy was assessed by using two different types of extractants, a mixture of trialkylphosphine oxides (Cyanex 923) and bis(2-ethylhexyl)phosphoric acid (D2EHPA). Based on the distribution ratios, separation factors and the ease of subsequent stripping, Cyanex 923 was chosen as the most effective extractant. The purified Dy present in the less polar phase was easily recovered by stripping with water, while the Nd present in the deep-eutectic solvent was recovered by precipitation stripping with a stoichiometric amount of oxalic acid. Nd2O3 and Dy2O3 were recovered with a purity of 99.87% and 99.94%, respectively. The feasibility to scale up this separation process was corroborated by a setup of mixer-settlers and highlighted by the possibility to fully recover and reuse the deep-eutectic solvent and the less polar phases employed in the extractions. The new proposed system based on a deep-eutectic solvent combined with traditional organic extraction phases presented higher selectivities and efficiencies than the analogous aqueous system. Extended X-ray absorption fine structure (EXAFS) was employed to elucidate the different mechanisms for extraction of Co and Fe from the deep-eutectic solvent and from an aqueous solution.
2) was used for the leaching of rare earths and other metals from NdFeB magnets. A process for the separation of Fe, B and Co from Nd and Dy in the deep-eutectic solvent was developed by using the ionic liquid tricaprylmethylammonium thiocyanate (Aliquat 336 SCN, [A336][SCN]) diluted in toluene (0.9 M). The extraction parameters were optimized and stripping of B was efficiently carried out by HCl, while EDTA was employed for the recovery of Fe and Co. The separation of Nd and Dy was assessed by using two different types of extractants, a mixture of trialkylphosphine oxides (Cyanex 923) and bis(2-ethylhexyl)phosphoric acid (D2EHPA). Based on the distribution ratios, separation factors and the ease of subsequent stripping, Cyanex 923 was chosen as the most effective extractant. The purified Dy present in the less polar phase was easily recovered by stripping with water, while the Nd present in the deep-eutectic solvent was recovered by precipitation stripping with a stoichiometric amount of oxalic acid. Nd2O3 and Dy2O3 were recovered with a purity of 99.87% and 99.94%, respectively. The feasibility to scale up this separation process was corroborated by a setup of mixer-settlers and highlighted by the possibility to fully recover and reuse the deep-eutectic solvent and the less polar phases employed in the extractions. The new proposed system based on a deep-eutectic solvent combined with traditional organic extraction phases presented higher selectivities and efficiencies than the analogous aqueous system. Extended X-ray absorption fine structure (EXAFS) was employed to elucidate the different mechanisms for extraction of Co and Fe from the deep-eutectic solvent and from an aqueous solution.
Introduction
The recycling of rare-earth permanent magnets continues to be of high importance since it represents a promising strategy against the global supply crisis of rare-earth elements.1 During the last years, the environmental and economic benefits of NdFeB magnet recycling have been discussed, analyzed and investigated.2,3 Diverse methodologies have been proposed, including the use of mechano-chemical treatment without external heating,4 the recovery of neodymium from NdFeB magnets at high temperatures using molten magnesium metal as extractant5 and hydrogen gas to separate the NdFeB magnets from waste.6–9 As an alternative to these processes, rare earths and other valuable metals present in magnet scrap can be recovered and purified through leaching and solvent extraction.2,10–12 The separation and purification of metals by solvent extraction is a well-known strategy that has been used at laboratory and industrial scale for decades.13–20 To start the separation process, a leachate or a concentrate containing the metals of interest must be obtained. With this aim, strong mineral acids and bases, leaching agents such as cyanide or chelating agents are normally used to leach the metals into an aqueous solution that is later processed by solvent extraction.10,21–24 The metal ions are extracted into an organic phase that usually consists of an extractant, a molecular solvent, and if needed, a phase modifier (to avoid the formation of a third phase and/or to improve phase disengagement).16 In the cases where non-target metals are co-extracted together with the desired metals, scrubbing can be done to improve the purity of the previously obtained raffinate. The metal ions can be back extracted from the organic phase using an appropriate stripping agent. Afterwards, they can be precipitated and calcined to obtain metal oxides.In solvent extraction, attention has been paid to the replacement of commonly used organic solvents. New organic phases that provide new and tunable functionalities to improve the selectivity and efficiency of the extraction process are preferred, especially when they benefit workplace safety and are less harmful to the environment. With this aim, ionic liquids have been successfully employed as an organic phase in solvent extraction for the purification of metal ions, including transition metals and rare-earth elements.10–12,25–32 In contrast, less effort has been made in order to replace the aqueous phase by other liquid media able to facilitate the separation process. Deep-eutectic solvents (DESs) could act as a new functionalized, more polar phase for the leaching, solvent extraction, separation and purification of metal ions.33–36
DESs are systems formed from a eutectic mixture of Lewis or Brønsted acids and bases which can contain a variety of anionic and/or cationic species.35 DESs have been defined by Abbott et al.37 with the general formula R1R2R3R4N+X−·Y and can be classified into four different groups: (1) type I, in which Y corresponds to ZnCl2, SnCl2, AlCl3, GaCl3, FeCl3; (2) type II, in which hydrated metal halides are used as for instance Y = MClx·yH2O, M = Cr, Co, Cu, Ni, Fe; (3) type III, in which Y is an hydrogen bond donor (HBD) that can be an amide, alcohol or carboxylic acid; (4) type IV, in which DESs are composed of metal chlorides mixed with HBDs such us urea, ethylene glycol, acetamide or hexanediol. DESs are structurally different from ionic liquids (ILs) which are composed entirely by ions and usually contain only one type of anion and cation.35,38 Ionic liquids and DESs share some common physical properties such as low vapor pressure and non-flammability. Besides these properties, DESs are easier and cheaper to prepare than ionic liquids since their synthesis relies on the mixture of two components which is 100% atom economic (beneficial for their scaling up and industrial use). Moreover, DESs have excellent dissolution properties due to their ability to donate or accept electrons or protons to form hydrogen bonds.35,38 DESs are able to selectively dissolve various metal oxides depending on the deep eutectic solvent that is employed.33,39 This makes DESs good candidates for the dissolution of end-of-life NdFeB magnets. The composition of a NdFeB magnet varies depending on the type of application it is used for. Generally, cobalt is added to the magnets to increase the Curie temperature, while dysprosium and gadolinium are added when the magnets have to be used at high temperatures as these elements increase the coercivity of the magnet.40,41 Praseodymium is often present together with neodymium to reduce production costs since its complete separation from neodymium requires multiple steps and these two elements share similar magnetic properties.42
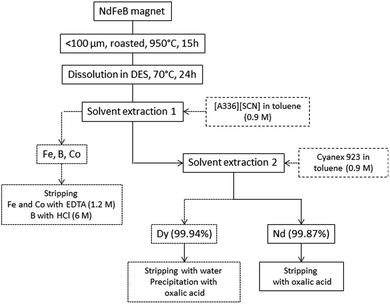
In this paper the preparation of a NdFeB magnet leachate with a deep-eutectic solvent (choline chloride and lactic acid, molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and a subsequent solvent extraction procedure for the separation of neodymium, dysprosium, iron, cobalt and boron from the leachate in two steps are reported for the first time. This DES was chosen because its components are easily available and the protons present in the lactic acid can react with the metal oxides to dissolve them. In the first step, the ionic liquid [A336][SCN] diluted in toluene (0.9 M) is employed for the extraction of iron, cobalt and boron. Afterwards, Cyanex 923 diluted in toluene (0.9 M) is employed for the separation of neodymium and dysprosium. One of the main advantages of this process is that the leaching can be carried out using a cheap and easily available solvent that can be re-used or easily discarded since its components are biodegradable. Moreover, higher distribution ratios are obtained when extracting from deep-eutectic solvent media than from conventional aqueous feeds. The system is versatile. First, only the transition metals and boron are separated and afterwards, dysprosium can be separated. Neodymium remaining in the deep-eutectic solvent can be easily stripped. The feasibility of the proposed extraction was tested in larger scale by using a mixer-settler setup.
2) and a subsequent solvent extraction procedure for the separation of neodymium, dysprosium, iron, cobalt and boron from the leachate in two steps are reported for the first time. This DES was chosen because its components are easily available and the protons present in the lactic acid can react with the metal oxides to dissolve them. In the first step, the ionic liquid [A336][SCN] diluted in toluene (0.9 M) is employed for the extraction of iron, cobalt and boron. Afterwards, Cyanex 923 diluted in toluene (0.9 M) is employed for the separation of neodymium and dysprosium. One of the main advantages of this process is that the leaching can be carried out using a cheap and easily available solvent that can be re-used or easily discarded since its components are biodegradable. Moreover, higher distribution ratios are obtained when extracting from deep-eutectic solvent media than from conventional aqueous feeds. The system is versatile. First, only the transition metals and boron are separated and afterwards, dysprosium can be separated. Neodymium remaining in the deep-eutectic solvent can be easily stripped. The feasibility of the proposed extraction was tested in larger scale by using a mixer-settler setup.
Experimental
Information concerning the chemicals and the equipment can be found in the ESI.†Synthesis of DES
Batches of approximately 1 liter of DES based on choline chloride and lactic acid as hydrogen bond donor were prepared by mixing choline chloride (1 molar eq.) and lactic acid (2 molar eq.) and shaking manually in a bottle until the mixture was completely homogeneous. Viscosity of the DES at 25 °C was 156 cP, water content: 13 wt%.Leaching
Two different samples of small NdFeB magnets (one round-shaped of approximately 25D × 10H mm and one rectangular-shaped of approximately 25W × 10H × 33L mm) were kindly provided by BEC Gesellschaft für Produktmanagement GmbH (Moers, Germany) and Magneti Ljubljana (Ljubljana, Slovenia), respectively. The magnets were demagnetized by heating them during 5 h at 300 °C in a furnace. Afterwards, they were crushed, milled and sieved to collect the fraction <400 μm from which a part was further ball-milled and sieved to obtain the <100 μm fraction. The chemical composition of the magnet samples was determined by carefully dissolving a sample of the magnet powder in concentrated hydrochloric acid and quantifying the solution with ICP-OES (Table S1, ESI†). For the roasting process, a magnet powder sample of 5 g was placed in a porcelain crucible without lid and heated at 950 °C in a muffle furnace during 15 h.Magnet samples of 100 mg were dissolved in 5 mL of deep-eutectic solvent and heated at 70 °C during 12 h, unless stated otherwise. Afterwards, the leachate was centrifuged and filtered. A sample of the leachate was diluted with 1 M HNO3 and the metal concentration was measured with ICP-OES. The solid residue was dissolved in 6 M HNO3 and quantified by ICP-OES.
Solvent extraction
The chloride ion in the ionic liquid Aliquat 336 was exchanged by a thiocyanate anion by equilibrating it three times with a 2.5 M KSCN solution. Afterwards, the thiocyanate ionic liquid was washed. For the solvent extraction experiments, a synthetic solution was prepared by dissolving NdCl3·6H2O, DyCl3·6H2O, CoCl2·6H2O, FeCl3 and H3BO3 in the DES and stirring the mixture while heating at 40 °C. The final concentrations ± the standard deviation were: 0.016 M for Nd, 0.122 M for Fe, 0.0054 M for B, 0.0007 M for Dy and 0.0026 M for Co. These concentrations mimic an average of the composition of different leachates of NdFeB magnets. For the removal of iron, cobalt and boron, batch experiments were carried out by contacting 1 mL of DES with 1 mL of the less polar phase [A336][SCN] diluted in toluene (0.9 M) in closed 4 mL vials. The vials were shaken at 25 °C and 2000 rpm during 20 min, unless otherwise is stated. Viscosity of the of the less polar phase [A336][SCN] diluted in toluene (0.9 M) at 25 °C was 6.1 cP. Batch experiments for the separation of Nd and Dy were carried out using a synthetic solution which was prepared by dissolving NdCl3·6H2O and DyCl3·6H2O in the deep-eutectic solvent (choline chloride and lactic acid, molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The final concentrations ± the standard deviation were 0.016 M for Nd and 0.0007 M for Dy. A sample of 1 mL of this solution was put in contact with 1 mL of less polar phase (Cyanex 923 or D2EHPA diluted in toluene 0.9 M unless otherwise is stated) and shaken during 20 min at 25 °C and 2000 rpm. For the stripping experiments, the loaded less polar phase was put in contact with 1 mL of stripping agent (different concentrations of HCl, oxalic acid, citric acid or EDTA) in 4 mL closed vials, and shaken during 60 min at 25 °C. A sample of the aqueous phase was taken, diluted and measured with ICP-OES.
2). The final concentrations ± the standard deviation were 0.016 M for Nd and 0.0007 M for Dy. A sample of 1 mL of this solution was put in contact with 1 mL of less polar phase (Cyanex 923 or D2EHPA diluted in toluene 0.9 M unless otherwise is stated) and shaken during 20 min at 25 °C and 2000 rpm. For the stripping experiments, the loaded less polar phase was put in contact with 1 mL of stripping agent (different concentrations of HCl, oxalic acid, citric acid or EDTA) in 4 mL closed vials, and shaken during 60 min at 25 °C. A sample of the aqueous phase was taken, diluted and measured with ICP-OES.
For comparison reasons, solvent extraction experiments from aqueous solutions containing 0.015 M for Nd, 0.114 M for Fe, 0.0061 M for B, 0.0006 M for Dy and 0.0025 M for Co were carried out. CaCl2 was used as source of chlorides and its concentration in the aqueous phase was 1.5 M, unless otherwise stated. For the separation of Nd and Dy the concentrations in the aqueous phase were 0.015 M for Nd and 0.0007 M for Dy. The extraction was carried out in 4 mL vials at 25 °C and 2000 rpm during 60 min to assure that equilibrium was achieved.
A counter-current mixer-settler system comprised of five extraction units (PVDF) was used to evaluate the scaling up feasibility of the developed process for the separation of iron, cobalt and boron from neodymium and dysprosium and subsequently dysprosium from neodymium. The mixer-settler unit consists of: (a) the mixing chamber, where the less polar phase and the more polar phase are mixed using a motor stirrer, (b) the settling chamber, where the phases are separated by density difference and (c) a more polar phase outlet compartment, from which the more polar phase exits the unit.43 The more and less polar phases were pumped into the mixer-settlers using electromagnetic pumps (Iwaki). Each mixer-settler unit has a volume of 120 mL per unit (MYMEKO, Sweden).
The light phase![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) heavy phase ratio (LP
heavy phase ratio (LP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HP) was 1
HP) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The light phase corresponds to 0.9 M [A336][SCN] or Cyanex 923 in toluene, while the heavy phase corresponds to the DES chlorine chloride
1. The light phase corresponds to 0.9 M [A336][SCN] or Cyanex 923 in toluene, while the heavy phase corresponds to the DES chlorine chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid (molar ratio 1
lactic acid (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The flow rates for the deep-eutectic solvent and organic feeds were 1.5 mL min−1, the mixing speed in the mixer chambers was 900 rpm. For the separation of dysprosium from neodymium, the LP
2). The flow rates for the deep-eutectic solvent and organic feeds were 1.5 mL min−1, the mixing speed in the mixer chambers was 900 rpm. For the separation of dysprosium from neodymium, the LP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HP phase ratio was 2
HP phase ratio was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The flow rates for the deep eutectic solvent and organic feeds were 3 and 1.5 mL min−1, respectively, the mixing speed in the mixer chambers was 900 rpm.
1. The flow rates for the deep eutectic solvent and organic feeds were 3 and 1.5 mL min−1, respectively, the mixing speed in the mixer chambers was 900 rpm.
EXAFS
Extended X-ray Absorption Fine Structure (EXAFS) spectra of the Fe K-edge (7112 eV) and Co K-edge (7709 eV) were collected at the Dutch-Belgian Beamline (DUBBLE, BM26A) at the European Synchrotron Radiation Facility (ESRF) in Grenoble (France). The energy of the X-ray beam was tuned by a double-crystal monochromator operating in fixed-exit mode using a Si(111) crystal pair. The measurements were done in transmission mode using ionization chambers filled with Ar/He gas at ambient pressure. A brass sample holder with Kapton® windows and a flexible polymeric spacer (VITON®) with a thickness of 2 mm was used as a sample holder. Samples of the less polar phase (0.9 M [Aliquat 336][SCN]) were taken after equilibrating it with solutions of approximately 0.09 M of Fe and 0.08 M of Co in DES. For the aqueous solutions the same concentrations of Fe and Co were employed and the total concentration of CaCl2 was 5 M. Standard procedures were used for pre-edge subtraction and data normalization in order to isolate the EXAFS function (χ). The isolated EXAFS oscillations were accomplished by a smoothing spline as realized in the program VIPER.44 The data were fitted using the ab initio code FEFF 7.0 which was used to calculate the theoretical phase and amplitude functions that subsequently were used in the non-linear least-squares refinement of the experimental data.45 The chloride concentration in both phases was determined with a bench top total reflection X-ray fluorescence (TXRF) spectrometer (S2 Picofox, Bruker). X-ray diffraction powder patterns were recorded at room temperature with a Seifert 3003 T/T X-ray diffractometer equipped with a scintillation detector. X-ray type: Cu Kα operating at 40 kV and 40 mA, scanning range: 10–80° (2θ), step width: 0.02°, step scan: 2.00 s. The X-ray diffractograms were processed by “X'pert HighScore Plus” PANalytical software with ICDD database.Results and discussion
Dissolution
For the dissolution experiments, two NdFeB magnets were tested. Both magnets contained four different rare earths, Nd, Dy, Pr and Gd. Magnet 2 contained higher amounts of Gd and Pr but a lower amount of Dy compared to magnet 1.The percentage metal leached (% L) was calculated as follows,
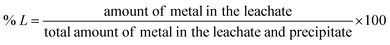 | (1) |
To the best of our knowledge, the solubility of rare-earth oxides has not been reported in any DESs yet. Magnet samples (<100 μm particle size) were roasted and leached using three different deep-eutectic solvents based on choline chloride (ChCl), ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea, ChCl
urea, ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylene glycol and ChCl
ethylene glycol and ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid, all of them with a molar ratio 1
lactic acid, all of them with a molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. High % L were obtained when using the deep-eutectic solvent composed of ChCl
2. High % L were obtained when using the deep-eutectic solvent composed of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid (1
lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and no significant deviations were obtained between both roasted magnets. The mixtures ChCl
2) and no significant deviations were obtained between both roasted magnets. The mixtures ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1
urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and ChCl
2) and ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylene glycol (1
ethylene glycol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) allowed the poor dissolution of Nd and Fe and did not dissolve any of the other metal oxides from the roasted magnets. Since physicochemical properties of deep-eutectic solvents depend on the kind of moieties and substituents present in the mixture, the high solubility of the oxides in the choline chloride
2) allowed the poor dissolution of Nd and Fe and did not dissolve any of the other metal oxides from the roasted magnets. Since physicochemical properties of deep-eutectic solvents depend on the kind of moieties and substituents present in the mixture, the high solubility of the oxides in the choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid (1
lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) DES can be explained by the fact that the protons present in the lactic acid react with the oxides to form water (eqn (2)) and probably the coordinating abilities of lactic acid and the choline help dissolution.
2) DES can be explained by the fact that the protons present in the lactic acid react with the oxides to form water (eqn (2)) and probably the coordinating abilities of lactic acid and the choline help dissolution.
| Nd2O3(s) + 6CH3CHOHCOOH(DES) → 2Nd(CH3CHOHCOO)3(DES) + 3H2O(DES) | (2) |
Since the best results were obtained with the DES based on choline chloride and lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), it was decided to carry out the dissolution of metals from the NdFeB magnets with this solvent. The effect of the liquid
2), it was decided to carry out the dissolution of metals from the NdFeB magnets with this solvent. The effect of the liquid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solid (L/S) ratio on the two different roasted magnet powders was investigated (Fig. 1).
solid (L/S) ratio on the two different roasted magnet powders was investigated (Fig. 1).
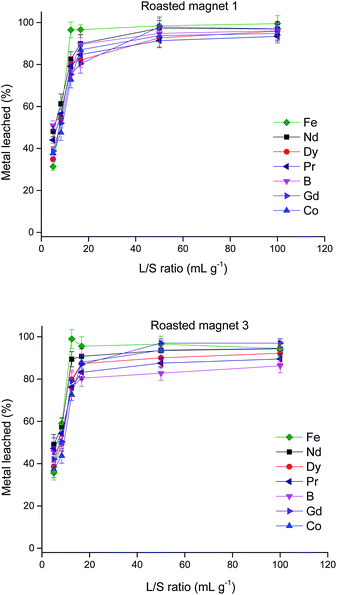 | ||
Fig. 1 Effect of the liquid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solid ratio (L/S) on the percentage metal leached from two different roasted magnet powders (<100 μm particle size), 70 °C leaching temperature, 24 h leaching duration. solid ratio (L/S) on the percentage metal leached from two different roasted magnet powders (<100 μm particle size), 70 °C leaching temperature, 24 h leaching duration. | ||
As expected, the percentage of metal leached increased when using L/S ratios larger than 20 mL g−1. When trying to dissolve the magnet using lower quantities of DES, low % L values were obtained. This can be explained because of the poor agitation due to the low amount of liquid and the large amount of solid, which probably makes it difficult for the DES to reach all the metals in the magnet powder. At high L/S ratios, all metals were dissolved almost completely. No selectivity was observed at any ratio and there were not significant differences when using magnets with different compositions.
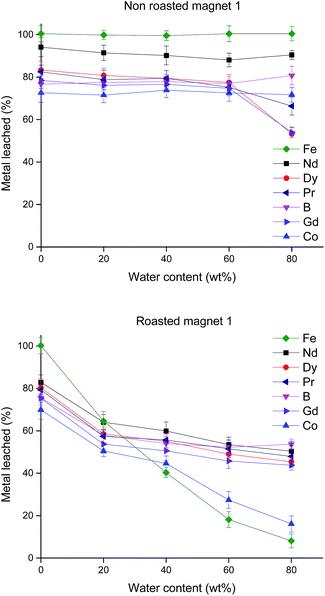
The effect of the water content in the DES choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid (1
lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) on the percentage of metal leached was evaluated (Fig. 2).
2) on the percentage of metal leached was evaluated (Fig. 2).
The effect of roasting the magnets was investigated by comparing the leaching of non-roasted and roasted magnets. The magnet powders (<100 μm particle size) were roasted at 950 °C before dissolving them in the DES at 70 °C during 24 h. In the case of non-roasted magnets (<100 μm particle size), the dissolution was started by contacting the non-roasted magnets with DES. We point out as a safety note that the addition of non-roasted magnet to the DES should be done slowly in an open vial since evolution of hydrogen can take place (eqn (3)).
| 2Nd(s) + 6CH3CHOHCOOH(DES) → 2Nd(CH3CHOHCOO)3(DES) + 3H2(g) | (3) |
For the dissolution of the non-roasted magnet with the DES choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid (1
lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), an increase in water content (up to 60 wt%) had no significant effect on the percentage metal leached. As expected, no dissolution of metals occurred when pure water was used.
2), an increase in water content (up to 60 wt%) had no significant effect on the percentage metal leached. As expected, no dissolution of metals occurred when pure water was used.
For the leaching of the roasted magnets, selectivity can be achieved when adding water to the DES (Fig. 2). During the roasting process, different kinds of metal oxides such as Fe2O3, NdBO3 and NdFeO3 are formed.46 In the same way as explained in eqn (2), lactic acid reacts with the corresponding oxides to form water (eqn (4)):
| Fe2O3 + 6CH3CHOHCOOH(DES) → 2Fe(CH3CHOHCOO)3(DES) + 3H2O | (4) |
In the case of non-roasted magnets, iron is released into the DES as iron(II), as follows,
| Fe(s) + 2CH3CHOHCOOH(DES) → Fe(CH3CHOHCOO)2(DES) + H2(g) | (5) |
Iron(II) is more stable against hydrolysis than iron(III), thus, in the case of non-roasted magnets, no selectivity can be achieved since iron(II) can go in solution as long as there is not an oxidizing environment. In the case of roasted magnets (eqn (4)), as the lactic acid is consumed and water is produced, the pH increases allowing the hydrolysis of iron(III) and precipitation of Fe(OH)3. The advantage of such situation is not only the selectivity but also that the eutectic mixture is not decomposed since no redox reactions occur upon dissolution of the roasted magnets. The dissolution of magnets in mixtures of water and DESs is promising and might result after optimizing some leaching parameters (e.g. kinetics, temperature, nature of the carboxylic acid in the DES) in leachates containing mostly rare earths. Still, since the highest % L was achieved with the DES choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid (1
lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) without the addition of water, this DES was employed as the more polar phase for the further solvent extractions.
2) without the addition of water, this DES was employed as the more polar phase for the further solvent extractions.
Solvent extraction
The leachate from the roasted magnet produced with the DES choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid (1
lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) without water was employed as the more polar phase for solvent extraction. Foreman has studied the solvent extraction of transition metals and lanthanides from DES using Aliquat 336 and D2EHPA diluted in an aromatic solvent and a saturated aliphatic hydrocarbon respectively.36 Since the recovery of metals from NdFeB magnets using DES has not been widely explored yet, it was decided to carry out the separation of only the most typical elements present in NdFeB magnets (i.e. Nd, Dy, Fe, B, Co) through solvent extraction.
2) without water was employed as the more polar phase for solvent extraction. Foreman has studied the solvent extraction of transition metals and lanthanides from DES using Aliquat 336 and D2EHPA diluted in an aromatic solvent and a saturated aliphatic hydrocarbon respectively.36 Since the recovery of metals from NdFeB magnets using DES has not been widely explored yet, it was decided to carry out the separation of only the most typical elements present in NdFeB magnets (i.e. Nd, Dy, Fe, B, Co) through solvent extraction.
To evaluate and quantify the efficiency of the solvent extraction, parameters such as, percentage extraction (% E), distribution ratio (D) and separation factor (SFA,B) were evaluated. The percentage extraction (% E) is defined as the initial amount of metal ion in the more polar phase ([M]i) minus the amount of metal ion in the more polar phase after extraction ([M]f) over the initial amount of metal ion ([M]i). In case of equal volumes it can be expressed as:
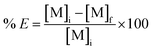 | (6) |
The distribution ratio (D) of a metal is defined in eqn (7) as the ratio of its concentration in the less polar phase by its concentration in the more polar phase ([M]f) after extraction and phase separation.
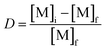 | (7) |
The separation factor (SFA,B) between two metals is the ratio of the distribution ratios of the metals A and B, where A and B are chosen so that SF > 1 (eqn (8)).
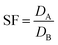 | (8) |
Ionic liquids were chosen as the less polar phase for the solvent extraction because they are effective extractants for metal ions.10–12,26,28,31,32 Since the available mixer settlers operated exclusively at room temperature, ionic liquids were diluted with organic solvents to avoid longer equilibration times due to the relatively high viscosities. Toluene and Solvent 70 (one of the fractions of kerosene) were tested because they are very common diluents for solvent extraction systems.47–54 Despite the fact that toluene is not a green solvent,55 it was chosen as diluent since it allowed higher separation factors as well as faster and better phase disengagements in comparison to solvent 70. Besides this, no problems with third phase formation were encountered when using toluene.
Table 1 shows the results obtained for the extraction of Nd, Fe, B, Dy and Co from a synthetic solution, mimicking the concentrations of a leachate of NdFeB magnet, where two ionic liquids (Aliquat 336 with two different anions, chloride and thiocyanate, diluted in toluene) were employed.
| D for [A336][Cl] in toluene (0.7 M) | D for [A336][SCN] in toluene (0.7 M) | |
|---|---|---|
| a Shaking time: 60 min, 2000 rpm, 25 °C. Each value represents the average of three measurements. Concentrations in the aqueous phase: 16 mM Nd, 122 mM Fe, 5.4 mM B, 0.7 mM Dy and 2.6 mM Co. | ||
| Nd | 0.041 ± 0.009 | 0.029 ± 0.006 |
| Fe | 187 ± 6 | 47.8 ± 0.7 |
| B | 2.73 ± 0.30 | 0.78 ± 0.08 |
| Dy | 0.023 ± 0.005 | 0.026 ± 0.007 |
| Co | 0.74 ± 0.07 | 192 ± 10 |
Both [A336][SCN] and [A336][Cl] extracted Fe and higher distribution ratios were obtained for B when [A336][Cl] was employed, while [A336][SCN] allowed a more efficient extraction of Co. In both cases, there was almost no extraction of the rare earths. Since in general, high D were obtained for Co with less co-extraction of the rare earths when using [A336][SCN], this extractant was chosen to carry out the first separation stage.
In terms of equilibration time, it was found that a period between 15 and 20 min was adequate to reach equilibrium. An equilibration time of 20 min was chosen as the optimal time to make sure that equilibrium was reached. This relatively long equilibration time is needed because of the slow mass transfer process occurring under the operating conditions.
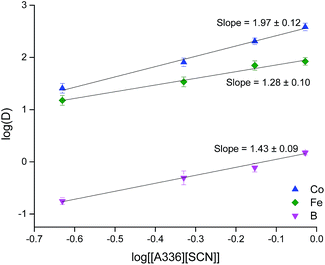
Dilutions of [A336][SCN] in toluene were prepared in order to optimize the concentration of the extractant. Poor phase mixing and slow phase disengagement were observed when working with concentrations of [A336][SCN] higher than 0.9 M. A concentration of 0.9 M of [A336][SCN] in toluene was chosen as optimal since it allowed the highest D for B, Fe and Co without facing problems with viscosity. It is important to notice that little if any extraction of the rare earths during this step (Fig. 3).
As chloride is a good inner-sphere ligand it is possible to generate extractable chlorometallate complexes as expressed as follows,
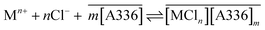 | (9) |
The equilibrium constant can be expressed as
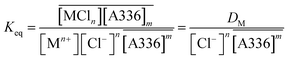 | (10) |
By plotting log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D vs. log[A336] when the chloride concentration is constant, it is possible to determine the number of molecules of ionic liquid that are involved in the extraction of the metal ion (eqn (11)).
D vs. log[A336] when the chloride concentration is constant, it is possible to determine the number of molecules of ionic liquid that are involved in the extraction of the metal ion (eqn (11)).
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D = m D = m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[A336] + n log[A336] + n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[Cl−] + log log[Cl−] + log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Keq Keq
| (11) |
It can be seen that the distribution ratios for B, Fe and Co increase with the [A336][SCN] concentration. The distribution ratios of Co and Fe are in concordance with what has been reported previously for the extraction of these metals from the same DES.36 The stoichiometric ratios between Fe, B and Co and the extractant were determined by plotting the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D vs. log[[A336][SCN]] and then doing the linear regression to determine the stoichiometric ratio between the metal ion and the extractant (Fig. 3). The results show that the slope values are close to 2 for Co and B and 1 for Fe, this would suggest that the complexes formed could correspond to [FeCl4][A336] and [CoCl4][A336]2. In the case of boron, a value of 1.43 was obtained for the slope, indicating that most likely more than one kind of complex is formed. It is known that boric acid can form 1
D vs. log[[A336][SCN]] and then doing the linear regression to determine the stoichiometric ratio between the metal ion and the extractant (Fig. 3). The results show that the slope values are close to 2 for Co and B and 1 for Fe, this would suggest that the complexes formed could correspond to [FeCl4][A336] and [CoCl4][A336]2. In the case of boron, a value of 1.43 was obtained for the slope, indicating that most likely more than one kind of complex is formed. It is known that boric acid can form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 anionic complexes with lactic acid, [B(OH)2(C4H5O3)]− or [B(C4H5O3)2]−.56 In the presence of an excess of lactic acid, the complex [B(C4H5O3)2]− would be predominant. This complex could be extracted by the ionic liquid present in the less polar phase as [B(C4H5O3)2][A336]. However, estimations by slope analysis do not give complete information about the structure of the extracted metal complex. EXAFS was used to determine the environment of the extracted complexes of Fe and Co and the results will be discussed in the section EXAFS.
2 anionic complexes with lactic acid, [B(OH)2(C4H5O3)]− or [B(C4H5O3)2]−.56 In the presence of an excess of lactic acid, the complex [B(C4H5O3)2]− would be predominant. This complex could be extracted by the ionic liquid present in the less polar phase as [B(C4H5O3)2][A336]. However, estimations by slope analysis do not give complete information about the structure of the extracted metal complex. EXAFS was used to determine the environment of the extracted complexes of Fe and Co and the results will be discussed in the section EXAFS.
Since industrial processes using ambient temperature are usually less expensive and preferred over those that use high temperatures, the effect of the temperature was not studied. However, it must be stressed that working at higher temperatures could help to reduce the viscosity of the system and thus, to increase the mass transfer of the extraction process. Additionally, it could also allow the use of the ionic liquid in its undiluted form, which will possibly increase the extraction of boron.
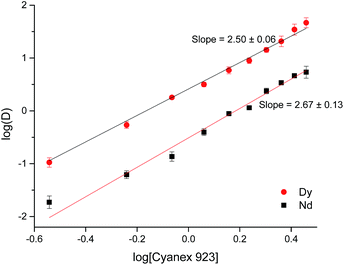
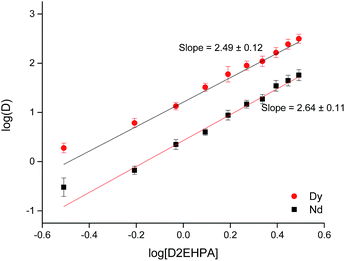
After the removal of Fe, Co and B (i.e. by countercurrent solvent extraction in mixer settlers), a process for the separation of Nd and Dy present in the DES was developed. Two conventional extractants were tested: Cyanex 923 and D2EHPA. D2EHPA is a well-known acidic extractant that is widely employed for the separation of rare earths due to its high selectivity and extractant capacity.16,57 Cyanex 923 is a solvating extractant that has been widely used for the separation of rare earths in nitrate media.16,57 Table 2 shows the distribution ratios of Nd and Dy when extracted from the DES using Cyanex 923 and D2EHPA.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)a
2)a
| DNd | DDy | |
|---|---|---|
| a Shaking speed: 2000 rpm, equilibration time: 60 min, 25 °C. | ||
| Cyanex 923 (0.9 M in toluene) | 0.14 ± 0.07 | 1.79 ± 0.10 |
| D2EHPA (0.9 M in toluene) | 2.23 ± 0.09 | 28.85 ± 2.53 |
D2EHPA allowed the obtention of higher distribution ratios than Cyanex 923 but the separation factors were similar, SFDy/Nd = 12.93 and 12.79 for D2EHPA and Cyanex 923 respectively. Since solvent extraction processes must not be analyzed only in terms of their distribution ratios, but also considering the stripping procedure and their feasibility to be scaled up, it was decided to study both systems to compare them and choose the most adequate one. The kinetics of extraction of both systems was studied at 25 °C and in both cases, the equilibrium is reached somewhere in between 5 and 10 min. Taking this into account, 15 min was chosen as the optimal time to carry out further experiments.
The next parameter investigated was the effect of the concentration of extractant in the less polar phase. In both cases the distribution ratios for both rare-earth metals increase as the concentration of extractant increases (Fig. 4 and 5). Concentrations of extractant higher than 0.9 M resulted in higher distribution ratios but also in a loss of the selectivity.
In general, the extraction of rare earths by organophosphorous acids, such as D2EHPA, can be expressed as follows,16
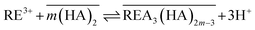 | (12) |
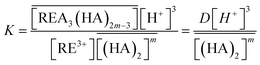 | (13) |
Taking logarithm on both sides, the following equation is obtained,
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D = −3 D = −3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[H+] + m log[H+] + m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[(HA)2] + log log[(HA)2] + log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K(HA)2 K(HA)2
| (14) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D against log[(HA)2] should give a straight line with a slope of the value of m.
D against log[(HA)2] should give a straight line with a slope of the value of m.
On the other hand, the extraction reaction using Cyanex 923 can be expressed by eqn (15),
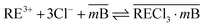 | (15) |
The equilibrium constant can be written as,
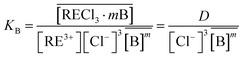 | (16) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D = 3 D = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[Cl−] + m log[Cl−] + m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[B] + log log[B] + log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KB KB
| (17) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D against log[B] should give a slope of the value of m.
D against log[B] should give a slope of the value of m.
From Fig. 4 and 5 it can be observed that the slopes had values close to 2.5 for both Nd and Dy extracted from DES with Cyanex 923 or D2EHPA. The slopes are non-integer values, indicating that more than one type of complex is involved in the extraction or that there is a molecular aggregation of the extractants in the solvent. These slopes are in concordance with what has been published previously by other authors.16,58–62 Cyanex 923 has been widely used in the extraction of rare earths from nitrate media.62–64 In general, solvating extractants have been mostly used when extracting from nitrate media because they offer higher distribution ratios and separation factors than from chloride media.65 Therefore, the use of solvating extractants to extract rare earths from chloride media has been barely reported.62,65–67 When using Cyanex 923 to extract rare earths from chloride media, the distribution ratios for heavy rare earths were higher than those for light rare earths, which is in agreement with what has been found in this work for the extraction of Nd and Dy from DES and also with other works on the extraction of REEs with Cyanex 923 from different aqueous feeds.60–62
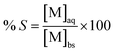 | (18) |
The stripping of Fe, Co and B from the less polar phase consisting of the ionic liquid [A336][SCN] diluted in toluene (0.9 M) was studied. Diluted and concentrated acids were employed as well as NaOH, EDTA and citric acid (Table 3).
| Stripping agent | Concentration (M) | DFe ± SD (% S) | DB ± SD (% S) | DCo ± SD (% S) |
|---|---|---|---|---|
| a Equilibration time 60 min, 25 °C, 2000 rpm.b Not detected (below detection limit). In the cases where D are higher than 200, the value of the % S can be considered as very close to zero. | ||||
| NaOH | 8.0 | 0.66 ± 0.02 (60.3) | 2.20 ± 0.13 (31.2) | 0.47 ± 0.01 (68.2) |
| 2.0 | 1.18 ± 0.04 (45.8) | n.d.b | 3.95 ± 0.33 (20.2) | |
| 1.0 | 1.31 ± 0.02 (43.2) | n.d.b | 13.5 ± 1.6 (6.9) | |
| 0.5 | 3.78 ± 0.11 (20.9) | n.d.b | >200 | |
| HCl | 6.0 | >200 | 0.32 ± 0.01 (75.8) | 122 ± 8 (0.81) |
| 3.0 | >200 | 0.53 ± 0.02 (65.2) | 107 ± 10 (0.92) | |
| 2.0 | 42.5 ± 7 (0.26) | 0.90 ± 0.05 (52.4) | 82.3 ± 6.2 (1.2) | |
| 1.0 | 26.8 ± 2.9 (1.6) | 1.20 ± 0.06 (45.5) | 17.9 ± 6.1 (5.3) | |
| EDTA | 1.2 | 0.03 ± 0.01 (98.7) | n.d.b | 0.03 ± 0.01 (99.1) |
| 0.4 | 1.44 ± 0.03 (40.9) | n.d.b | 1.35 ± 0.03 (42.5) | |
| 0.1 | 2.52 ± 0.12 (28.4) | n.d.b | 61.5 ± 1.5 (1.6) | |
| 0.05 | 5.13 ± 0.28 (16.3) | n.d.b | >200 | |
| Citric acid | 0.4 | 8.43 ± 0.23 (10.6) | n.d.b | 29.3 ± 3.7 (3.3) |
The use of NaOH as stripping agent allowed precipitation stripping of Fe and Co. B was only back-extracted to the aqueous phase when using high concentrations of NaOH (8 M). It has been reported that at very high concentrations of NaOH or KOH, B can be stripped by the formation of Na2B4O7 and K2B4O7 at high pH.68 One disadvantage of this method is the formation of the non-coarse precipitate of hydroxides of iron and cobalt that is difficult to filter, besides the fact that high concentrations of NaOH are required to precipitate all the metals. HCl was also tested but it was not a good stripping agent for Fe(III) and Co(II) due to the strong complexes formed between the thiocyanate ions and these metal ions. When HCl was used as stripping agent, Fe and Co were not back-extracted, however, B could easily be stripped since it is less strongly extracted. As expected, EDTA was a good complexing agent for stripping Fe and Co, but a poor one for B. Citric acid was not a better alternative than EDTA because the stability constants for the formation of the complexes are smaller with citric acid (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KCo = 4.4 and log
KCo = 4.4 and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KFe = 11.8) than with EDTA (log
KFe = 11.8) than with EDTA (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KCo = 16.21 and log
KCo = 16.21 and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KFe = 14.3).69,70 To achieve complete stripping, the solution can be treated with 6 M HCl to remove B, followed by a treatment with 1.2 M EDTA to remove Fe and Co.
KFe = 14.3).69,70 To achieve complete stripping, the solution can be treated with 6 M HCl to remove B, followed by a treatment with 1.2 M EDTA to remove Fe and Co.
After the removal of Fe, Co and B, Dy can be separated from Nd by using either Cyanex 923 or D2EHPA diluted in toluene (0.9 M) as previously discussed. Since Nd is one of the main components of the magnet, it is present in the leachate in higher concentrations (i.e. 0.016 M) than Dy (i.e. 0.001 M). Therefore, Nd is also co-extracted during the removal of Dy using the above mentioned extractants. To remove the Nd that is co-extracted with Dy to the less polar phase, a scrubbing procedure is necessary. CaCl2 at different concentrations was tested as scrubbing agent (Table 4).
| CaCl2 (M) | DNd ± SD | DDy ± SD |
|---|---|---|
| a 25 °C, 2000 rpm, 40 min. Concentrations in the less polar phase: 0.003 M for Nd and 0.002 M for Dy (concentrations of the raffinate obtained from the mixer settler). | ||
| 5.6 | 0.64 ± 0.06 | >200 |
| 4.2 | 0.73 ± 0.10 | 57.8 ± 3.8 |
| 2.8 | 1.05 ± 0.07 | 3.69 ± 0.13 |
| 1.4 | 0.65 ± 0.07 | 0.38 ± 0.06 |
| 0.7 | 0.45 ± 0.12 | 0.16 ± 0.09 |
From Table 4 it can be seen that no selectivity can be achieved at very low concentrations of CaCl2 since both Nd and Dy are stripped. However, selectivity can be achieved when working at higher concentrations of CaCl2 because the presence of chloride anions increases the distribution of heavy rare earths towards the less polar phase. When D2EHPA was used as extractant, this scrubbing step with CaCl2 could not be carried out since the metals are not easily stripped by water and the presence of high chloride concentrations favours their distribution in the less polar phase. Thus, 5.6 M CaCl2 was selected as scrubbing agent and was employed to purify the Dy-rich less polar phase obtained after running the mixer-settlers using 0.9 M Cyanex 923 in toluene. No co-extraction of Ca was observed when using Cyanex 923 as extractant. The stripping of Dy was evaluated using different stripping agents and the results are summarized in Table 5.
| Stripping agent | DDy from Cyanex 923 in toluene 0.9 M (% S) | DNd from D2EHPA in toluene 0.9 M (% S) |
|---|---|---|
| a Equilibration time: 40 min, 25 °C, 2000 rpm. Concentration of Dy in the less polar phase after the scrubbing: 0.002 M. In the cases where D are higher than 200, the value of the % S can be considered as very close to zero. | ||
| Water | 0.016 ± 0.007 (99.4) | >200 |
| 0.5 M HCl | 0.012 ± 0.010 (99.6) | 0.31 ± 0.09 (75.9) |
| 0.1 M citric acid | 0.019 ± 0.009 (99.0) | >200 |
| 0.1 M EDTA | 0.24 ± 0.03 (81.0) | >200 |
From Table 5, a clear difference in the stripping behaviour of the two systems is observed since the D were lower for all the stripping agents that were used when stripping from the Cyanex 923 in comparison to stripping from the D2EHPA phase. This can be explained because of the high affinity of the acidic extractant D2EHPA for the rare earths. With this extractant very high distribution ratios can be achieved for heavy rare earths, but the stripping is difficult. Because of the easiness of scrubbing and stripping when Cyanex 923 is used, Cyanex 923 was chosen as optimal extractant for the separation of Dy from Nd and water was selected as stripping agent. Once Dy was removed from the DES phase (i.e. by counter-current extraction), it was necessary to find a way to strip Nd from the DES. With this aim, precipitation stripping with oxalic acid was explored. Different amounts of oxalic acid were contacted with the DES and then stirred at 25 °C and 2000 rpm during 25 min. After this time, the precipitate was removed and the aqueous phase quantified by ICP-OES. A stoichiometric amount of oxalic acid was needed to precipitate all Nd present in the DES, as stated in eqn (19). Using an excess of oxalic acid is not recommended since the deep eutectic solvent choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxalic acid can be formed (Fig. S1†).
oxalic acid can be formed (Fig. S1†).
| 2Nd3+ + 3H2C2O4 → Nd2(C2O4)3 + 6H+ | (19) |
Separation by mixer-settlers
The feasibility of scaling up the extraction processes was tested using small mixer-settlers. Besides determining the extractant, the composition of the less polar phases, the contact time, the stripping agent and evaluating other parameters such as phase disengagement, the number of stages needed for the separation of Fe, B and Co from Nd and Dy was also determined. McCabe Thiele diagrams showing the loading of Fe, Co and B at different light phase![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) heavy phase ratios (LP
heavy phase ratios (LP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HP) from a solution containing all the metals (Fe, B, Co, Nd and Dy) were constructed. It was estimated that one stage was needed for Fe and Co extraction, approximately three for B extraction when using an LP
HP) from a solution containing all the metals (Fe, B, Co, Nd and Dy) were constructed. It was estimated that one stage was needed for Fe and Co extraction, approximately three for B extraction when using an LP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HP ratio of 1
HP ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. At a LP
1. At a LP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HP ratio of 2
HP ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 one stage is needed for Fe and Co and two for B (Fig. S2–S4†). In total, three stages would be necessary for the complete extraction of Fe, Co and B from Nd and Dy. However two more stages were put as precaution in case more stages were needed due to the relative high viscosity of the more polar phase.
1 one stage is needed for Fe and Co and two for B (Fig. S2–S4†). In total, three stages would be necessary for the complete extraction of Fe, Co and B from Nd and Dy. However two more stages were put as precaution in case more stages were needed due to the relative high viscosity of the more polar phase.
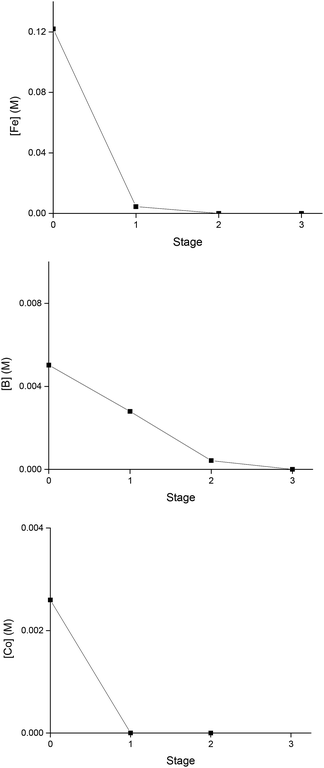
The extraction behaviour of Fe, Co and B in the mixer-settler is presented in Fig. 6. Every hour a sample was taken from each extraction chamber throughout the day and analysed by ICP-OES. The extraction system showed a good stability over time, with minor variations in metal concentrations between the samples. In the first stage, the concentration of Fe and Co in the DES decreased considerably. On the other hand, B required three stages to be completely recovered from the DES. During the whole time of operation, no precipitate or third phase formation was observed. The experimental data obtained with the mixer-settlers showed that the fourth and fifth stages were not needed since at those stages Fe, Co and B were not detected in the more polar phase.
After the removal of Fe, Co and B, Nd and Dy remained in the DES solution. As it was previously discussed, the extractant, its concentration, the kinetics and the stripping agent were evaluated. Cyanex 923 (0.9 M in toluene) was selected as the less polar phase to carry out the separation of Dy from Nd in the mixer settlers since good separation factors were achieved, the stripping could be carried out efficiently using water and the system presented fast and good phase disengagement (important when running mixer settlers). Fig. S5 in the ESI† shows the extraction isotherm of Dy at different LP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HP ratios from an eutectic mixture of choline chloride and lactic acid (molar ratio 1
HP ratios from an eutectic mixture of choline chloride and lactic acid (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) containing only Nd and Dy. It was estimated that approximately three stages were needed when working at an LP
2) containing only Nd and Dy. It was estimated that approximately three stages were needed when working at an LP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HP phase ratio of 1
HP phase ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and two stages when using an LP
1 and two stages when using an LP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HP phase ratio of 2
HP phase ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. An LP
1. An LP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HP phase ratio of 2
HP phase ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was chosen since it allows the less number of stages. No formation of a third phase, precipitate or gel was observed at any point of the separation study. One extra stage was added to the system in order to assure complete extraction of the Dy in the case that two stages were not enough when testing the separation system in the mixer settlers.
1 was chosen since it allows the less number of stages. No formation of a third phase, precipitate or gel was observed at any point of the separation study. One extra stage was added to the system in order to assure complete extraction of the Dy in the case that two stages were not enough when testing the separation system in the mixer settlers.
The extraction behaviour of Dy is presented in Fig. 7. Every hour a sample was taken from each extraction chamber throughout the day and analysed with ICP-OES. The extraction showed good stability over time, with minor variations in metal concentrations between the samples. In the first stage, the concentration of Dy in the more polar phase was decreased by almost the half and on the second stage it was close to 0 M. No Dy was detected on the third stage. The experimental data with the mixer settlers showed that two stages, when working at an LP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HP phase ratio of 2
HP phase ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were needed to successfully separate Dy from the Nd.
1 were needed to successfully separate Dy from the Nd.
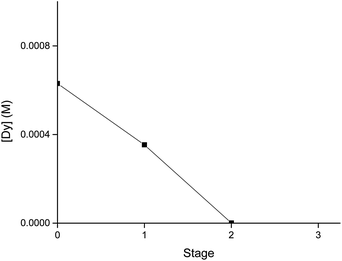 | ||
Fig. 7 Extraction behaviour of Dy from the deep-eutectic solvent choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid (1 lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The extraction was carried out with Cyanex 923 (0.9 M in toluene). The LP 2). The extraction was carried out with Cyanex 923 (0.9 M in toluene). The LP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HP phase ratio was 2 HP phase ratio was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
After extraction, part of the raffinate containing mainly Nd was contacted with a stoichiometric amount of oxalic acid and stirred at 25 °C during 30 min. The light pink precipitate that was obtained was filtered, washed with water and ethanol and then calcined at 950 °C during 3 hours. The blue powder that was obtained was analyzed by XRD and corresponded to Nd2O3.71 The dissolution of this powder in concentrated HCl and its quantification with ICP-OES indicated a purity of 99.87% and 0.13% of Dy. The less polar phase obtained from the mixer settler, rich in Dy and containing Nd was scrubbed manually in a separatory funnel, 5 times, with 6 M CaCl2 and then the Dy was stripped with water, precipitated with oxalic acid and calcined at 950 °C. The obtained white powder was analyzed by XRD which indicated that it corresponded to Dy2O3,72 the purity was determined by ICP and it corresponded to 99.94% Dy and 0.06% Nd as impurity.
Recycling of the less polar phases
After the removal of Fe, B and Co from the feed solution with the ionic liquid [A336][SCN] (0.9 M in toluene) and the stripping of these elements using 6 M HCl and 1.5 M EDTA pH 5.6, the less polar phase, without any further treatment, was put in contact with a fresh DES mixture containing Fe, B, Co, Nd and Dy. In a similar experiment, the less polar phase was equilibrated with KSCN before its reuse in a new extraction. Results are presented in Table 6.| Less polar phase | DFe ± SD | DB± SD | DCo ± SD |
|---|---|---|---|
| a 25 min equilibration time, 2000 rpm, 25 °C. | |||
| Without treatment | 30.3 ± 1.4 | 2.6 ± 0.2 | 65.7 ± 2.4 |
| Equilibrated with [SCN]− | 52.9 ± 4.9 | 0.84 ± 0.07 | 199 ± 8 |
Table 6 shows how after the stripping of Fe, B and Co with HCl and EDTA, the less polar phase can be re-used for the extraction of these metals. The percentages of extraction for Fe and Co are comparable to the ones obtained when carrying out the extraction for the first time (Table 1). However, in the case of B, higher percentages extraction were obtained and they are comparable to the ones obtained when [A336][Cl] was used as extractant. When the less polar phase is equilibrated with 2.5 M KSCN before its re-use, the percentages extraction that are obtained are similar to those observed when working with [A336][SCN]. This could indicate that there is an anionic exchange mechanism when using [A336][SCN], therefore, after extraction, the less polar phase is richer in chloride ions, which explains why the higher percentages of extraction for boron when the less polar phase is reused without equilibration with KSCN. In fact, the analysis of the less polar phase with TXRF after extraction indicated the presence of chloride peaks.
After stripping with water, the less polar phase (Cyanex 923 diluted in toluene 0.9 M), was re-used to extract Dy from the mixture of Nd and Dy in the DES of choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid (molar ratio 1
lactic acid (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and no significant deviations from the results obtained when using the extractant for the first time were encountered.
2) and no significant deviations from the results obtained when using the extractant for the first time were encountered.
Comparison with conventional systems
The developed system was compared to the traditional liquid–liquid extraction systems composed by an aqueous phase and an organic phase. Since the concentration of chloride anions in the DES is close to 3 M, it was decided to carry out the experiments with an aqueous phase containing 1.5 M CaCl2 to have a concentration of chloride anions close to the one present in the DES, a concentration of 5 M CaCl2 in the aqueous phase was also tested for comparison (Table 7).| More polar phase | DFe ± SD | DB ± SD | DCo ± SD | DNd ± SD | DDy ± SD |
|---|---|---|---|---|---|
| a Equilibration time: 60 min, 2000 rpm, 25 °C.b n.d. = not detected (below limit of detection). | |||||
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid (molar ratio 1 lactic acid (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
44.5 ± 2.6 | 0.78 ± 0.10 | 193 ± 2 | 0.029 ± 0.007 | 0.025 ± 0.008 |
| Aqueous phase (without CaCl2) | 5.7 ± 0.3 | n.d.b | 10.0 ± 1.4 | n.d.b | 0.080 ± 0.009 |
| Aqueous phase (CaCl2 1.5 [M]) | 20.0 ± 1.6 | n.d.b | 29.8 ± 1.2 | 0.10 ± 0.02 | 0.23 ± 0.04 |
| Aqueous phase (CaCl2 5 [M]) | 49.6 ± 1.5 | 0.09 ± 0.03 | 72 ± 1 | 0.31 ± 0.08 | 0.45 ± 0.07 |
From Table 7 it can be seen how higher D for Fe and Co are obtained when extracting from DESs. Moreover, the extraction of B is not possible from the aqueous system. The extraction of metal ions from the aqueous system can be improved by addition of CaCl2 but by doing this, the selectivity is lost since Dy and Nd are co-extracted. The use of a DES as the more polar phase was also beneficial for the separation of Nd and Dy in comparison to conventional aqueous systems (Table 8). When extracting Nd and Dy at these concentrations from aqueous systems, low D and SF are obtained. When extracting from DESs, better separation factors are obtained. This can be due to the presence of lactic acid in the more polar phase. It has been reported how a complexing agent that can form complexes with rare-earth cations (with different equilibrium constants) can significantly increase the possibility of selectively separate the different rare earth cations. The complexation of rare-earth cations with enhances the sorption performance and improves the selectivity.73–75 This highlights the importance of these new systems and the importance of studying and improving them. Fig. 8 shows the flow sheet for a process to separate Fe, B and Co from Nd and Dy in a DES and afterwards to separate Dy from Nd using [A336][SCN] (0.9 M in toluene) and Cyanex 923 (0.9 M in toluene), respectively.
| More polar phase | DNd ± SD | DDy ± SD |
|---|---|---|
| a Equilibration time: 60 min, 2000 rpm, 25 °C. | ||
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid (molar ratio 1 lactic acid (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
0.17 ± 0.09 | 1.89 ± 1.2 |
| Aqueous phase (without CaCl2) | 0.10 ± 0.02 | 0.23 ± 0.06 |
| Aqueous phase (CaCl2 1.5 [M]) | 0.29 ± 0.06 | 0.55 ± 0.12 |
| Aqueous phase (CaCl2 5 [M]) | 0.45 ± 0.05 | 0.77 ± 0.13 |
EXAFS
Understanding of the mechanism of extraction is crucial in every single extraction process. When it is understood how the extraction occurs, the process can be optimized and redesigned in order to create better systems in terms of efficiency and consumption of chemicals. The extraction of transition metals and rare earths using conventional extractants from aqueous systems is described extensively in the literature.14,16,57 However, the mechanism of extraction from DESs has not been studied yet. The mechanism of extraction of Fe and Co with [A336][SCN] 0.9 M in toluene was studied from the DES phase and compared against the extraction of the same metals from aqueous solutions.A fit of the first coordination shell of iron extracted from the aqueous to the [A336][SCN] phase showed 6 nitrogen atoms. Therefore, a model containing 6 coordinating thiocyanate anions was used to fit the experimental data. A model including a linear Fe–N–C bond showed bad fits. In case of a non-linear Fe–N–C bond, four-leg scattering paths can be excluded if the bond angle is not close to 180°. A model including three two-leg scattering paths (Fe–N, Fe–C and Fe–S) and three three-leg scattering paths resulted in a good fit of the data. The degeneracy of the different paths were constrained but S0 was allowed to vary resulting in a value of 0.95. The distances to N, C and S also proof the non-linearity of the Fe–N–C bond as the total sum of the bond lengths in Fe–N–C–S is around 5 Å and the fact that the three leg scattering paths to carbon and sulphur are significantly larger in distance than the two leg scattering paths to carbon and sulphur, respectively (Table S2, Fig. S6 and S7†).
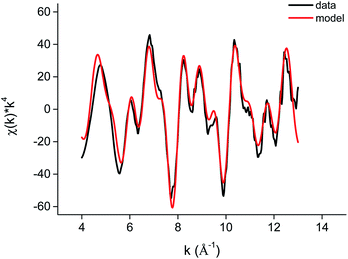
The edge structure of the iron(III) complex extracted from the DES looked very similar to the edge structure previously observed for [FeCl4]− complexes (Fig. S8†).76,77 A fit of the EXAFS region, including only Fe–Cl single scattering paths resulted in a fit degeneracy of 3.4, a bond distance of 2.208 Å and a Debye Waller factor of 0.004 Å2. A slightly better fit was obtained when including also one Fe–O path. However, this is contradictory to the rest of the Fourier transform as coordinating oxygen atoms can only come from lactic acid or choline. This suggests that there should be contributions to the FT at slightly higher R values as well, which is not the case. Therefore, it can be concluded that iron extracts as a [FeCl4]− complex to the less polar phase (Fig. 9 and 10).
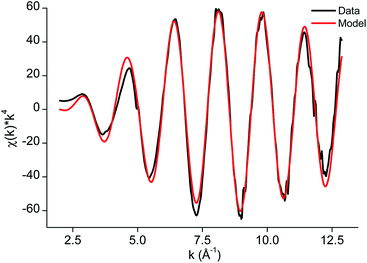 | ||
| Fig. 9 EXAFS function χ(k) × k4 and model of the [FeCl4]− complex in [A336][SCN] diluted in toluene 0.9 M, as extracted from the DES. | ||
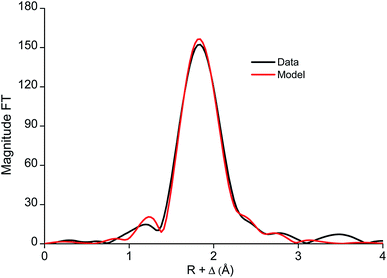 | ||
| Fig. 10 Fourier transform and model of the [FeCl4]− complex in [A336][SCN] diluted in toluene (0.9 M), as extracted from DES. | ||
Fitting of the first shell of the Co complex in the [A336][SCN] phase, as extracted from an aqueous solution, showed the presence of four nitrogen atoms. Therefore, a model consisting of four thiocyanate ligands was used to fit the experimental data. Three single scattering paths and three three-leg scattering paths were included, and of which the degeneracy was constrained. The amplitude reduction factor S0 was set to 0.95. Similarly to the [Fe(SCN)6]3− complex in [A336][SCN], the three-leg scattering paths Co–C–N, Co–S–C and Co–S–N are larger than the distances between Co and N, C and Co, which indicates the non-linearity between the Co–N–C angle and the N–C–S angle (Table S3†).
The EXAFS function of the Co complex extraction from the DES and from 5 M CaCl2 was very similar to the EXAFS function of Co extracted from an aqueous solution, which indicates that the [Co(SCN)4]2− complex is formed in both cases (Fig. S9†). This result is in contrast to the extraction of iron, where [Fe(SCN)6]3− complex is formed when extraction occurred from an aqueous solution, while a [FeCl4]− complex is formed when extracted from DES (Fig. 11 and 12).
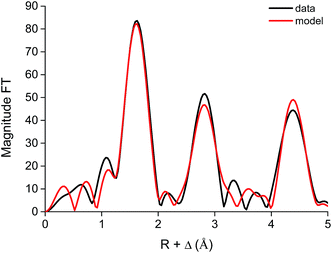 | ||
| Fig. 12 Fourier transform and model of the [Co(SCN)4]2− complex in the ionic liquid [A336][SCN], as extracted from aqueous solution. | ||
Furthermore, samples of the organic and the DES phase after extraction of Fe were taken and analyzed by 1H NMR. Components of the DES were not detected in the less polar phase nor the Aliquat 336 cation was detected in the DES. The latter results in addition with the ones obtained by EXAFS, suggest that the mechanism of extraction of Fe from the DES choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lactic acid (1
lactic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) corresponds to an anionic exchange (eqn (20)).
2) corresponds to an anionic exchange (eqn (20)).
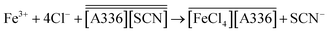 | (20) |
This means that part of the thiocyanate anions are being exchanged by chloride anions from the DES phase and thus, the ionic liquid in the less polar phase is being degraded. This issue can be solved by contacting the less polar phase with [SCN]− and regenerating the ionic liquid after extraction (Table 6).
Conclusions
For the first time a DES composed of choline chloride and lactic acid (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was successfully employed for the complete dissolution of NdFeB magnets. Fe, Co and B were removed from this leachate using the ionic liquid [A336][SCN] as extractant diluted in toluene (0.9 M). Cyanex 923 diluted in toluene (0.9 M) was a better extractant for the separation of Dy from Nd than D2EHPA diluted in toluene, since it allowed easier scrubbing and stripping. As demonstrated by EXAFS measurements, the extraction mechanism from DESs is different from those from aqueous solutions, thus different distribution ratios and separation ratios were achieved. As a result, the use of DESs allowed higher selectivity for the removal of Fe, Co and B from Nd and Dy and also for the separation of Dy from Nd in comparison with conventional liquid–liquid extraction systems. The feasibility of scaling up the proposed process was further confirmed by its implementation in a mixer-settler setup. It was found that the studied DES can be used at room temperature without any issue related to high viscosity, poor phase disengagement or third-phase formation. The metals were stripped by HCl and EDTA (for Fe, B and Co) and water (for Dy) from the less polar phase and a stoichiometric amount of oxalic acid for the removal of Nd from the DES. Nd2O3 and Dy2O3 were recovered with purities of 99.87% and 99.94%, respectively.
2) was successfully employed for the complete dissolution of NdFeB magnets. Fe, Co and B were removed from this leachate using the ionic liquid [A336][SCN] as extractant diluted in toluene (0.9 M). Cyanex 923 diluted in toluene (0.9 M) was a better extractant for the separation of Dy from Nd than D2EHPA diluted in toluene, since it allowed easier scrubbing and stripping. As demonstrated by EXAFS measurements, the extraction mechanism from DESs is different from those from aqueous solutions, thus different distribution ratios and separation ratios were achieved. As a result, the use of DESs allowed higher selectivity for the removal of Fe, Co and B from Nd and Dy and also for the separation of Dy from Nd in comparison with conventional liquid–liquid extraction systems. The feasibility of scaling up the proposed process was further confirmed by its implementation in a mixer-settler setup. It was found that the studied DES can be used at room temperature without any issue related to high viscosity, poor phase disengagement or third-phase formation. The metals were stripped by HCl and EDTA (for Fe, B and Co) and water (for Dy) from the less polar phase and a stoichiometric amount of oxalic acid for the removal of Nd from the DES. Nd2O3 and Dy2O3 were recovered with purities of 99.87% and 99.94%, respectively.
Acknowledgements
The research leading to these results has received funding from the European Community's Seventh Framework Programme ([FP7/2007–2013]) under grant agreement no. 607411 (MC-ITN EREAN: European Rare Earth Magnet Recycling Network). This publication reflects only the author's view, exempting the Community from any liability. Project website: http://www.erean.eu. Mehmet Ali Recai Önal (KU Leuven) is acknowledged for milling the magnets. The authors thank Stefan Möwius (BEC Gesellschaft für Produktmanagement GmbH) and Žiga Erman (Magneti Ljubljana) for providing the NdFeB magnet samples, the FWO for the postdoctoral fellowship of TVDH and the VLAIO for the PhD scholarship to BO.References
- J. H. Rademaker, R. Kleijn and Y. Yang, Environ. Sci. Technol., 2013, 47, 10129–10136 CrossRef CAS PubMed.
- K. Binnemans, P. T. Jones, B. Blanpain, T. Van Gerven, Y. Yang, A. Walton and M. Buchert, J. Cleaner Prod., 2013, 51, 1–22 CrossRef CAS.
- Y. Yang, A. Walton, R. Sheridan, K. Güth, R. Gauß, O. Gutfleisch, M. Buchert, B.-M. Steenari, T. Van Gerven, P. T. Jones and K. Binnemans, J. Sustain. Metall., 2017, 3, 122–149 CrossRef.
- R. Sasai and N. Shimamura, J. Asian. Cer. Soc., 2016, 4, 155–158 CrossRef.
- O. Takeda, T. H. Okabe and Y. Umetsu, J. Alloys Compd., 2006, 408–412, 387–390 CrossRef CAS.
- A. Walton, H. Yi, N. A. Rowson, J. D. Speight, V. S. J. Mann, R. S. Sheridan, A. Bradshaw, I. R. Harris and A. J. Williams, J. Cleaner Prod., 2015, 104, 236–241 CrossRef CAS.
- M. Zakotnik, I. R. Harris and A. J. Williams, J. Alloys Compd., 2008, 450, 525–531 CrossRef CAS.
- M. Zakotnik, I. R. Harris and A. J. Williams, J. Alloys Compd., 2009, 469, 314–321 CrossRef CAS.
- X. Li, M. Yue, M. Zakotnik, W. Liu, D. Zhang and T. Zuo, J. Rare Earths, 2015, 33, 736–739 CrossRef CAS.
- D. Dupont and K. Binnemans, Green Chem., 2015, 17, 856–868 RSC.
- S. Riano and K. Binnemans, Green Chem., 2015, 17, 2931–2942 RSC.
- T. Vander Hoogerstraete, S. Wellens, K. Verachtert and K. Binnemans, Green Chem., 2013, 15, 919–927 RSC.
- D. Beltrami, G. Cote, H. Mokhtari, B. Courtaud, B. A. Moyer and A. Chagnes, Chem. Rev., 2014, 114, 12002–12023 CrossRef CAS PubMed.
- S. Nishihama, T. Hirai and I. Komasawa, Ind. Eng. Chem. Res., 2001, 40, 3085–3091 CrossRef CAS.
- P. J. Panak and A. Geist, Chem. Rev., 2013, 113, 1199–1236 CrossRef CAS PubMed.
- F. Xie, T. A. Zhang, D. Dreisinger and F. Doyle, Miner. Eng., 2014, 56, 10–28 CrossRef CAS.
- Z. Zhu, W. Zhang and C. Y. Cheng, Hydrometallurgy, 2011, 105, 304–313 CrossRef CAS.
- M. K. Jha, D. Gupta, J.-C. Lee, V. Kumar and J. Jeong, Hydrometallurgy, 2014, 142, 60–69 CrossRef CAS.
- V. S. Kislik, in Solvent Extraction, Elsevier, Amsterdam, 2012, ch. 1, pp. 3–67 Search PubMed.
- V. S. Kislik, in Solvent Extraction, Elsevier, Amsterdam, 2012, ch. 3, pp. 113–156 Search PubMed.
- M. A. R. Önal, C. R. Borra, M. Guo, B. Blanpain and T. Gerven, J. Sustain. Metall., 2015, 1, 199–215 CrossRef.
- M. Al-Harahsheh and S. W. Kingman, Hydrometallurgy, 2004, 73, 189–203 CrossRef CAS.
- S. S. Konyratbekova, A. Baikonurova and A. Akcil, Miner. Process. Extr. Metall. Rev., 2015, 36, 198–212 CrossRef CAS.
- M. Sahin, A. Akcil, C. Erust, S. Altynbek, C. S. Gahan and A. Tuncuk, Sep. Sci. Technol., 2015, 50, 2587–2595 CAS.
- Y. Dong, X. Sun, Y. Wang and Y. Chai, Hydrometallurgy, 2015, 157, 256–260 CrossRef CAS.
- T. Vander Hoogerstraete, B. Onghena and K. Binnemans, J. Phys. Chem. Lett., 2013, 4, 1659–1663 CrossRef PubMed.
- X. Sun, C.-L. Do-Thanh, H. Luo and S. Dai, Chem. Eng. J., 2014, 239, 392–398 CrossRef CAS.
- X. Sun, H. Luo and S. Dai, Talanta, 2012, 90, 132–137 CrossRef CAS PubMed.
- J. Wang, J. Zhao, D. Feng, X. Kang, Y. Sun, L. Zhao and H. Liang, J. Rare Earths, 2016, 34, 83–90 CrossRef CAS.
- H.-L. Yang, W. Wang, H.-M. Cui and J. Chen, Chin. J. Anal. Chem., 2011, 39, 1561–1566 CrossRef CAS.
- K. Larsson and K. Binnemans, Hydrometallurgy, 2015, 156, 206–214 CrossRef CAS.
- A. Rout and K. Binnemans, Dalton Trans., 2014, 43, 1862–1872 RSC.
- A. P. Abbott, G. Capper, D. L. Davies, K. J. McKenzie and S. U. Obi, J. Chem. Eng. Data, 2006, 51, 1280–1282 CrossRef CAS.
- G. R. T. Jenkin, A. Z. M. Al-Bassam, R. C. Harris, A. P. Abbott, D. J. Smith, D. A. Holwell, R. J. Chapman and C. J. Stanley, Miner. Eng., 2016, 87, 18–24 CrossRef CAS.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- M. R. S. Foreman, Cogent Chem., 2016, 2, 1139289 Search PubMed.
- A. P. Abbott, J. C. Barron, K. S. Ryder and D. Wilson, Chem.–Eur. J., 2007, 13, 6495–6501 CrossRef CAS PubMed.
- Q. Zhang, K. De Oliveira Vigier, S. Royer and F. Jerome, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and P. Shikotra, Inorg. Chem., 2005, 44, 6497–6499 CrossRef CAS PubMed.
- B. Sprecher, Y. Xiao, A. Walton, J. Speight, R. Harris, R. Kleijn, G. Visser and G. J. Kramer, Environ. Sci. Technol., 2014, 48, 3951–3958 CrossRef CAS PubMed.
- W. Yan, S. Yan, D. Yu, K. Li, H. Li, Y. Luo and H. Yang, J. Rare Earths, 2012, 30, 133–136 CrossRef CAS.
- P. C. Dent, J. Appl. Phys., 2012, 111, 07A721 CrossRef.
- J. O. Liljenzin, G. Persson, I. Svantesson and S. Wingefors, in Transplutonium Elements—Production and Recovery, American Chemical Society, 1981, vol. 161, ch. 13, pp. 203–221 Search PubMed.
- K. V. Klementev, Nucl. Instrum. Methods Phys. Res., Sect. A, 2000, 448, 299–301 CrossRef CAS.
- M. Newville, J. Synchrotron Radiat., 2001, 8, 96–100 CrossRef CAS PubMed.
- L. K. Jakobsson, G. Tranell and I.-H. Jung, Metall. Trans. B, 2017, 48, 60–72 CrossRef CAS.
- M. I. Saleh, M. F. Bari and B. Saad, Hydrometallurgy, 2002, 63, 75–84 CrossRef CAS.
- A. A. Mhaske and P. M. Dhadke, Hydrometallurgy, 2001, 61, 143–150 CrossRef CAS.
- G. A. Clark, R. M. Izatt and J. J. Christensen, Sep. Sci. Technol., 1983, 18, 1473–1482 CrossRef CAS.
- A. Cieszynska and M. Wisniewski, Sep. Purif. Technol., 2010, 73, 202–207 CrossRef CAS.
- J. Yang, T. Retegan and C. Ekberg, Hydrometallurgy, 2013, 137, 68–77 CrossRef CAS.
- Y. Jin, Y. Ma, Y. Weng, X. Jia and J. Li, J. Ind. Eng. Chem., 2014, 20, 3446–3452 CrossRef CAS.
- N. Panda, N. Devi and S. Mishra, J. Rare Earths, 2012, 30, 794–797 CrossRef CAS.
- S. M. Liu, H. H. Liu, Y. J. Huang and W. J. Yang, Trans. Nonferrous Met. Soc. China, 2015, 25, 329–334 CrossRef CAS.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- R. Pizer and R. Selzer, Inorg. Chem., 1984, 23, 3023–3026 CrossRef CAS.
- M. K. Jha, A. Kumari, R. Panda, J. Rajesh Kumar, K. Yoo and J. Y. Lee, Hydrometallurgy, 2016, 165, 2–26 CrossRef CAS.
- K. Ohto, M. Yano, K. Inoue, T. Yamamoto, M. Goto, F. Nakashio, S. Shinkai and T. Nagasaki, Anal. Sci., 1995, 11, 893–902 CrossRef CAS.
- D. Zheng, N. B. Gray and G. W. Stevens, Solvent Extr. Ion Exch., 1991, 9, 85–102 CrossRef CAS.
- C. Tunsu, C. Ekberg, M. Foreman and T. Retegan, Solvent Extr. Ion Exch., 2014, 32, 650–668 CrossRef CAS.
- N. K. Batchu, T. Vander Hoogerstraete, D. Banerjee and K. Binnemans, Sep. Purif. Technol., 2017, 174, 544–553 CrossRef CAS.
- B. Gupta, P. Malik and A. Deep, Solvent Extr. Ion Exch., 2003, 21, 239–258 CrossRef CAS.
- Y. A. El-Nadi, J. Rare Earths, 2010, 28, 215–220 CrossRef CAS.
- M. L. P. Reddy, R. Luxmi Varma, T. R. Ramamohan, S. K. Sahu and V. Chakravortty, Solvent Extr. Ion Exch., 1998, 16, 795–812 CrossRef CAS.
- D. F. Peppard, J. P. Faris, P. R. Gray and G. W. Mason, J. Phys. Chem., 1953, 57, 294–301 CrossRef CAS.
- J. S. Kim, B. N. Kumar, S. Radhika, M. L. Kantam and B. R. Reddy, Int. J. Miner. Process., 2012, 112–113, 37–42 CrossRef CAS.
- D. Li and C. Wang, Hydrometallurgy, 1998, 48, 301–312 CrossRef CAS.
- A. Fortuny, M. T. Coll, C. S. Kedari and A. M. Sastre, J. Chem. Technol. Biotechnol., 2014, 89, 858–865 CrossRef CAS.
- T. E. Furia, in CRC Handbook of Food Additives, ed. C. Press, CRC Press, Boca Raton, Florida, 1972 Search PubMed.
- G. Schwarzenbach, Analyst, 1955, 80, 713–729 RSC.
- T. Liu, Y. H. Zhang, H. Y. Shao and X. G. Li, Langmuir, 2003, 19, 7569–7572 CrossRef CAS.
- A. Bassano, V. Buscaglia, M. Sennour, M. T. Buscaglia, M. Viviani and P. Nanni, J. Nanopart. Res., 2010, 12, 623–633 CrossRef CAS.
- F. V. P. Blondet and T. Guibal, in Ion Exch. Solvent Extr., CRC Press, Boca Raton, Florida, 2007, pp. 151–292 Search PubMed.
- S. Yin, W. Wu, X. Bian, Y. Luo and F. Zhang, Ind. Eng. Chem. Res., 2013, 52, 8558–8564 CrossRef CAS.
- S. Yin, W. Wu, X. Bian and F. Zhang, Hydrometallurgy, 2013, 131–132, 133–137 CrossRef CAS.
- A. Kiyotaka, N. Masaharu and K. Haruo, Bull. Chem. Soc. Jpn., 1985, 58, 1543–1550 CrossRef.
- M. Bauer and C. Gastl, Phys. Chem. Chem. Phys., 2010, 12, 5575–5584 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra06540j |
| This journal is © The Royal Society of Chemistry 2017 |